Adiposity is Associated with Decreased Serum 17-Hydroxyprogesterone Levels in Non-Diabetic Obese Men Aged 18–49: A Cross-Sectional Study
Abstract
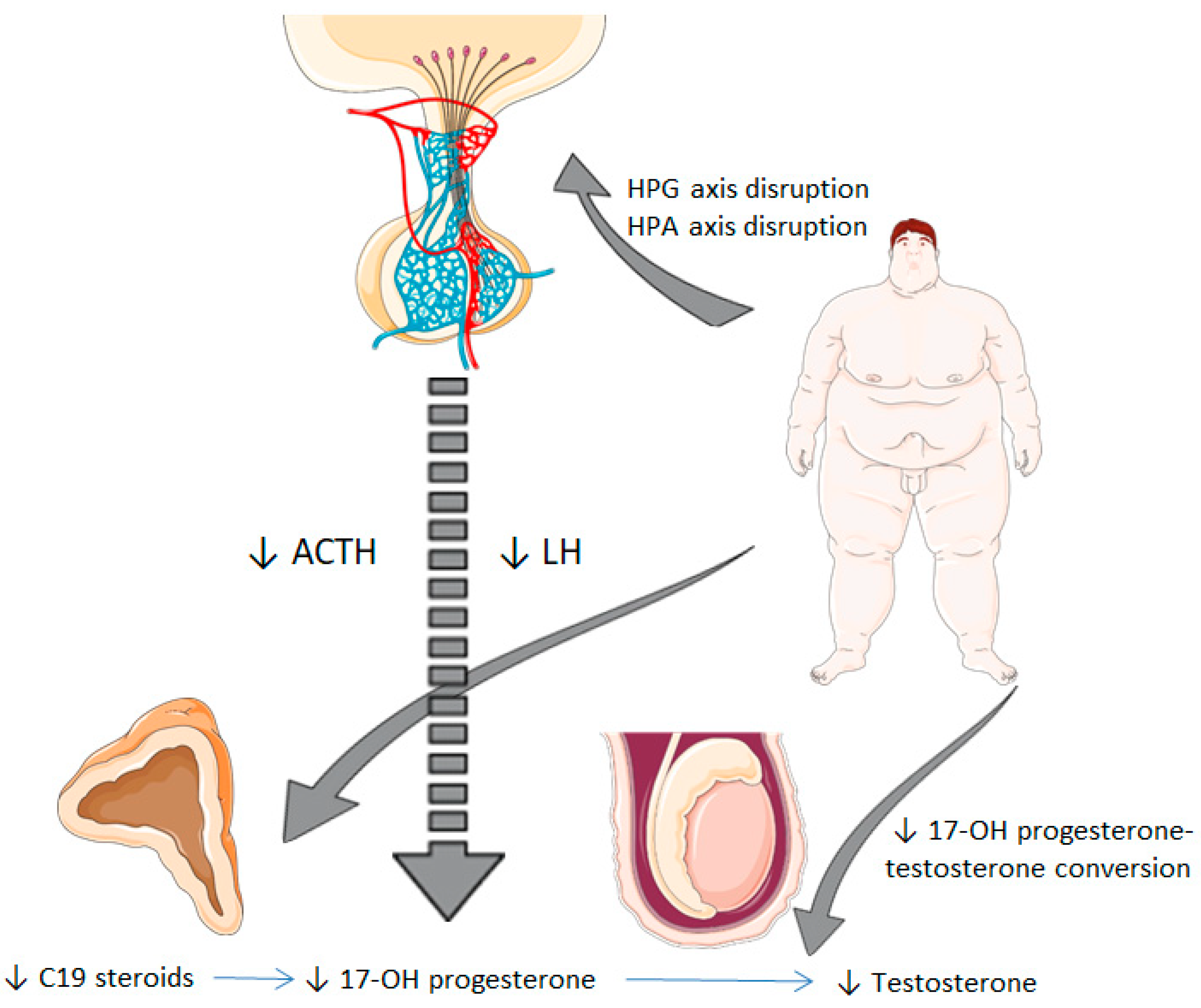
1. Introduction
2. Experimental Section
2.1. Study Design and Participants
2.2. Biochemical Evaluation
2.3. Body Composition Analysis
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characteristics of the Study Population
3.3. Correlation Analysis between 17-OH Progesterone and Other Variables
3.4. Factors Associated with 17-OH Progesterone Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| BMI | Body mass index |
| FT | Free testosterone |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| HPLC-MS | High-performance liquid chromatography mass spectrometry |
| hS-CRP | High-sensitivity C-reactive protein |
| LH | Luteinizing hormone |
| 17-OHprogesterone | 17-hydroxyprogesterone |
| TT | Total testosterone |
| VFR | Visceral fat rating |
| WC | Waist circumference |
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The science of obesity management: An endocrine society scientific statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- Saboor Aftab, S.A.; Kumar, S.; Barber, T.M. The role of obesity and type 2 diabetes mellitus in the development of male obesity-associated secondary hypogonadism. Clin. Endocrinol. 2013, 78, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Molina-Vega, M.; Muñoz-Garach, A.; Damas-Fuentes, M.; Fernández-García, J.C.; Tinahones, F.J. Secondary male hypogonadism: A prevalent but overlooked comorbidity of obesity. Asian J. Androl. 2018, 20, 531–538. [Google Scholar]
- Molina-Vega, M.; Asenjo-Plaza, M.; García-Ruiz, M.C.; Varea-Marineto, E.; Casal-Nievas, N.; Álvarez-Millán, J.J.; Cabezas-Sanchez, P.; Cardona-Díaz, F.; Queipo-Ortuño, M.I.; Castellano-Castillo, D.; et al. Cross-Sectional, Primary Care–Based Study of the Prevalence of Hypoandrogenemia in Nondiabetic Young Men with Obesity. Obesity 2019, 27, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Hydroxyprogesterone|C21H30O3-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/hydroxyprogesterone (accessed on 25 April 2020).
- Roth, M.Y.; Lin, K.; Bay, K.; Amory, J.K.; Anawalt, B.D.; Matsumoto, A.M.; Marck, B.T.; Bremner, W.J.; Page, S.T. Serum insulin-like factor 3 is highly correlated with intratesticular testosterone in normal men with acute, experimental gonadotropin deficiency stimulated with low-dose human chorionic gonadotropin: A randomized, controlled trial. Fertil. Steril. 2013, 99, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, K.; Stege, R. Adrenocortical function in prostatic cancer patients: Effects of orchidectomy or different modes of estrogen treatment on basal steroid levels and on the response to exogenous adrenocorticotropic hormone. Urol. Int. 1990, 45, 160–163. [Google Scholar] [CrossRef]
- Stege, R.; Eriksson, A.; Henriksson, P.; Carlstrom, K. Orchidectomy or oestrogen treatment in prostatic cancer: Effects on serum levels of adrenal androgens and related steroids. Int. J. Androl. 1987, 10, 581–587. [Google Scholar] [CrossRef]
- Allan, C.A.; McLachlan, R.I. Androgens and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 224–232. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.-P.; Bélanger, A.; Dupont, A.; Prud’Homme, D.; Moorjani, S.; Lupien, P.J.; Labrie, F. Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism 1995, 44, 513–519. [Google Scholar] [CrossRef]
- Patel, A.; Patel, P.; Bitran, J.; Ramasamy, R. Can serum 17-hydroxyprogesterone and insulin-like factor 3 be used as a marker for evaluation of intratesticular testosterone? Transl. Androl. Urol. 2019, 8, S58–S63. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.K.; Coviello, A.D.; Page, S.T.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J. Serum 17-hydroxyprogesterone strongly correlates with intratesticular testosterone in gonadotropin-suppressed normal men receiving various dosages of human chorionic gonadotropin. Fertil. Steril. 2008, 89, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, S.; Marceau, P.; Biron, S.; Brochu, C.; Tchernof, A. Circulating progesterone and obesity in men. Horm. Metab. Res. 2006, 38, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Caprio, M.; Strollo, F.; Moretti, C.; Frajese, G.; Isidori, A.; Fabbri, A. Leptin and androgens in male obesity: Evidence for leptin contribution to reduced androgen levels. J. Clin. Endocrinol. Metab. 1999, 84, 3673–3680. [Google Scholar] [CrossRef]
- Damgaard-Olesen, A.; Johannsen, T.; Holmboe, S.A.; Søeborg, T.; Petersen, J.H.; Andersson, A.-M.; Aadahl, M.; Linneberg, A.; Juul, A. Reference ranges of 17-hydroxyprogesterone, DHEA, DHEAS, androstenedione, total and free testosterone determined by TurboFlow-LC-MS/MS and associations to health markers in 304 men. Clin. Chim. Acta 2016, 454, 82–88. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef]
- Strain, G.W.; Wang, J.; Gagner, M.; Pomp, A.; Inabnet, W.B.; Heymsfield, S.B. Bioimpedance for severe obesity: Comparing research methods for total body water and resting energy expenditure. Obesity 2008, 16, 1953–1956. [Google Scholar] [CrossRef]
- Ayeser, T.; Basak, M.; Arslan, K.; Sayan, I. Investigating the correlation of the number of diagnostic criteria to serum adiponectin, leptin, resistin, TNF-alpha, EGFR levels and abdominal adipose tissue. Diabetol. Metab. Syndr. 2016, 10, 165–169. [Google Scholar] [CrossRef]
- Nordenström, A.; Falhammar, H. Management of endocrine disease: Diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur. J. Endocrinol. 2019, 180, R127–R145. [Google Scholar] [CrossRef]
- Merke, D.P.; Bornstein, S.R. Congenital adrenal hyperplasia. Lancet 2005, 365, 2125–2136. [Google Scholar] [CrossRef]
- Bachega, T.A.S.S.; Billerbeck, A.E.C.; Marcondes, J.A.M.; Madureira, G.; Arnhold, I.J.P.; Mendonca, B.B. Influence of different genotypes on 17-hydroxyprogesterone levels in patients with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin. Endocrinol. 2000, 52, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R. Obesity and androgens: Facts and perspectives. Fertil. Steril. 2006, 85, 1319–1340. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef] [PubMed]
- Lamm, S.; Chidakel, A.; Bansal, R. Obesity and Hypogonadism. Urol. Clin. N. Am. 2016, 43, 239–245. [Google Scholar] [CrossRef]
- Kokkoris, P.; Pi-Sunyer, F.X. Obesity and endocrine disease. Endocrinol. Metab. Clin. N. Am. 2003, 32, 895–914. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Uhlmann, K.; Haidan, A.; Ehrhart-Bornstein, M.; Scherbaum, W.A. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: Leptin inhibits cortisol release directly. Diabetes 1997, 46, 1235–1238. [Google Scholar] [CrossRef]
- Pitteloud, N.; Hardin, M.; Dwyer, A.A.; Valassi, E.; Yialamas, M.; Elahi, D.; Hayes, F.J. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J. Clin. Endocrinol. Metab. 2005, 90, 2636–2641. [Google Scholar] [CrossRef]
- Tsai, E.C.; Boyko, E.J.; Leonetti, D.L.; Fujimoto, W.Y. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int. J. Obes. 2000, 24, 485–491. [Google Scholar] [CrossRef]
- Simon, M.; Charles, M.-A.; Nahoul, K.; Orssaud, G.; Kremski, J.; Hully, V.; Joubert, E.; Papoz, L.; Eschwege, E. Association between Plasma Total Testosterone and Cardiovascular Risk Factors in Healthy Adult Men: The Telecom Study 1. J. Clin. Endocrinol. Metab. 1997, 82, 682–685. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Li, S.; Zhou, W.; Ye, L.; Wang, L.; Tao, T.; Gu, J.; Yang, Z.; Zhao, D.; et al. Steroid hormone profiling in obese and nonobese women with polycystic ovary syndrome. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Tertile 1 (n-84) | Tertile 2 (n-84) | Tertile 3 (n-85) | p Value | |
|---|---|---|---|---|
| Age (years) | 38.4 ± 6.3 | 36± 8.5 | 36.2 ± 7.6 | 0.320 |
| Weight (kg) | 103.4 ± 10.2 a | 117.7 ± 11.3 b | 141.4 ± 17.8 c | <0.001 |
| BMI (kg/m2) | 33.2 ± 2.8 a | 37.3 ± 2.5 b | 46.8 ± 5.6 c | <0.001 |
| WC (cm) | 111.2 ± 5.7 a | 121.5 ± 7.7 b | 140.8 ± 12.3 c | <0.001 |
| Fat mass (kg) | 29.2 ± 3.7 a | 40.1 ± 4.7 b | 59.4 ± 11.9 c | <0.001 |
| Fat mass (%) | 28.2 ± 2.1 a | 34 ± 1.2 b | 41.6 ± 3.4 c | <0.001 |
| Fat-free mass (kg) | 72.9 ± 5.6 a | 77.1 ± 7.1 b | 81.8 ± 7.3 c | <0.001 |
| Fat- free mass (%) | 70.7 ± 3.7 a | 65.5 ± 1.8 b | 58.2 ± 3.4 c | <0.001 |
| VFR (points) | 12.6 ± 2 a | 16.7 ± 2.5 b | 26.1 ± 5.4 c | <0.001 |
| Glucose (mg/dL) | 90.7 ± 8.2 a | 92.6 ± 11.2 a,b | 94.5 ± 9.8 b | 0.048 |
| HbA1c (%) | 5.3 ± 0.3 a | 5.4 ± 0.4 a | 5.5 ± 0.4 b | 0.035 |
| Triglycerides (mg/dL) | 150.7 ± 81.1 | 149 ± 75.8 | 157.5 ± 83.5 | 0.773 |
| HDL-c (mg/dL) | 42.3 ± 7.6 | 42.2 ± 10.4 | 41.1 ± 9.5 | 0.676 |
| LDL-c (mg/dL) | 118 ± 28 | 112 ± 31.4 | 110.5 ± 29.4 | 0.229 |
| hs-CRP (mg/dL) | 1.6 ± 1.9 a | 2.9 ± 4.5 a | 5.7 ± 8.5 b | <0.001 |
| Insulin (uIU/mL) | 13.5 ± 6.4 a | 20 ± 13.2 b | 27.1 ± 21.5 c | <0.001 |
| HOMA-IR | 3.1 ± 1.6 a | 4.8 ± 3.9 b | 6.5 ± 6 c | <0.001 |
| ACTH (pg/mL) | 25.2 ± 15.9 | 27 ± 14.2 | 29.7 ± 15.1 | 0.172 |
| LH (mUI /mL) | 3.8 ± 1.7 | 4 ± 2.7 | 3.5 ± 1.7 | 0.371 |
| TT (ng/mL) | 4.1± 1.4 a | 3.8 ± 1.2 a | 3.2 ± 1.2 b | <0.001 |
| FT (pg/mL) | 94.6 ± 28.3 a | 92.3 ± 27.7 a | 77.7 ± 24.5 b | <0.001 |
| 17-OH progesterone (ng/mL) | 0.94 ± 0.4 a | 0.89 ± 0.33 a | 0.74 ± 0.31 b | <0.001 |
| Tertile 1 | Tertile 2 | Tertile 3 | p Value | |
|---|---|---|---|---|
| BMI | 0.94 ± 0.38 a | 0.88 ± 0.33 a | 0.73 ± 0.36 b | <0.001 |
| WC | 0.94 ± 0.36 a | 0.89 ± 0.35 a | 0.71 ± 0.31 b | <0.001 |
| VFR | 0.93 ± 0.39 a | 0.91 ± 0.34 a | 0.70 ± 0.36 b | <0.001 |
| 17-OH Progesterone | ||
|---|---|---|
| r | p Value | |
| Age (years) | −0.120 | 0.058 |
| Weight (kg) | −0.245 | <0.001 |
| BMI (kg/m2) | −0.299 | <0.001 |
| WC (cm) | −0.297 | <0.001 |
| VFR (points) | −0.322 | <0.001 |
| Fat mass (kg) | −0.284 | <0.001 |
| Fat mass (%) | −0.290 | <0.001 |
| Fat-free mass (%) | 0.270 | <0.001 |
| Glucose (mg/dL) | −0.086 | 0.171 |
| Insulin (uIU/mL) | −0.256 | <0.001 |
| HOMA-IR | −0.243 | <0.001 |
| hs-CRP (mg/dL) | −0.095 | 0.137 |
| ACTH (pg/mL) | 0.077 | 0.232 |
| LH (mUI/mL) | 0.214 | 0.001 |
| TT (mg/dL) | 0.537 | <0.001 |
| FT (pg/mL) | 0.450 | <0.001 |
| B (SE) | Beta | T Statistic | p Value | |
|---|---|---|---|---|
| Age (years) | −0.001 (0.003) | −0.031 | −0.536 | 0.592 |
| Body fat (%) | −0.008 (0.004) | −0.128 | −2.029 | 0.044 |
| ACTH (pg/mL) | 0.003 (0.001) | 0.125 | 2.158 | 0.032 |
| HOMA-IR | −0.012 (0.005) | −0.148 | −2.514 | 0.013 |
| LH (mUI/mL) | 0.017 (0.010) | 0.102 | 1.771 | 0.078 |
| FT (pg/mL) | 0.005 (0.001) | 0.382 | 6.020 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Montoro, J.I.; Molina-Vega, M.; Asenjo-Plaza, M.; García-Ruiz, M.C.; Varea-Marineto, E.; Plaza-Andrade, I.; Álvarez-Millán, J.J.; Cabezas-Sánchez, P.; Tinahones, F.J.; Fernández-García, J.C. Adiposity is Associated with Decreased Serum 17-Hydroxyprogesterone Levels in Non-Diabetic Obese Men Aged 18–49: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 3873. https://doi.org/10.3390/jcm9123873
Martínez-Montoro JI, Molina-Vega M, Asenjo-Plaza M, García-Ruiz MC, Varea-Marineto E, Plaza-Andrade I, Álvarez-Millán JJ, Cabezas-Sánchez P, Tinahones FJ, Fernández-García JC. Adiposity is Associated with Decreased Serum 17-Hydroxyprogesterone Levels in Non-Diabetic Obese Men Aged 18–49: A Cross-Sectional Study. Journal of Clinical Medicine. 2020; 9(12):3873. https://doi.org/10.3390/jcm9123873
Chicago/Turabian StyleMartínez-Montoro, José Ignacio, María Molina-Vega, Maite Asenjo-Plaza, María Concepción García-Ruiz, Enrique Varea-Marineto, Isaac Plaza-Andrade, Juan J. Álvarez-Millán, Pablo Cabezas-Sánchez, Francisco J. Tinahones, and José Carlos Fernández-García. 2020. "Adiposity is Associated with Decreased Serum 17-Hydroxyprogesterone Levels in Non-Diabetic Obese Men Aged 18–49: A Cross-Sectional Study" Journal of Clinical Medicine 9, no. 12: 3873. https://doi.org/10.3390/jcm9123873
APA StyleMartínez-Montoro, J. I., Molina-Vega, M., Asenjo-Plaza, M., García-Ruiz, M. C., Varea-Marineto, E., Plaza-Andrade, I., Álvarez-Millán, J. J., Cabezas-Sánchez, P., Tinahones, F. J., & Fernández-García, J. C. (2020). Adiposity is Associated with Decreased Serum 17-Hydroxyprogesterone Levels in Non-Diabetic Obese Men Aged 18–49: A Cross-Sectional Study. Journal of Clinical Medicine, 9(12), 3873. https://doi.org/10.3390/jcm9123873





