Cerebrospinal Fluid and Serum d-Serine Levels in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
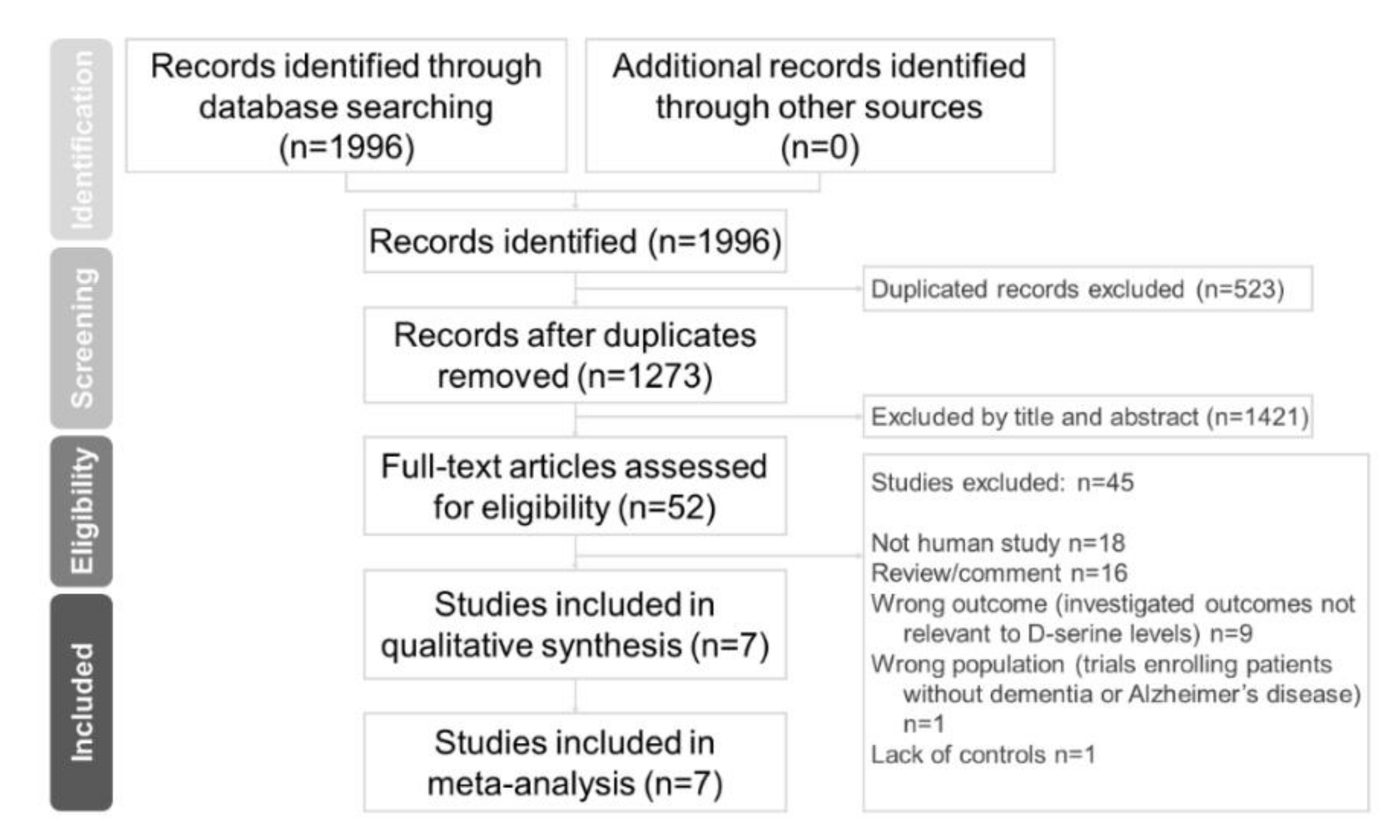
2.1. Search Strategy and Inclusion Criteria
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Meta-Analysis Procedure
2.6. Heterogeneity and Publication Bias
2.7. Sensitivity Test
2.8. Subgroup Meta-Analysis and Metaregression
3. Results
3.1. Characteristics of Included Studies
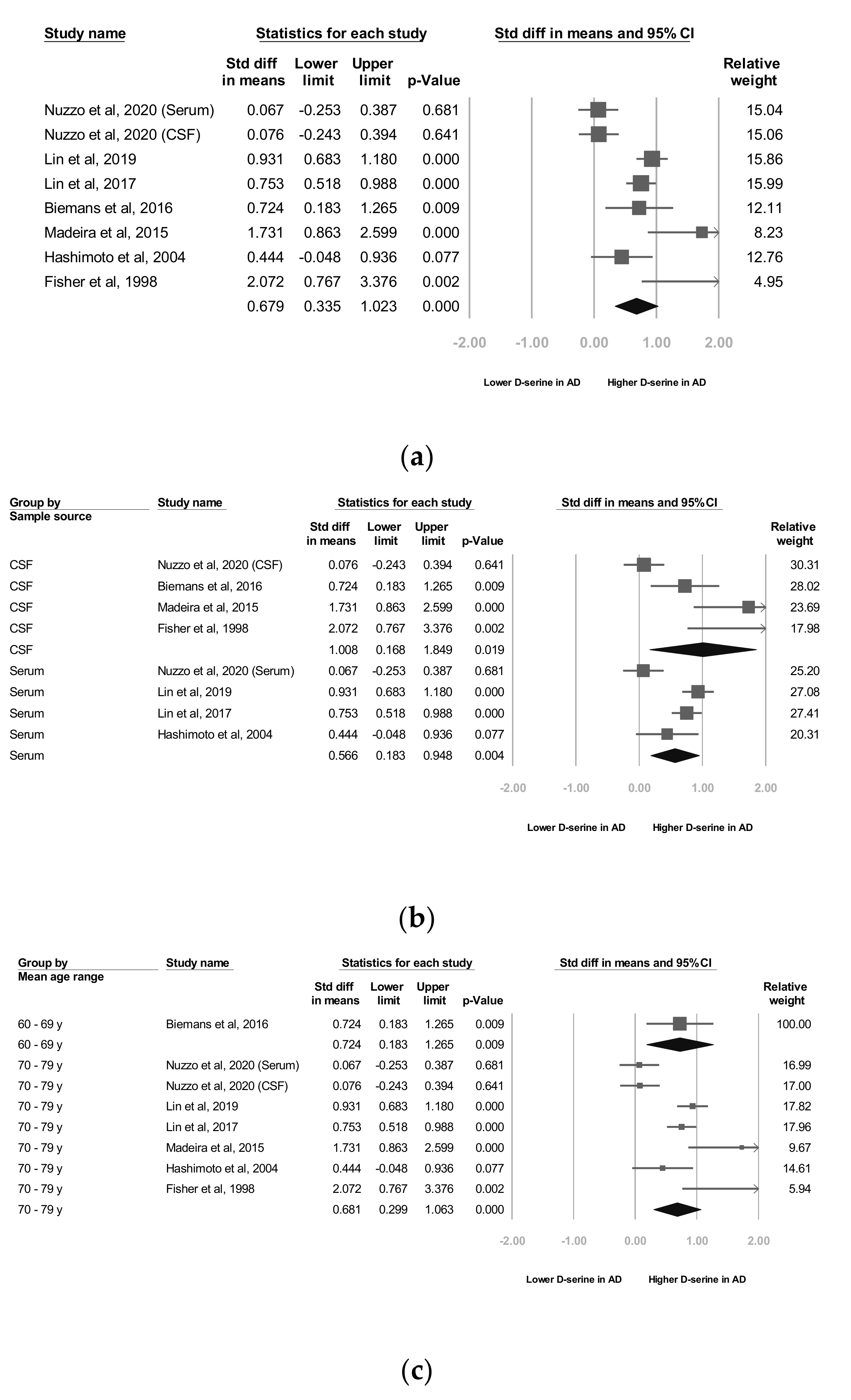
3.2. Meta-Analyses of Overall D-Serine Levels
3.3. Subgroup Analyses of Overall D-Serine-Level Moderators
3.4. A Subgroup Analyses by D-Serine Source
3.5. B Subgroup Analyses by Mean Age Range
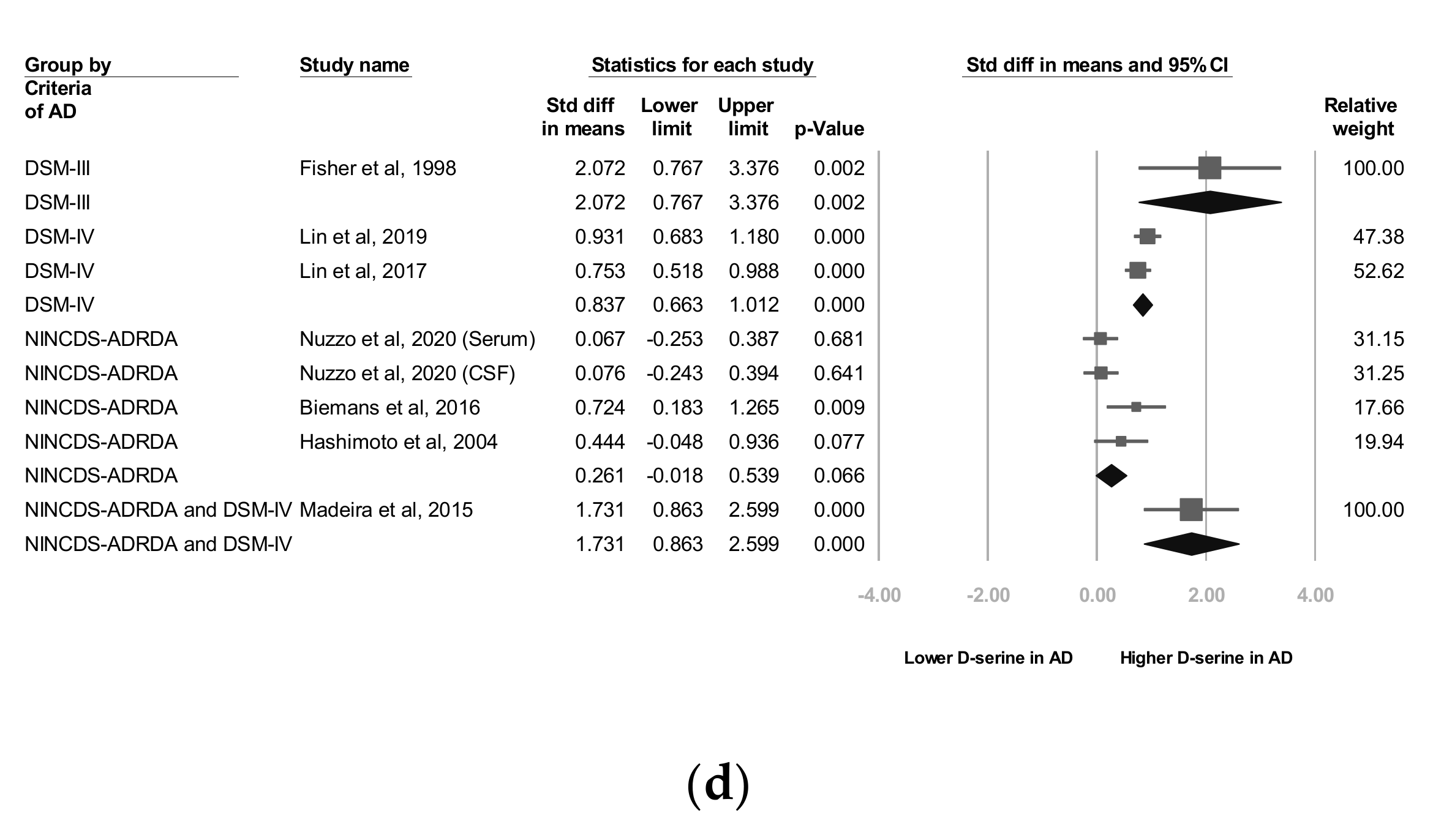
3.6. C Subgroup Analyses by AD Criteria
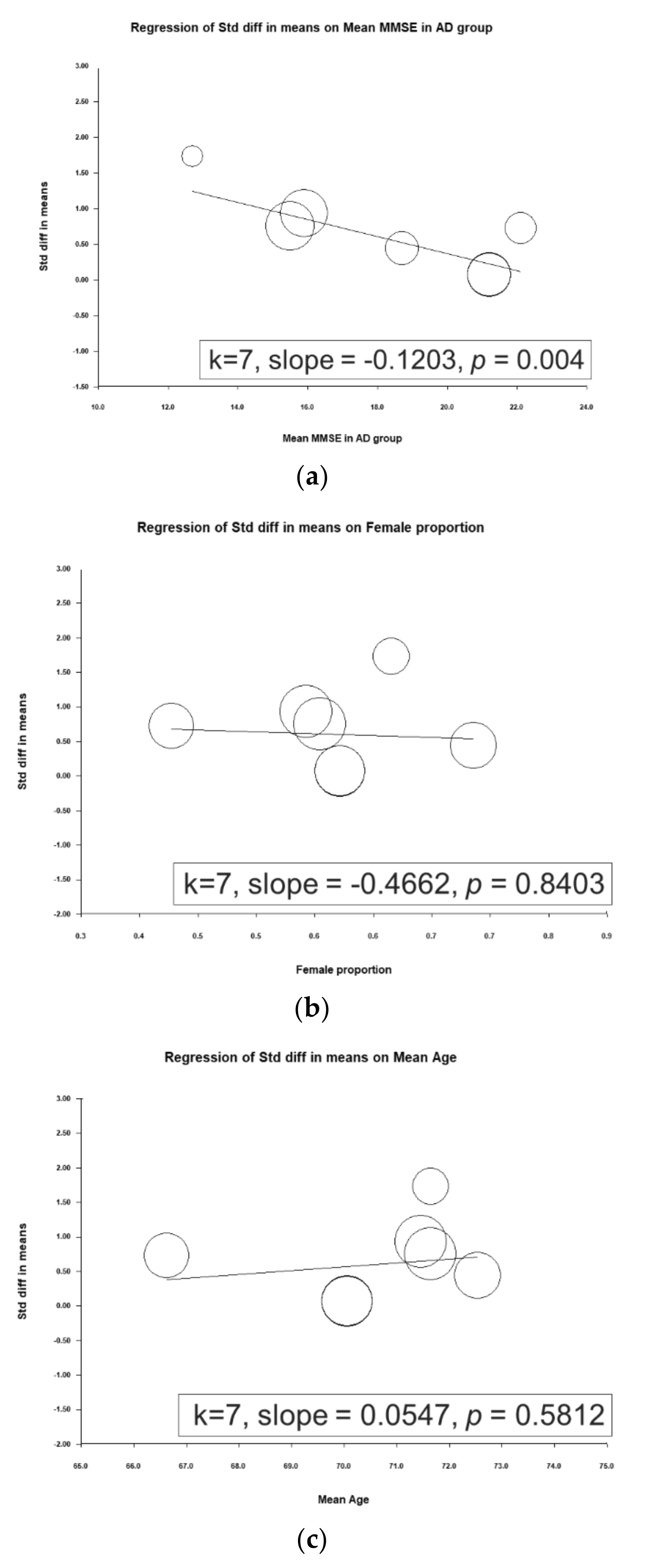
3.7. Metaregression Analyses of D-Serine Levels
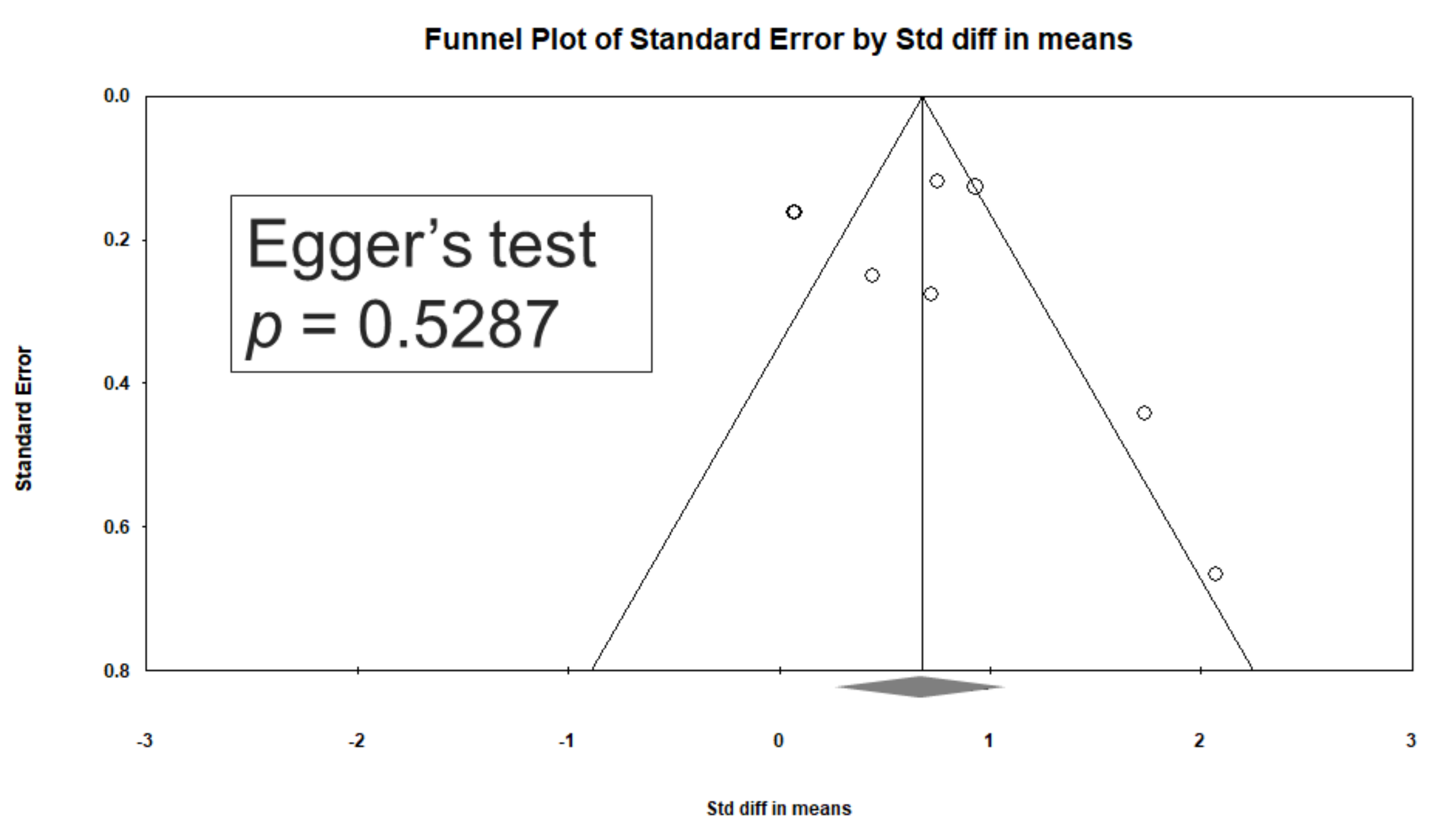
3.8. Heterogeneity and Publication Bias
3.9. Sensitivity Analysis
4. Discussion
5. Strengths
6. Implications
7. Limitations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nat. Cell Biol. 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, J.; Meng, J.; Numthuam, S.; Naruse, K. Application of Multi-Modal Imaging Mediated by Iron Carbon Nanoparticles Based on Reinforcement Learning in the Diagnosis of Breast Nodules. J. Nanosci. Nanotechnol. in press. [CrossRef] [PubMed]
- D’Abramo, C.; D’Adamio, L.; Giliberto, L. Significance of Blood and Cerebrospinal Fluid Biomarkers for Alzheimer’s Disease: Sensitivity, Specificity and Potential for Clinical Use. J. Pers. Med. 2020, 10, 116. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.W.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum. Med. Clin. North Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Davda, N.; Corkill, R. Biomarkers in the diagnosis and prognosis of Alzheimer’s disease. J. Neurol. 2020, 267, 2475–2477. [Google Scholar] [CrossRef]
- Jack, C.R.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, W.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef]
- Hampel, H.; Bürger, K.; Teipel, S.J.; Bokde, A.L.; Zetterberg, H.; Blennow, K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimer’s Dement. 2008, 4, 38–48. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Body Fluid Biomarkers for Alzheimer’s Disease—An Up-To-Date Overview. Biomedicines 2020, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Ausó, E.; Gómez-Vicente, V.; Esquiva, G. Biomarkers for Alzheimer’s Disease Early Diagnosis. J. Pers. Med. 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, M.; Koeglsperger, T.; Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Soluble a Oligomers Inhibit Long-Term Potentiation through a Mechanism Involving Excessive Activation of Extrasynaptic NR2B-Containing NMDA Receptors. J. Neurosci. 2011, 31, 6627–6638. [Google Scholar] [CrossRef]
- Li, S.; Hong, S.; Shepardson, N.E.; Walsh, D.M.; Shankar, G.M.; Selkoe, D.J. Soluble Oligomers of Amyloid β Protein Facilitate Hippocampal Long-Term Depression by Disrupting Neuronal Glutamate Uptake. Neuron 2009, 62, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Bloodgood, B.L.; Townsend, M.; Walsh, D.M.; Selkoe, D.; Sabatini, B.L. Natural Oligomers of the Alzheimer Amyloid- Protein Induce Reversible Synapse Loss by Modulating an NMDA-Type Glutamate Receptor-Dependent Signaling Pathway. J. Neurosci. 2007, 27, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.-I.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E.; et al. A induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Danysz, W.; Dekundy, A.; Pulte, I. Memantine and Cholinesterase Inhibitors: Complementary Mechanisms in the Treatment of Alzheimer’s Disease. Neurotox. Res. 2013, 24, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Radzishevsky, I.; Sason, H.; Wolosker, H. D-Serine. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 72–75. [Google Scholar] [CrossRef]
- Wolosker, H.; Dumin, E.; Balan, L.; Foltyn, V.N. d-Amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008, 275, 3514–3526. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Ahmad, A.S.; Zeynalov, E.; Gazi, S.K.; Sikka, G.; Ehmsen, J.T.; Barrow, R.K.; Coyle, J.T.; Snyder, S.H.; Doré, S. Serine Racemase Deletion Protects Against Cerebral Ischemia and Excitotoxicity. J. Neurosci. 2010, 30, 1413–1416. [Google Scholar] [CrossRef]
- Esultan, S.; Gebara, E.G.; Emoullec, K.; Toni, N. D-serine increases adult hippocampal neurogenesis. Front. Neurosci. 2013, 7, 155. [Google Scholar] [CrossRef]
- Madeira, C.; Lourenco, M.V.; Vargaslopes, C.; Suemoto, C.; Brandao, C.O.; Reis, T.; Leite, R.E.P.; Laks, J.; Jacobfilho, W.; Pasqualucci, C.A.; et al. D-serine levels in Alzheimer’s disease: Implications for novel biomarker development. Transl. Psychiatry 2015, 5, e561. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, S.; Yang, C.; Jia, H.; Gu, Z.; Tu, X.; Tian, S.; Liu, J.; Li, G.; Ma, Y. D-serine Ameliorates Motor and Cognitive Impairments in β-amyloid 1-42 Injected Mice by Inhibiting JNK Signaling Pathway. J. Chem. Neuroanat. 2020, 109, 101852. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Lorenzo, N.; Abe, H.; Fujita, E.; Frey, W.H.; Emory, C.; di Fiore, M.M.; D’Aniello, A. Free D- and L-amino acids in ventricular cerebrospinal fluid from Alzheimer and normal subjects. Amino Acids 1998, 15, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, T.; Miroballo, M.; Casamassa, A.; Mancini, A.; Gaetani, L.; Nisticò, R.; Eusebi, P.; Katane, M.; Homma, H.; Calabresi, P.; et al. Cerebrospinal fluid and serum d-serine concentrations are unaltered across the whole clinical spectrum of Alzheimer’s disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140537. [Google Scholar] [CrossRef]
- Biemans, E.A.; Verhoeven-Duif, N.M.; Gerrits, J.; Claassen, J.A.; Kuiperij, H.B.; Verbeek, M.M. CSF d-serine concentrations are similar in Alzheimer’s disease, other dementias, and elderly controls. Neurobiol. Aging 2016, 42, 213–216. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chiu, C.-C.; Huang, C.-H.; Yang, H.-T.; Lane, H.-Y. pLG72 levels increase in early phase of Alzheimer’s disease but decrease in late phase. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yang, H.-T.; Chiu, C.-C.; Lane, H.-Y. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Okada, S.-I.; Komatsu, N.; Okamura, N.; Koike, K.; Koizumi, H.; Kumakiri, C.; Imai, K.; et al. Possible role of d-serine in the pathophysiology of Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 385–388. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Cho, S.-E.; Na, K.-S.; Cho, S.-J.; Kang, S.G. Low d-serine levels in schizophrenia: A systematic review and meta-analysis. Neurosci. Lett. 2016, 634, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Moskalewicz, A.; Oremus, M. No clear choice between Newcastle–Ottawa Scale and Appraisal Tool for Cross-Sectional Studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J. Clin. Epidemiol. 2020, 120, 94–103. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.T.; Hedges, L.V.; Rothstein, H.R. Basics of meta-analysis: I2is not an absolute measure of heterogeneity. Res. Synth. Methods 2017, 8, 5–18. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biomedicines 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Davey, J.; Turner, R.M.; Clarke, M.; Higgins, J.P. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: A cross-sectional, descriptive analysis. BMC Med. Res. Methodol. 2011, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Dolci, G.A.M.; Damanti, S.; Scortichini, V.; Galli, A.; Rossi, P.D.; Abbate, C.; Arosio, B.; Mari, D.; Arighi, A.; Fumagalli, G.G.; et al. Alzheimer’s Disease Diagnosis: Discrepancy between Clinical, Neuroimaging, and Cerebrospinal Fluid Biomarkers Criteria in an Italian Cohort of Geriatric Outpatients: A Retrospective Cross-sectional Study. Front. Med. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; DeKosky, S.T.; Barberger-Gateau, P.; Cummings, J.L.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.A.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yang, H.-T.; Lane, H.-Y. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 2019, 185, 172760. [Google Scholar] [CrossRef] [PubMed]
- Bado, P.; Madeira, C.; Vargas-Lopes, C.; Moulin, T.C.; Wasilewska-Sampaio, A.P.; Maretti, L.; de Oliveira, R.V.; Amaral, O.B.; Panizzutti, R. Effects of low-dose d-serine on recognition and working memory in mice. Psychopharmacology 2011, 218, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Filali, M.; la Londe, R. The effects of subchronic d-serine on left–right discrimination learning, social interaction, and exploratory activity in APPswe/PS1 mice. Eur. J. Pharmacol. 2013, 701, 152–158. [Google Scholar] [CrossRef]
- Esposito, S.; Pristerà, A.; Maresca, G.; Cavallaro, S.; Felsani, A.; Florenzano, F.; Manni, L.; Ciotti, M.T.; Pollegioni, L.; Borsello, T.; et al. Contribution of serine racemase/d-serine pathway to neuronal apoptosis. Aging Cell 2012, 11, 588–598. [Google Scholar] [CrossRef]
| Study (First Author, Year) | Criteria of AD | Diagnosis | Sample Source | Comparison | Subject | Mean Age (y) | Female (%) | Cognitive Measures | Mean MMSE in AD Group | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Nuzzo et al., 2020 [25] | NINCDS-ADRDA | AD | Serum and CSF | AD Control | 166 | 70.1 (7.6) | 59.6 | CDR, MMSE | AD: 21.2 (3.8) (pre-AD: 27.3 (0.9) AD-MCI: 23.7 (3.1) AD-Dem: 16.0 (5.1)) | No statistically significant differences |
| Lin et al., 2019 [27] | DSM-IV | AD | Serum | AD Control | 376 | 71.5 (8.5) | 56.4 | CDR, MMSE | AD: 15.9 (4.3) (Mild AD: 18.9 (4.4) Moderate AD: 11.5 (3.7) Severe AD: 8.1 (4.2)) | Higher in AD group |
| Lin et al., 2017 [28] | DSM-IV | AD | Serum | AD Control | 397 | 71.6 (8.6) | 57.7 | CDR, MMSE | AD: 15.4 (4.5) (Mild AD: 18.5 (4.4) Moderate to severe AD: 10.4(4.6)) | Higher in AD group |
| Biemans et al., 2016 [26] | NINCDS-ADRDA | AD | CSF | AD Control | 79 | 66.9 (7.4) | 43.6 | MMSE | AD: 22.1 (3.3) | No statistically significant differences |
| Madeira et al., 2015 [22] | NINCDS-ADRDA and DSM-IV | AD | CSF | AD Control | 71 | 71.7 (7.8) | 64.5 | MMSE | AD: 12.7 (6.2) | Higher in AD group |
| Hashimoto et al., 2004 [29] | NINCDS-ADRDA | AD | Serum | AD Control | 65 | 72.5 (6.1) | 72.3 | MMSE | AD: 18.7 (5.0) | No statistically significant differences |
| Fisher et al., 1998 [24] | DSM-III | AD | CSF | AD Control | 32 | 73.4 (9.6) | NA | NA | NA | Higher in AD group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Kuo, H.-L.; Ma, W.-F.; Tsai, H.-C. Cerebrospinal Fluid and Serum d-Serine Levels in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 3840. https://doi.org/10.3390/jcm9123840
Chang C-H, Kuo H-L, Ma W-F, Tsai H-C. Cerebrospinal Fluid and Serum d-Serine Levels in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(12):3840. https://doi.org/10.3390/jcm9123840
Chicago/Turabian StyleChang, Chun-Hung, Hsiao-Lun Kuo, Wei-Fen Ma, and Hsin-Chi Tsai. 2020. "Cerebrospinal Fluid and Serum d-Serine Levels in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 12: 3840. https://doi.org/10.3390/jcm9123840
APA StyleChang, C.-H., Kuo, H.-L., Ma, W.-F., & Tsai, H.-C. (2020). Cerebrospinal Fluid and Serum d-Serine Levels in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(12), 3840. https://doi.org/10.3390/jcm9123840





