NO Synthesis Markers Are Not Significantly Associated with Blood Pressure and Endothelial Dysfunction in Patients with Arterial Hypertension: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| ADMA | Asymmetric dimethylarginine |
| AGXT2 | Alanine-glyoxylate aminotransferase 2 |
| ANOVA | Analysis of variance |
| BMI | Body mass index |
| CD | Cardiovascular disease |
| cGMP | Cyclic guanosinmonophosphate |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CV | Coefficient of variation |
| DDAH I/II | Dimethylarginine dimethylaminohydrolase I/II |
| GFR | Glomerular filtration rate |
| HbA1c | Glycated hemoglobin |
| HDL | High Density Lipoprotein |
| HPLC | High-performance liquid chromatography |
| LDL | Low Density Lipoprotein |
| NO | Nitric oxide |
| PRMT I/II | Protein arginine methyltransferases I/II |
| PWV | Pulse wave velocity |
| SDMA | Symmetric dimethylarginine |
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Huffman, M.D.; Moran, A.E.; Feigin, V.L.; Mensah, G.A.; Naghavi, M.; Murray, C.J. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015, 132, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A.A. Homoarginine and all-cause mortality: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12960. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Yanchev, G.R.; Kayacelebi, A.A.; Hanff, E.; Bledau, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; Weissenborn, K.; Bauersachs, J.; et al. The role of L-arginine/L-homoarginine/nitric oxide pathway for aortic distensibility and intima-media thickness in stroke patients. Amino Acids 2017, 110, 1111–1121. [Google Scholar] [CrossRef]
- Dominiczak, A.F.; Bohr, D.F. Nitric oxide and its putative role in hypertension. Hypertension 1995, 25, 1202–1211. [Google Scholar] [CrossRef]
- Kayacelebi, A.A.; Willers, J.; Pham, V.V.; Hahn, A.; Schneider, J.Y.; Rothmann, S.; Frölich, J.C.; Tsikas, D. Plasma homoarginine, arginine, asymmetric dimethylarginine and total homocysteine interrelationships in rheumatoid arthritis, coronary artery disease and peripheral artery occlusion disease. Amino Acids 2015, 47, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Karetnikova, E.S.; Jarzebska, N.; Markov, A.G.; Weiss, N.; Lentz, S.R.; Rodionov, R.N. Is Homoarginine a Protective Cardiovascular Risk Factor? Arter. Thromb. Vasc. Biol. 2019, 39, 869–875. [Google Scholar] [CrossRef]
- Pilz, S.; Meinitzer, A.; Gaksch, M.; Grübler, M.; Verheyen, N.; Drechsler, C.; Hartaigh, B.Ó.; Lang, F.; Alesutan, I.; Voelkl, J.; et al. Homoarginine in the renal and cardiovascular systems. Amino Acids 2015, 47, 1703–1713. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Androulakis, E.; Papaioannou, S.; Antoniades, C.; Tousoulis, D. Homoarginine in the shadow of asymmetric dimethylarginine: From nitric oxide to cardiovascular disease. Amino Acids 2015, 47, 1741–1750. [Google Scholar] [CrossRef]
- Atzler, D.; McAndrew, D.J.; Cordts, K.; Schneider, J.E.; Zervou, S.; Schwedhelm, E.; Neubauer, S.; Lygate, C.A. Dietary supplementation with homoarginine preserves cardiac function in a murine model of post-myocardial infarction heart failure. Circulation 2017, 135, 400–402. [Google Scholar] [CrossRef]
- Dellera, F.; Ganzetti, G.S.; Froio, A.; Manzini, S.; Busnelli, M.; Meinitzer, A.; Sirtori, C.R.; Chiesa, G.; Parolini, C. L-homoarginine administration reduces neointimal hyperplasia in balloon-injured rat carotids. Thromb. Haemost. 2016, 116, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Stockebrand, M.; Hornig, S.; Neu, A.; Atzler, R.; Cordts, K.; Böger, R.H.; Isbrandt, D.; Schwedhelm, E.; Choe, C.-U. Homoarginine supplementation improves blood glucose in diet-induced obese mice. Amino Acids 2015, 47, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Edelmann, F.; Meinitzer, A.; Gelbrich, G.; Döner, U.; Düngen, H.-D.; Tomaschitz, A.; Kienreich, K.; Gaksch, M.; Duvinage, A.; et al. Associations of methylarginines and homoarginine with diastolic dysfunction and cardiovascular risk factors in patients with preserved left ventricular ejection fraction. J. Card. Fail. 2014, 20, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Jud, P.; Hafner, F.; Verheyen, N.; Meinitzer, A.; Gary, T.; Brodmann, M.; Seinost, G.; Hackl, G. Homoarginine/ADMA ratio and homoarginine/SDMA ratio as independent predictors of cardiovascular mortality and cardiovascular events in lower extremity arterial disease. Sci Rep. 2018, 8, 14197. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H. Asymmetric dimethylarginine (ADMA) and cardiovascular disease: Insights from prospective clinical trials. Vasc. Med. 2005, 10 (Suppl. S2), S19–S25. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J. Am. Soc. Nephrol. 2006, 17, 1128–1134. [Google Scholar] [CrossRef]
- Cooke, J.P. Does ADMA cause endothelial dysfunction? Arter. Thromb. Vasc. Biol. 2000, 20, 2032–2037. [Google Scholar] [CrossRef]
- Tsikas, D.; Bollenbach, A.; Hanff, E.; Kayacelebi, A.A. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): The ADMA, SDMA and hArg paradoxes. Cardiovasc Diabetol. 2018, 17, 1. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef]
- Michel, T. R is for arginine: Metabolism of arginine takes off again, in new directions. Circulation 2013, 128, 1400–1404. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Salpeter, S.R.; Bode-Boeger, S.M.; Cooke, J.P.; Fliser, D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function--a meta-analysis. Nephrol. Dial. Transplant. 2006, 21, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.; Vallance, P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999, 43, 542–548. [Google Scholar] [CrossRef]
- Hermenegildo, C.; Medina, P.; Peiroó, M.; Segarra, G.; Vila, J.M.; Ortega, J.; Lluch, S. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in hyperthyroid patients. J. Clin. Endocrinol. Metab. 2002, 87, 5636–5640. [Google Scholar] [CrossRef] [PubMed]
- Goonasekera, C.D.; Rees, D.D.; Woolard, P.; Frend, A.; Shah, V.; Dillon, M.J. Nitric oxide synthase inhibitors and hypertension in children and adolescents. J. Hypertens. 1997, 15, 901–909. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef]
- MacAllister, R.J.; Rambausek, M.H.; Vallance, P.; Williams, D.; Hoffmann, K.H.; Ritz, E. Concentration of dimethyl-L-arginine in the plasma of patients with end-stage renal failure. Nephrol. Dial. Transplant. 1996, 11, 2449–2452. [Google Scholar] [CrossRef]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grübler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; Hartaigh, B.Ó.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef]
- Grosse, G.M.; Schwedhelm, E.; Worthmann, H.; Choe, C.U. Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2020, 21, 1798. [Google Scholar] [CrossRef]
- Grübler, M.R.; Gaksch, M.; Kienreich, K.; Verheyen, N.; Schmid, J.; Hartaigh, B.Ó.; Richtig, G.; Scharnagl, H.; Meinitzer, A.; Fahrleitner-Pammer, A.; et al. Effects of vitamin D supplementation on glycated haemoglobin and fasting glucose levels in hypertensive patients: A randomized controlled trial. Diabetes Obes. Metab. 2016, 18, 1006–1012. [Google Scholar] [CrossRef]
- Grübler, M.R.; Gaksch, M.; Kienreich, K.; Verheyen, N.; Schmid, J.; Hartaigh, B.W.Ó.; Richtig, G.; Scharnagl, H.; Meinitzer, A.; Pieske, B.; et al. Effects of Vitamin D Supplementation on Plasma Aldosterone and Renin-A Randomized Placebo-Controlled Trial. J. Clin. Hypertens. 2016, 18, 608–613. [Google Scholar] [CrossRef]
- Meinitzer, A.; Puchinger, M.; Winklhofer-Roob, B.M.; Rock, E.; Ribalta, J.; Roob, J.M.; Sundl, I.; Halwachs-Baumann, G.; Marz, W. Reference values for plasma concentrations of asymmetrical dimethylarginine (ADMA) and other arginine metabolites in men after validation of a chromatographic method. Clin. Chim. Acta 2007, 384, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, L.P.; Davids, M.; Scheffer, P.G.; Dekker, J.M.; Stehouwer, C.D.; Teerlink, T. L-Homoarginine and L-arginine are antagonistically related to blood pressure in an elderly population: The Hoorn study. J. Hypertens. 2013, 31, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Kayacelebi, A.A.; Nguyen, T.H.; Neil, C.; Horowitz, J.D.; Jordan, J.; Tsikas, D. Homoarginine and 3-nitrotyrosine in patients with takotsubo cardiomyopathy. Int. J. Cardiol. 2014, 173, 546–547. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.U.; Atzler, D.; Wild, P.S.; Carter, A.M.; Böger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine levels are regulated by L-arginine:glycine amidinotransferase and affect stroke outcome: Results from human and murine studies. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef]
- Chen, P.Y.; Sanders, P.W. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J. Clin. Investig. 1991, 88, 1559–1567. [Google Scholar] [CrossRef]
- Altun, Z.S.; Uysal, S.; Guner, G.; Yilmaz, O.; Posaci, C. Effects of oral L-arginine supplementation on blood pressure and asymmetric dimethylarginine in stress-induced preeclamptic rats. Cell Biochem. Funct. 2008, 26, 648–653. [Google Scholar] [CrossRef]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arter. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef]
- Tsikas, D. A critical review and discussion of analytical methods in the L-arginine/nitric oxide area of basic and clinical research. Anal. Biochem. 2008, 379, 139–163. [Google Scholar] [CrossRef] [PubMed]

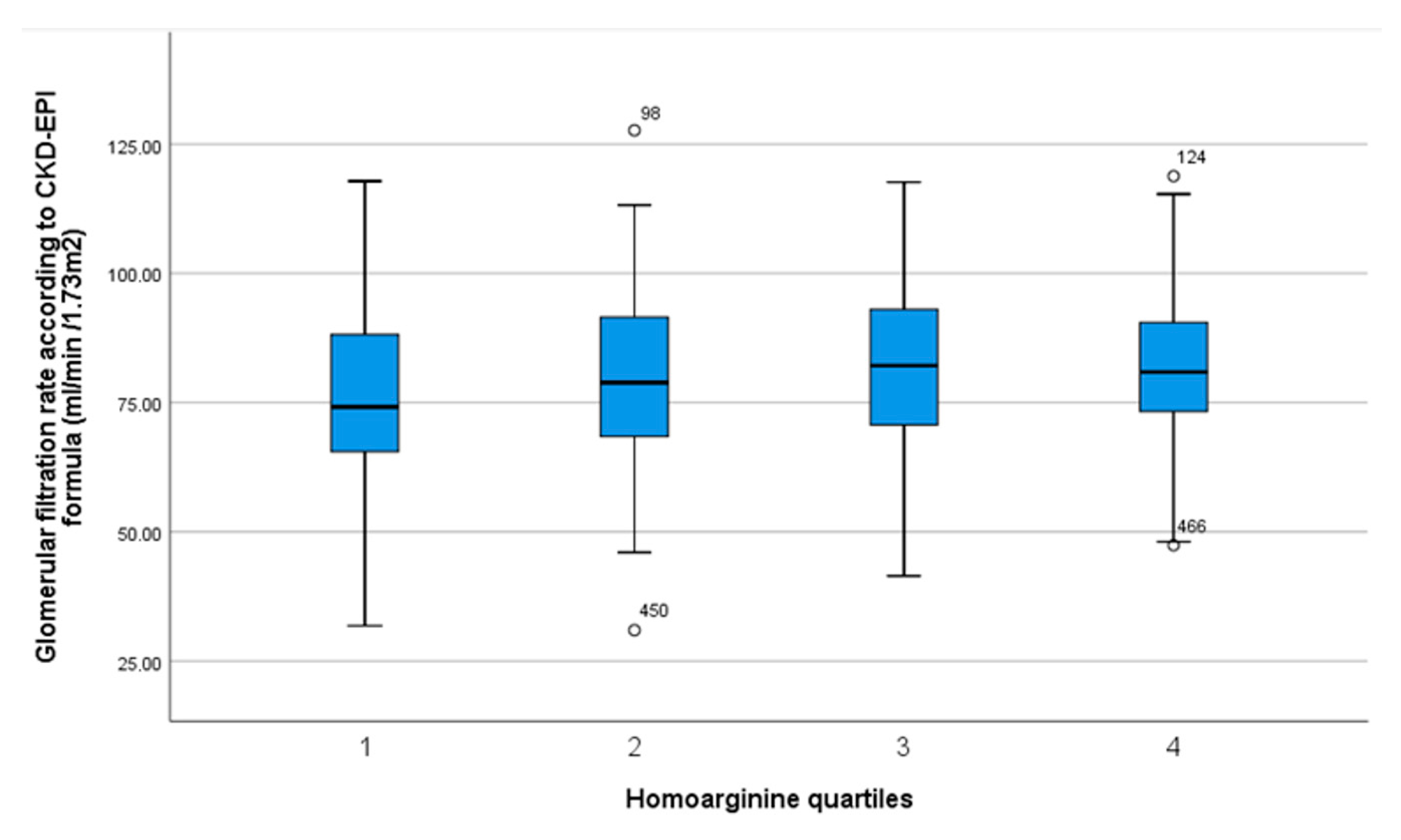
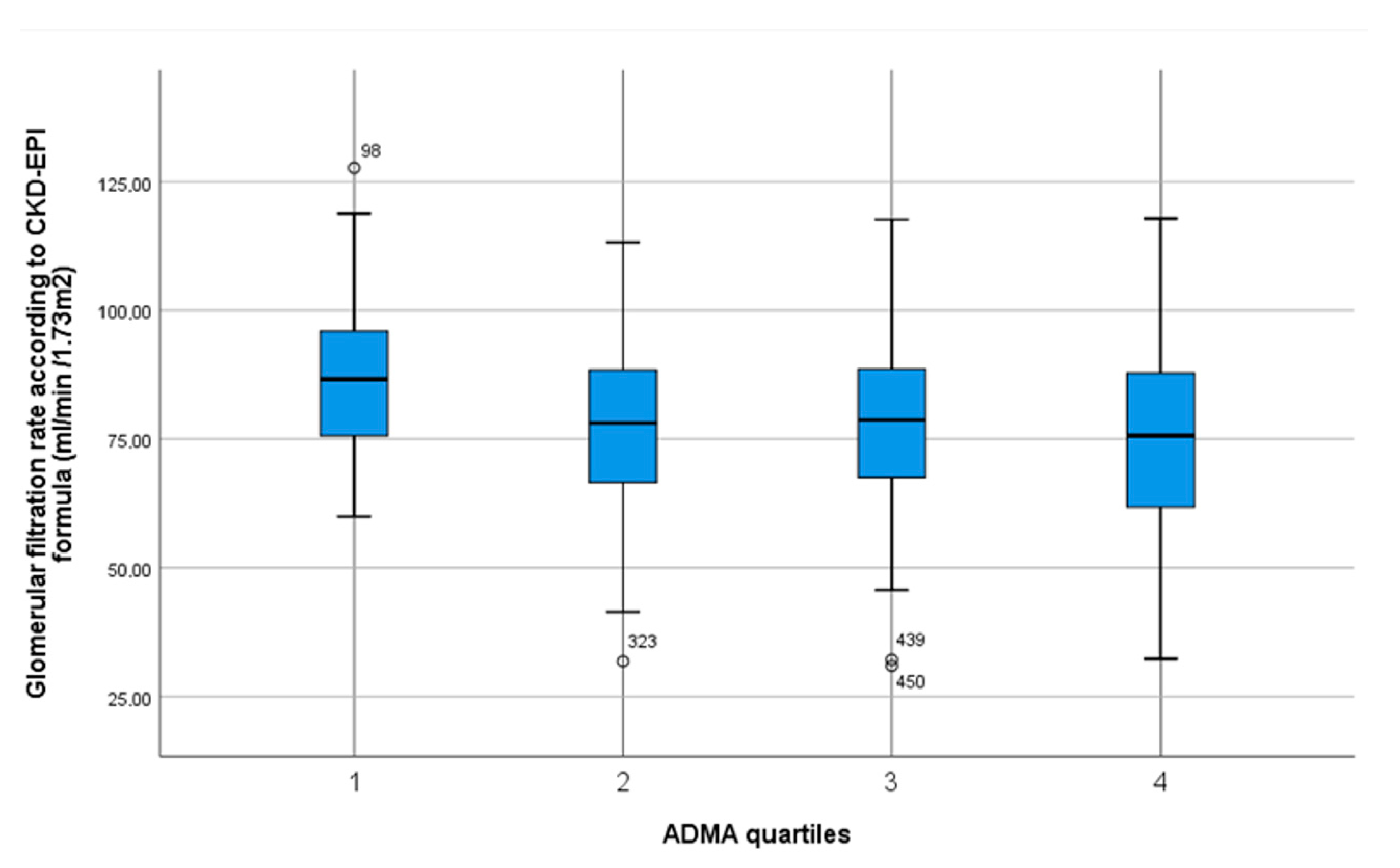
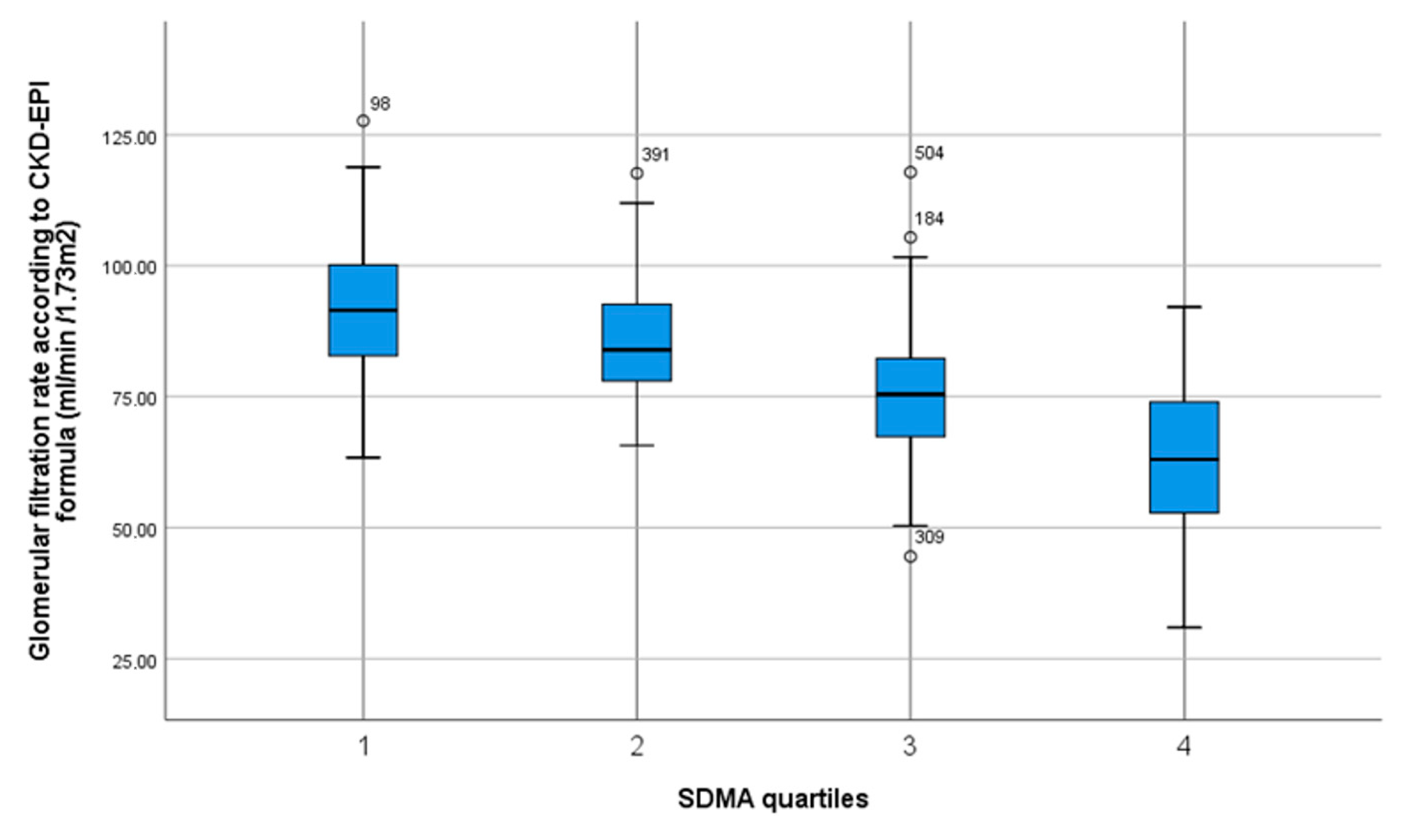
| Homoarginine Quartiles [µmol/L] | 0.58–1.45 n = 127 | 1.46–1.77 n = 131 | 1.78–2.23 n = 124 | 2.24–4.45 n = 127 | p Value Trend |
|---|---|---|---|---|---|
| Female [%] | 71.7 | 56.5 | 44.4 | 36.2 | p < 0.001 * |
| Age [years] | 63.5 ± 9.9 | 61.1 ± 1.1 | 60.9 ± 10.3 | 59.2 ± 10.5 | p = 0.002 * |
| BMI [kg/m2] | 28.6 ± 5.4 | 29.4 ± 4.8 | 30.0 ± 5.1 | 30.4 ± 5.1 | p = 0.004 * |
| Laboratory results | |||||
| Glomerular filtration rate (CKD-EPI formula) | 75.0 ± 18.6 | 80.3 ± 17.5 | 80.8 ± 17.3 | 81.7 ± 14.9 | p = 0.022 * |
| HbA1c [mmol/mol] | 44.9 ± 12.6 | 43.6 ± 11.9 | 41.9 ± 9.8 | 41.8 ± 12.1 | p = 0.29 |
| Total cholesterol [mg/dL] | 206 (169.8–228) | 198 (163.8–231) | 199 (171–224.5) | 186 (162–217) | p = 0.09 |
| HDL [mg/dL] | 60.5 (51.8–75) | 55 (46–69) | 55.5 (45–68) | 54 (44–65) | p = 0.006 * |
| LDL [mg/dL] | 114.5 (88.5–143.3) | 112.5 (82.3–148.3) | 115 (90–140) | 104 (87–133.5) | p = 0.81 |
| Triglycerides [mg/dL] | 99 (70.8–142.5) | 110 (76.5–145.3) | 112 (78.3–164.5) | 117 (80–169) | p = 0.06 |
| Medication [%] | |||||
| Beta blockers | 46.8 | 49.6 | 50 | 50 | p = 0.95 |
| ACE inhibitors | 38.1 | 35.9 | 24.2 | 40.5 | p = 0.035 * |
| Angiotensin II receptor antagonists | 30.2 | 34.4 | 34.7 | 30.2 | p = 0.78 |
| Calcium channel antagonists | 27.0 | 24.4 | 29.8 | 23.0 | p = 0.10 |
| Diuretics | 48.4 | 45.0 | 41.9 | 41.3 | p = 0.65 |
| Aldosterone antagonists | 4.8 | 3.1 | 4.0 | 4.0 | p = 0.92 |
| Other antihypertensive drugs | 12.0 | 16.0 | 11.3 | 17.5 | p = 0.42 |
| Glucocorticoids | 3.2 | 1.5 | 4.9 | 1.6 | p = 0.32 |
| Pulse wave velocity [m/sec] | 8.9 ± 2.5 | 8.8 ± 2.2 | 8.0 ± 2.1 | 8.5 ± 2.5 | p = 0.03 * |
| Mean 24 h systolic blood pressure [mm Hg] | 125.2 ± 14.9 | 127.9 ± 13.5 | 127.9 ± 13.4 | 129.2 ± 13.4 | p = 0.03 * |
| Mean 24 h diastolic blood pressure [mm Hg] | 74.9 ± 8.7 | 75.3 ± 8.9 | 77.1 ± 9.4 | 78.2 ± 10.5 | p = 0.002 * |
| Mean 24 h heart rate [bpm] | 71.6 ± 9.5 | 72.5 ± 10.5 | 70.6 ± 9.0 | 72.8 ± 10.0 | p = 0.68 |
| Mean night systolic blood pressure [mm Hg] | 116.4 ± 16.9 | 116.8 ± 16.3 | 115.8 ± 19.6 | 117.6 ± 14.7 | p = 0.72 |
| Mean night diastolic blood pressure [mm Hg] | 66.9 ± 9.0 | 66.6 ± 9.5 | 68.5 ± 9.4 | 68.6 ± 9.3 | p = 0.07 |
| Mean night heart rate [bpm] | 64.4 ± 9.3 | 65.7 ± 9.7 | 63.8 ± 9.6 | 64.8 ± 8.9 | p = 0.86 |
| Mean Systolic Dipping [%] | −11.0 ± 9.5 | −11.9 ± 9.6 | −11.7 ± 8.0 | −12.2 ± 9.2 | p = 0.39 |
| Mean Diastolic Dipping [%] | −15.6 ± 9.1 | −15.3 ± 10.0 | −15.1 ± 8.6 | −15.7 ± 8.8 | p = 0.98 |
| ADMA Quartiles [µmol/L] | 0.47–0.64 n = 127 | 0.65–0.70 n = 138 | 0.71–0.77 n = 125 | 0.78–0.98 n = 119 | p Value Trend |
|---|---|---|---|---|---|
| Female [%] | 49.6 | 47.4 | 56.8 | 56.3 | p = 0.33 |
| Age [years] | 57.6 ± 11.4 | 60.9 ± 9.8 | 63.3 ± 9.9 | 63.2 ± 10.1 | p < 0.001 * |
| BMI [kg/m2] | 28.8 ± 4.7 | 29.5 ± 4.7 | 29.3 ± 4.8 | 30.8 ± 6.0 | p = 0.004 * |
| Laboratory results | |||||
| Glomerular filtration rate (CKD-EPI formula) | 87.2 ± 14.5 | 76.9 ± 17.1 | 77.8 ± 16.5 | 75.0 ± 18.7 | p < 0.001 * |
| HbA1c [mmol/mol] | 42.7 ± 12.0 | 42.7 ± 11.7 | 44.6 ± 11.5 | 44.5 ± 11.4 | p = 0.12 |
| Total cholesterol [mg/dL] | 200 (171–231) | 205 (172–229.5) | 184 (158–219.5) | 194.5 (164–223) | p = 0.44 |
| HDL [mg/dL] | 58 (47–75) | 56 (45–66.5) | 57 (48–68) | 54 (42.8–67) | p = 0.03 * |
| LDL [mg/dL] | 115 (95–140.5) | 120 (93–143) | 99 (80–137) | 109.5 (88–141.3) | p = 0.31 |
| Triglycerides [mg/dL] | 108 (73–138) | 115 (77–175) | 94 (70.5–139.5) | 122.5 (84.8–166.5) | p = 0.24 |
| Medication [%] | |||||
| Beta blockers | 41.3 | 43.8 | 55.2 | 57.1 | p = 0.02 * |
| ACE inhibitors | 33.3 | 38.0 | 33.6 | 33.6 | p = 0.83 |
| Angiotensin II receptor antagonists | 35.7 | 27.7 | 34.4 | 31.9 | p = 0.53 |
| Calcium channel antagonists | 22.2 | 21.2 | 32.8 | 28.6 | p = 0.11 |
| Diuretics | 38.1 | 42.3 | 44.0 | 52.9 | p = 0.12 |
| Aldosterone antagonists | 4.0 | 4.4 | 3.2 | 4.2 | p = 0.97 |
| Other antihypertensive drugs | 11.1 | 16.8 | 14.4 | 14.4 | p = 0.63 |
| Glucocorticoids | 1.6 | 1.5 | 3.2 | 5.0 | p = 0.28 |
| Pulse wave velocity [m/sec] | 8.2 ± 2.0 | 8.7 ± 2.1 | 8.9 ± 2.4 | 8.4 ± 3.0 | p = 0.31 |
| Mean 24 h systolic blood pressure [mm Hg] | 127.7 ± 13.2 | 126.7 ± 13.7 | 126.4 ± 13.8 | 129.5 ± 14.7 | p = 0.38 |
| Mean 24 h diastolic blood pressure [mm Hg] | 78.7 ± 10.0 | 76.1 ± 9.7 | 75.1 ± 8.8 | 75.6 ± 9.1 | p = 0.007 * |
| Mean 24 h heart rate [bpm] | 72.2 ± 9.3 | 72.0 ± 10.4 | 71.8 ± 9.5 | 71.6 ± 10.0 | p = 0.61 |
| Mean night systolic blood pressure [mm Hg] | 116.7 ± 19.0 | 115.1 ± 14.3 | 116.5 ± 19.5 | 118.8 ± 14.0 | p = 0.28 |
| Mean night diastolic blood pressure [mm Hg] | 69.1 ± 9.2 | 67.2 ± 9.7 | 67.1 ± 9.6 | 67.2 ± 8.4 | p = 0.12 |
| Mean night heart rate [bpm] | 64.8 ± 8.8 | 64.6 ± 10.0 | 64.6 ± 9.0 | 64.8 ± 9.6 | p = 0.99 |
| Mean Systolic Dipping [%] | −11.8 ± 9.6 | −13.1 ± 8.4 | −10.9 ± 9.2 | −10.8 ± 9.2 | p = 0.17 |
| Mean Diastolic Dipping [%] | −16.1 ± 9.2 | −15.9 ± 9.0 | −14.8 ± 9.3 | −14.8 ± 9.2 | p = 0.17 |
| SDMA Quartiles [µmol/L] | 0.44–0.60 n = 127 | 0.61–0.69 n = 138 | 0.70–0.79 n = 124 | 0.80–1.73 n = 120 | p Value Trend |
|---|---|---|---|---|---|
| Female [%] | 52.0 | 50.0 | 42.3 | 50.0 | p = 0.57 |
| Age [years] | 56.6 ± 10.2 | 59.4 ± 10.8 | 61.6 ± 10.2 | 67.6 ± 7.4 | p < 0.001 * |
| BMI [kg/m2] | 30.6 ± 5.3 | 29.6 ± 5.4 | 28.7 ± 4.2 | 29.6 ± 5.3 | p = 0.07 |
| Laboratory results | |||||
| Glomerular filtration rate (CKD-EPI formula) | 92.0 ± 13.4 | 85.7 ± 11.1 | 75.9 ± 13.3 | 62.5 ± 14.9 | p < 0.001 * |
| HbA1c [mmol/mol] | 46.4 ± 15.3 | 42.3 ± 9.9 | 41.7 ± 9.7 | 43.9 ± 10.6 | p = 0.08 |
| Total cholesterol [mg/dL] | 187 (165–218) | 206.5 (171.8–234.5) | 205 (169–229) | 186 (157–220) | p = 0.43 |
| HDL [mg/dL] | 54 (44.8–66) | 57 (48–73) | 57 (48–69) | 56 (44–69) | p = 0.32 |
| LDL [mg/dL] | 108 (84.5–137) | 122 (92.5–148.5) | 121 (93–145) | 101 (80.5–131.5) | p = 0.17 |
| Triglycerides [mg/dL] | 118 (81–176) | 109 (73–138) | 106 (78–144) | 104 (73–169) | p = 0.16 |
| Medication [%] | |||||
| Beta blockers | 43.3 | 43.9 | 48.4 | 55.8 | p = 0.27 |
| ACE inhibitors | 33.9 | 41.3 | 23.8 | 39.2 | p = 0.02 * |
| Angiotensin II receptor antagonists | 31.5 | 26.1 | 41.8 | 30.8 | p = 0.054 |
| Calcium channel antagonists | 26.0 | 19.6 | 33.6 | 25.8 | p = 0.09 |
| Diuretics | 39.4 | 39.9 | 40.2 | 58.3 | p = 0.01 * |
| Aldosterone antagonists | 2.4 | 0.0 | 3.3 | 10.8 | p < 0.001 * |
| Other antihypertensive drugs | 10.2 | 13.0 | 12.3 | 21.8 | p = 0.049 * |
| Glucocorticoids | 0.0 | 5.1 | 0.8 | 5.0 | p = 0.02 * |
| Pulse wave velocity [m/s] | 8.3 ± 2.0 | 8.4 ± 1.9 | 8.7 ± 2.7 | 8.9 ± 2.9 | p = 0.03 * |
| Mean 24 h systolic blood pressure [mm Hg] | 128.6 ± 13.2 | 127.5 ± 13.3 | 126.2 ± 12.8 | 127.8 ± 16.2 | p = 0.49 |
| Mean 24 h diastolic blood pressure [mm Hg] | 78.6 ± 9.5 | 77.0 ± 8.9 | 76.4 ± 9.8 | 73.2 ± 9.1 | p < 0.001 * |
| Mean 24 h heart rate [bpm] | 74.7 ± 9.8 | 71.2 ± 9.5 | 71.6 ± 10.4 | 70.1 ± 8.9 | p = 0.001 * |
| Mean night systolic blood pressure [mm Hg] | 116.2 ± 17.4 | 116.7 ± 15.7 | 115.8 ± 13.7 | 118.2 ± 20.4 | p = 0.45 |
| Mean night diastolic blood pressure [mm Hg] | 69.1 ± 9.4 | 68.0 ± 9.6 | 67.1 ± 8.1 | 66.2 ± 9.9 | p = 0.01 * |
| Mean night heart rate [bpm] | 67.2 ± 9.5 | 64.2 ± 8.8 | 63.4 ± 10.0 | 63.8 ± 8.9 | p = 0.003 * |
| Mean Systolic Dipping [%] | −12.8 ± 8.6 | −12.6 ± 8.7 | −11.5 ± 9.1 | −9.6 ± 9.9 | p = 0.003 * |
| Mean Diastolic Dipping [%] | −16.5 ± 8.9 | −16.2 ± 9.0 | −15.2 ± 8.9 | −13.6 ± 9.7 | p = 0.008 * |
| Bivariate Correlations | Homoarginine | ADMA | SDMA |
|---|---|---|---|
| Pulse wave velocity [m/s] | r = −0.09 p = 0.057 | r = −0.1 p = 0.83 | r = −0.01 p = 0.86 |
| Mean 24 h systolic blood pressure [mm Hg] | r = 0.07 p = 0.11 | r = 0.02 p = 0.59 | r = 0.02 p = 0.61 |
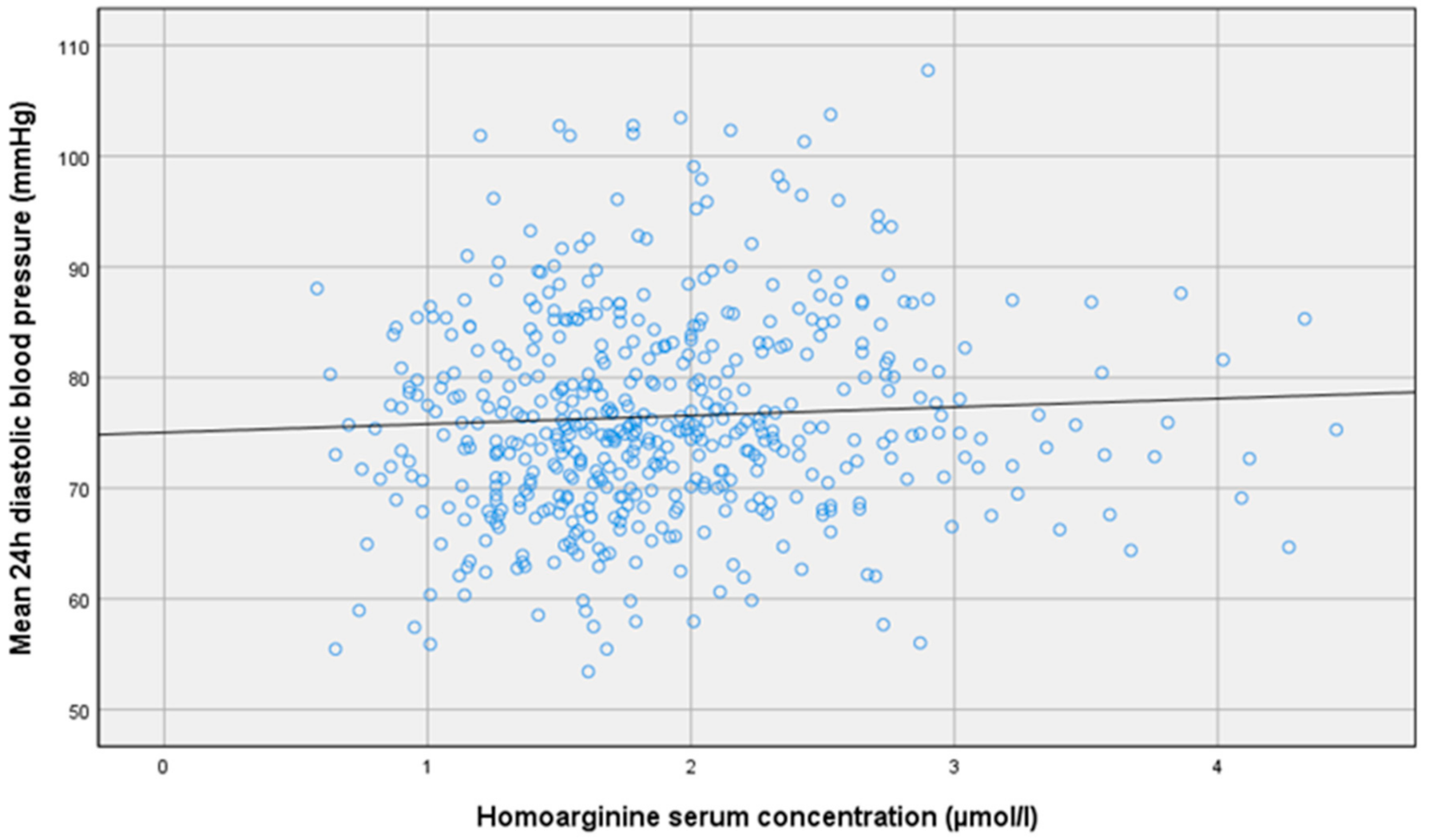
| Mean 24 h diastolic blood pressure [mm Hg] | r = 0.1 p = 0.02 * | r = −0.01 p = 0.86 | r = −0.02 p = 0.70 |
| Mean 24 h heart rate [bpm] | r = 0.01 p = 0.89 | r = 0.002 p = 0.96 | r = −0.01 p = 0.89 |
| Mean daytime systolic blood pressure [mm Hg] | r = 0.06 p = 0.18 | r = 0.03 p = 0.54 | r = −0.03 p = 0.56 |
| Mean daytime diastolic blood pressure [mm Hg] | r = 0.07 p = 0.12 | r = −0.01 p = 0.91 | r = −0.02 p = 0.73 |
| Mean night systolic blood pressure [mm Hg] | r = 0.02 p = 0.68 | r = 0.01 p = 0.85 | r = 0.01 p = 0.82 |
| Mean night diastolic blood pressure [mm Hg] | r = 0.06 p = 0.19 | r = −0.01 p = 0.76 | r = −0.02 p = 0.67 |
| Mean Systolic Dipping [%] | r = −0.05 p = 0.26 | r = −0.01 p = 0.75 | r = −0.01 p = 0.87 |
| Mean Diastolic Dipping [%] | r = −0.03 p = 0.51 | r = −0.01 p = 0.78 | r = −0.01 p = 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malle, O.; Trummer, C.; Theiler-Schwetz, V.; Meinitzer, A.; Keppel, M.H.; Grübler, M.R.; Tomaschitz, A.; Voelkl, J.; März, W.; Pilz, S. NO Synthesis Markers Are Not Significantly Associated with Blood Pressure and Endothelial Dysfunction in Patients with Arterial Hypertension: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 3895. https://doi.org/10.3390/jcm9123895
Malle O, Trummer C, Theiler-Schwetz V, Meinitzer A, Keppel MH, Grübler MR, Tomaschitz A, Voelkl J, März W, Pilz S. NO Synthesis Markers Are Not Significantly Associated with Blood Pressure and Endothelial Dysfunction in Patients with Arterial Hypertension: A Cross-Sectional Study. Journal of Clinical Medicine. 2020; 9(12):3895. https://doi.org/10.3390/jcm9123895
Chicago/Turabian StyleMalle, Oliver, Christian Trummer, Verena Theiler-Schwetz, Andreas Meinitzer, Martin H. Keppel, Martin R. Grübler, Andreas Tomaschitz, Jakob Voelkl, Winfried März, and Stefan Pilz. 2020. "NO Synthesis Markers Are Not Significantly Associated with Blood Pressure and Endothelial Dysfunction in Patients with Arterial Hypertension: A Cross-Sectional Study" Journal of Clinical Medicine 9, no. 12: 3895. https://doi.org/10.3390/jcm9123895
APA StyleMalle, O., Trummer, C., Theiler-Schwetz, V., Meinitzer, A., Keppel, M. H., Grübler, M. R., Tomaschitz, A., Voelkl, J., März, W., & Pilz, S. (2020). NO Synthesis Markers Are Not Significantly Associated with Blood Pressure and Endothelial Dysfunction in Patients with Arterial Hypertension: A Cross-Sectional Study. Journal of Clinical Medicine, 9(12), 3895. https://doi.org/10.3390/jcm9123895






