Precision Medicine in House Dust Mite-Driven Allergic Asthma
Abstract
1. Introduction
2. Phenotyping House Dust Mite-Driven Asthma
2.1. Allergic Asthma
2.2. Local Allergic Asthma
3. Endotyping House Dust Mite-Driven Asthma
3.1. Allergic Asthma
3.2. Local Allergic Asthma
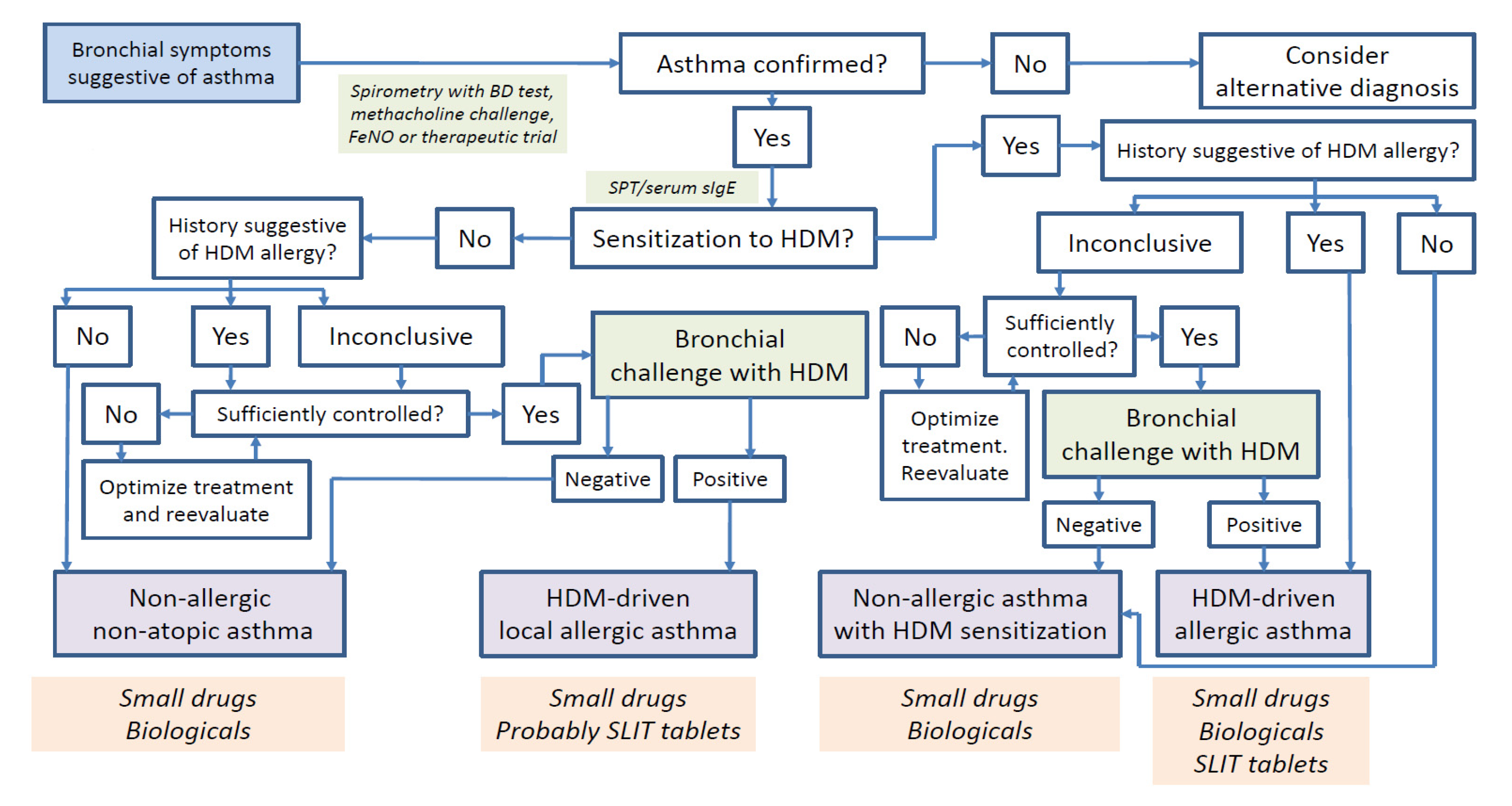
4. Diagnosis of House Dust Mite-Driven Asthma
4.1. Allergic Asthma
4.2. Local Allergic Asthma
5. Treatment Options for House Dust Mite-Driven Asthma
5.1. Small Drugs and Biologicals
5.2. Allergen Immunotherapy
5.2.1. Allergic Asthma
Clinical Studies
Positioning in Clinical Guidelines
The Ongoing Quest for Response Biomarkers
5.2.2. Local Allergic Asthma
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | allergic asthma |
| AHR | airway hyperresponsiveness |
| AIT | allergen immunotherapy |
| AR | allergic rhinitis |
| ARA | atopic respiratory allergy |
| BAC | bronchial allergen challenge |
| BAT | basophil activation test |
| BD test | bronchodilator test |
| ECP | eosinophil cationic protein |
| εCSR | class switch recombination to IgE |
| FcεRI | high affinity receptor for IgE |
| FeNO | fractional exhaled nitric oxide |
| FEV1 | forced expiratory volume in the 1st second |
| FnNO | fractional nasal nitric oxide |
| GINA | global initiative for asthma |
| HC | healthy non-atopic control |
| HDM | house dust mite |
| ICSIg | inhaled corticosteroidsimmunoglobulin |
| IL | interleukin |
| IL-4Rα | α subunit of IL-4 receptor |
| IL-5Rα | α subunit of IL-5 receptor |
| LAA | local allergic asthma |
| LAR | local allergic rhinitis |
| LRA | local respiratory allergy |
| NAC | nasal allergen challengenasal allergen challenge |
| NAR | non-allergic rhinitis |
| NK cell | natural killer cell |
| SQ-HDM | standardized quality brand-specific unit denoting the biological power of the extract |
| SCIT | subcutaneous immunotherapy |
| sIgE | allergen-specific IgE |
| sIgG4 | allergen-specific IgG4 |
| SLIT | sublingual immunotherapy |
| SPT | skin prick test |
| sT cell | allergen-specific T cell |
| sTh2 cell | allergen-specific Th2 cell |
| T2 | type 2 inflammation |
References
- Genuneit, J.; Seibold, A.M.; Apfelbacher, C.J.; Konstantinou, G.N.; Koplin, J.J.; La Grutta, S.; Logan, K.; Perkin, M.R.; Flohr, C. Task Force ‘Overview of Systematic Reviews in Allergy Epidemiology (OSRAE)’ of the EAACI Interest Group on Epidemiology. Overview of systematic reviews in allergy epidemiology. Allergy 2017, 72, 849–856. [Google Scholar] [CrossRef]
- Nurmagambetov, T.; Kuwahara, R.; Garbe, P. The Economic Burden of Asthma in the United States, 2008–2013. Ann. Am. Thorac. Soc. 2018, 15, 348–356. [Google Scholar] [CrossRef]
- Bousquet, J.; Van Cauwenberge, P.; Khaltaev, N. Allergic Rhinitis and Its Impact on Asthma. J. Allergy Clin. Immunol. 2001, 108 (Suppl. 5), S147–S334. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Tay, T.R.; Hew, M.; Escribese, M.M.; Barber, D.; O’Hehir, R.E.; Torres, M.J. Recent developments and highlights in biomarkers in allergic diseases and asthma. Allergy 2018, 73, 2290–2305. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Prim. 2015, 1, 15036. [Google Scholar] [CrossRef]
- Ruggieri, S.; Drago, G.; Longo, V.; Colombo, P.; Balzan, M.; Bilocca, D.; Zammit, C.; Montefort, S.; Scaccianoce, G.; Cuttitta, G.; et al. Sensitization to dust mite defines different phenotypes of asthma: A multicenter study. Pediatr. Allergy Immunol. 2017, 28, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Ollert, M.; Aalberse, R.; Austin, M.; Custovic, A.; DunnGalvin, A.; Eigenmann, P.A.; Fassio, F.; Grattan, C.; Hellings, P.W.; et al. A new framework for the interpretation of IgE sensitization tests. Allergy 2016, 71, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Campo, P.; Eguiluz-Gracia, I.; Plaza-Serón, M.C.; Salas, M.; Rodríguez, M.J.; Pérez-Sánchez, N.; González, M.; Molina, A.; Mayorga, C.; Torres, M.J.; et al. Bronchial asthma triggered by house dust mites in patients with local allergic rhinitis. Allergy 2019, 74, 1502–1510. [Google Scholar] [CrossRef]
- De Vries, M.P.; Bemt, L.V.D.; Van Der Mooren, F.; Muris, J.; Van Schayck, C. The prevalence of house dust mite (HDM) allergy and the use of HDM-impermeable bed covers in a primary care population of patients with persistent asthma in the Netherlands. Prim. Care Respir. J. 2005, 14, 210–214. [Google Scholar] [CrossRef]
- Kennedy, J.L.; Heymann, P.W.; Platts-Mills, T.A. The role of allergy in severe asthma. Clin. Exp. Allergy 2012, 42, 659–669. [Google Scholar] [CrossRef]
- Incorvaia, C.; Al-Ahmad, M.; Ansotegui, I.J.; Arasi, S.; Bachert, C.; Bos, C.; Bousquet, J.; Bozek, A.; Caimmi, D.P.; Calderón, M.A.; et al. Personalized medicine for allergy treatment: Allergen immunotherapy still a unique and unmatched model. Allergy 2020. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, M.J. Drug Development for Asthma. Am. J. Respir. Cell Mol. Biol. 2003, 29, 163–171. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Mathioudakis, A.; Bartel, S.; Vijverberg, S.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Dell’Albani, I.; Frati, F. Towards a global vision of molecular allergology: A map of exposure to airborne molecular allergens. Eur. Ann. Allergy Clin. Immunol. 2013, 45 (Suppl. 2), 17–23. [Google Scholar] [PubMed]
- Castner, J.; Barnett, R.; Huntington-Moskos, L.; Folz, R.J.; Polivka, B.J. Home environment allergen exposure scale in older adult cohort with asthma. Can. J. Public Health 2020. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Pérez-Sánchez, N.; Bogas, G.; Campo, P.; Rondón, C. How to Diagnose and Treat Local Allergic Rhinitis: A Challenge for Clinicians. J. Clin. Med. 2019, 8, 1062. [Google Scholar] [CrossRef]
- Rondón, C.; Campo, P.; Galindo, L.; Blanca-López, N.; Cassinello, M.S.; Rodriguez-Bada, J.L.; Torres, M.J.; Blanca, M. Prevalence and clinical relevance of local allergic rhinitis. Allergy 2012, 67, 1282–1288. [Google Scholar] [CrossRef]
- Campo, P.; Eguiluz-Gracia, I.; Bogas, G.; Salas, M.; Serón, C.P.; Pérez, N.; Mayorga, C.; Torres, M.J.; Shamji, M.; Rondón, C. Local allergic rhinitis: Implications for management. Clin. Exp. Allergy 2019, 49, 6–16. [Google Scholar] [CrossRef]
- Rondon, C.; Campo, P.; Eguiluz-Gracia, I.; Plaza, C.; Bogas, G.; Galindo, P.; Mayorga, C.; Torres, M.J. Local allergic rhinitis is an independent rhinitis phenotype: The results of a 10-year follow-up study. Allergy 2018, 73, 470–478. [Google Scholar] [CrossRef]
- Rondón, C.; Campo, P.; Togias, A.; Fokkens, W.; Durham, S.; Powe, D.G.; Mullol, J.; Blanca, M. Local allergic rhinitis: Concept, pathophysiology, and management. J. Allergy Clin. Immunol. 2012, 129, 1460–1467. [Google Scholar] [CrossRef]
- Bozek, A.; Winterstein, J.; Galuszka, B.; Jarzab, J. Different Development Forms of Local Allergic Rhinitis towards Birch. Biomed Res. Int. 2020, 2020, 3408561. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and Monocyte-Derived CD11b+ Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Eguíluz-Gracia, I.; Bosco, A.; Dollner, R.; Melum, G.R.; Lexberg, M.H.; Jones, A.C.; Dheyauldeen, S.A.; Holt, P.G.; Bækkevold, E.S.; Jahnsen, F.L. Rapid recruitment of CD14 + monocytes in experimentally induced allergic rhinitis in human subjects. J. Allergy Clin. Immunol. 2016, 137, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Gracia, I.; Malmström, K.; Dheyauldeen, S.A.; Lohi, J.; Sajantila, A.; Aaløkken, R.; Sundaram, A.Y.M.; Gilfillan, G.D.; Makela, M.; Baekkevold, E.S.; et al. Monocytes accumulate in the airways of children with fatal asthma. Clin. Exp. Allergy 2018, 48, 1631–1639. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Layhadi, J.A.; Rondon, C.; Shamji, M.H. Mucosal IgE immune responses in respiratory diseases. Curr. Opin. Pharmacol. 2019, 46, 100–107. [Google Scholar] [CrossRef]
- Tong, P.; Wesemann, D.R. Molecular Mechanisms of IgE Class Switch Recombination. Curr. Top Microbiol. Immunol. 2015, 388, 21–37. [Google Scholar] [CrossRef]
- Xiong, H.; Dolpady, J.; Wabl, M.; de Curotto Lafaille, M.A.; Lafaille, J.J. Sequential class switching is required for the generation ofhigh affinity IgE antibodies. J. Exp. Med. 2012, 13, 353–364. [Google Scholar] [CrossRef]
- Coker, H.A.; Durham, S.R.; Gould, H.J. Local Somatic Hypermutation and Class Switch Recombination in the Nasal Mucosa of Allergic Rhinitis Patients. J. Immunol. 2003, 171, 5602–5610. [Google Scholar] [CrossRef]
- Takhar, P.; Corrigan, C.J.; Smurthwaite, L.; O’Connor, B.J.; Durham, S.R.; Lee, T.H.; Gould, H.J. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 2007, 119, 213–218. [Google Scholar] [CrossRef]
- Balzar, S.; Strand, M.; Rhodes, D.; Wenzel, S.E. IgE expression pattern in lung: Relation to systemic IgE and asthma phenotypes. J. Allergy Clin. Immunol. 2007, 119, 855–862. [Google Scholar] [CrossRef][Green Version]
- Humbert, M.; Grant, J.A.; Taborda-Barata, L.; Durham, S.R.; Pfister, R.; Menz, G.; Barkans, J.; Ying, S.; Kay, A.B. High-affinity IgE receptor (FcepsilonRI)-bearing cells in bronchial biopsies from atopic and nonatopic asthma. Am. J. Respir. Crit. Care Med. 1996, 153 Pt 1, 1931–1937. [Google Scholar] [CrossRef]
- Pillai, P.; Fang, C.; Chan, Y.-C.; Shamji, M.H.; Harper, C.; Wu, S.-Y.; Ohm-Laursen, L.; Durham, S.R.; Menzies-Gow, A.; Rajakulasingam, R.K.; et al. Allergen-specific IgE is not detectable in the bronchial mucosa of nonatopic asthmatic patients. J. Allergy Clin. Immunol. 2014, 133, 1770–1772. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Jagger, C.; Kleinjan, A.; Carney, A.S.; Jenkins, D.; Jones, N. ‘Entopy’: Localized mucosal allergic disease in the absence of systemic responses for atopy. Clin. Exp. Allergy 2003, 33, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Mouthuy, J.; Viart, S.; Ladjemi, M.Z.; Detry, B.; Henket, M.; Bachert, C.; Louis, R.; Pilette, C. Mite allergen–specific IgE is detectable in bronchial secretions of patients with nonatopic asthma and correlates with mucosal expression of periostin. J. Allergy Clin. Immunol. 2015, 136, 1685–1688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mouthuy, J.; Detry, B.; Sohy, C.; Pirson, F.; Pilette, C. Presence in Sputum of Functional Dust Mite–Specific IgE Antibodies in Intrinsic Asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Rondón, C.; Romero, J.J.; López, S.; Antúnez, C.; Martín-Casañez, E.; Torres, M.J.; Mayorga, C.; R-Pena, R.; Blanca, M. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J. Allergy Clin. Immunol. 2007, 119, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Rondón, C.; Fernández, J.; López, S.; Campo, P.; Doña, I.; Torres, M.J.; Mayorga, C.; Blanca, M. Nasal inflammatory mediators and specific IgE production after nasal challenge with grass pollen in local allergic rhinitis. J. Allergy Clin. Immunol. 2009, 124, 1005–1011. [Google Scholar] [CrossRef]
- López, S.; Rondón, C.; Torres, M.J.; Campo, P.; Canto, G.; Fernandez, R.; Garcia, R.; Martínez-Cañavate, A.; Blanca, M. Immediate and dual response to nasal challenge with Dermatophagoides pteronyssinus in local allergic rhinitis. Clin. Exp. Allergy 2010, 40, 1007–1014. [Google Scholar] [CrossRef]
- Campo, P.; del Carmen Plaza-Seron, M.; Eguiluz-Gracia, I.; Verge, J.; Galindo, L.; Barrionuevo, E.; Fernandez, J.; Jurado, R.; Mayorga, C.; Torres, M.J.; et al. Direct intranasal application of the solid phase of ImmunoCAP(R) increases nasal specific immunoglobulin E detection in local allergic rhinitis patients. Int. Forum. Allergy Rhinol. 2018, 8, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rondón, C.; Eguiluz-Gracia, I.; Shamji, M.H.; Layhadi, J.A.; Salas, M.; Torres, M.J.; Campo, P. IgE Test in Secretions of Patients with Respiratory Allergy. Curr. Allergy Asthma Rep. 2018, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, Y.; Lou, H.; Wang, K.; Meng, N.; Zhang, L.; Wang, C. Specific immunoglobulin E in nasal secretions for the diagnosis of local allergic rhinitis. Rhinol. J. 2019, 57, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Bilo, M.; Braunstahl, G.J.; Delgado, L.; Demoly, P.; Eigenmann, P.; Gevaert, P.; Gomes, E.; Hellings, P.; Horak, F.; et al. In vivo diagnosis of allergic diseases--allergen provocation tests. Allergy 2015, 70, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Diamant, Z.; Gauvreau, G.M.; Cockcroft, D.W.; Boulet, L.-P.; Sterk, P.J.; De Jongh, F.H.C.; Dahlén, B.; O’Byrne, P.M. Inhaled allergen bronchoprovocation tests. J. Allergy Clin. Immunol. 2013, 132, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; El-Gammal, A.I.; O’Byrne, P.M. Allergen-induced airway responses. Eur. Respir. J. 2015, 46, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Vethanayagam, D.; Yoshida, M.; Watson, R.M.; Rerecich, T.; Inman, M.; O’Byrne, P.M. Effects of Montelukast and Budesonide on Airway Responses and Airway Inflammation in Asthma. Am. J. Respir. Crit. Care Med. 2002, 166, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Ruffin, R.E.; Dolovich, J.; Hargreave, F.E. Allergen-induced increase in non-allergic bronchial reactivity. Clin. Exp. Allergy 1977, 7, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Shannon, C.P.; Kim, Y.W.; Yang, C.X.; Balshaw, R.; Freue, G.V.C.; Gauvreau, G.M.; Fitzgerald, J.M.; Boulet, L.; O’Byrne, P.M.; et al. Novel Blood-based Transcriptional Biomarker Panels Predict the Late-Phase Asthmatic Response. Am. J. Respir. Crit. Care Med. 2018, 197, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.N.; Ledford, D.K. Nasal and ocular challenges. J. Allergy Clin. Immunol. 2018, 141, 1570–1577. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Testera-Montes, A.; González, M.; Pérez-Sánchez, N.; Ariza-Veguillas, A.; Salas, M.; Moreno-Aguilar, C.; Campo, P.; Torres, M.J.; Rondón, C. Safety and reproducibility of nasal allergen challenge. Allergy 2019, 74, 1125–1134. [Google Scholar] [CrossRef]
- Augé, J.; Vent, J.; Agache, I.; Airaksinen, L.; Mozo, P.C.; Chaker, A.; Cingi, C.; Durham, S.; Fokkens, W.; Gevaert, P.; et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy 2018, 73, 1597–1608. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Testera-Montes, A.; Salas, M.; Pérez-Sánchez, N.; Ariza, A.; Bogas, G.; Bartra, J.; Torres, M.J.; Rondón, C. Comparison of diagnostic accuracy of acoustic rhinometry and symptoms score for nasal allergen challenge monitoring. Allergy 2020. [Google Scholar] [CrossRef] [PubMed]
- Rondón, C.; Campo, P.; Herrera, R.; Blanca-Lopez, N.; Melendez, L.; Canto, G.; Torres, M.J.; Blanca, M. Nasal allergen provocation test with multiple aeroallergens detects polysensitization in local allergic rhinitis. J. Allergy Clin. Immunol. 2011, 128, 1192–1197. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Fernandez-Santamaria, R.; Testera-Montes, A.; Ariza, A.; Campo, P.; Prieto, A.; Pérez-Sánchez, N.; Salas, M.; Mayorga, C.; Torres, M.J.; et al. Coexistence of nasal reactivity to allergens with and without IgE sensitization in patients with allergic rhinitis. Allergy 2020, 75, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Kopferschmitt-Kubler, M.; Bigot, H.; Pauli, G. Allergen bronchial challenge tests: Variability and reproducibility of the early response. J. Allergy Clin. Immunol. 1987, 80, 730–740. [Google Scholar] [CrossRef]
- Inman, M.; Watson, R.; Cockcroft, D.; Wong, B.; Hargreave, F.; O’Byrne, P.M. Reproducibility of allergen-induced early and late asthmatic responses. J. Allergy Clin. Immunol. 1995, 95, 1191–1195. [Google Scholar] [CrossRef]
- Sanz, M.L.; Sanchez, G.; Gamboa, P.M.; Sexto, L.V.; Uasuf, C.G.; Chazot, M.; Dieguez, I.; De Weck, A.L. Allergen-induced basophil activation: CD63 cell expression detected by flow cytometry in patients allergic to Dermatophagoides pteronyssinus and Lolium perenne. Clin. Exp. Allergy 2001, 31, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Konradsen, J.R.; Nordlund, B.; Nilsson, O.B.; Van Hage, M.; Nopp, A.; Hedlin, G.; Grönlund, H. High basophil allergen sensitivity (CD-sens) is associated with severe allergic asthma in children. Pediatr. Allergy Immunol. 2012, 23, 376–384. [Google Scholar] [CrossRef]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef]
- Rondón, C.; Bogas, G.; Barrionuevo, E.; Blanca, M.; Torres, M.J.; Campo, P. Nonallergic rhinitis and lower airway disease. Allergy 2017, 72, 24–34. [Google Scholar] [CrossRef]
- Gómez, E.; Campo, P.; Rondón, C.; Barrionuevo, E.; Blanca-López, N.; Torres, M.J.; Herrera, R.; Galindo, L.; Mayorga, C.; Blanca, M. Role of the basophil activation test in the diagnosis of local allergic rhinitis. J. Allergy Clin. Immunol. 2013, 132, 975–976. [Google Scholar] [CrossRef]
- Ferreira, R.D.; Ornelas, C.; Silva, S.; Morgado, R.; Pereira, D.; Escaleira, D.; Moreira, S.; Valença, J.; Pedro, E.; Ferreira, M.B.; et al. Contribution of In Vivo and In Vitro Testing for the Diagnosis of Local Allergic Rhinitis. J. Investig. Allergol. Clin. Immunol. 2019, 29, 46–48. [Google Scholar] [CrossRef] [PubMed]
- The Global Initiative for Asthma (GINA). 2020. Available online: https://ginasthma.org/ (accessed on 25 October 2020).
- Agache, I.; Akdis, C.A.; Akdis, M.; Canonica, G.W.; Casale, T.B.; Chivato, T.; Corren, J.; Chu, D.K.; Del Giacco, S.; Eiwegger, T.; et al. EAACI Biologicals Guidelines—Recommendations for severe asthma. Allergy 2020. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Barnes, P.; Canonica, G.W.; Gaga, M.; Heaney, L.; Menzies-Gow, A.; Kritikos, V.; Fitzgerald, M. The evolving algorithm of biological selection in severe asthma. Allergy 2020, 75, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Caminati, M.; Bagnasco, D.; Rosenwasser, L.J.; Vianello, A.; Senna, G. Biologics for the Treatments of Allergic Conditions. Immunol. Allergy Clin. N. Am. 2020, 40, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Möller, C.; Dreborg, S.; Ferdousi, H.A.; Halken, S.; Høst, A.; Jacobsen, L.; Koivikko, A.; Koller, D.Y.; Niggemann, B.; Norberg, L.A.; et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J. Allergy Clin. Immunol. 2002, 109, 251–256. [Google Scholar] [CrossRef]
- Valovirta, E.; Petersen, T.H.; Piotrowska, T.; Laursen, M.K.; Andersen, J.S.; Sørensen, H.F.; Klink, R.; Varga, E.-M.; Huttegger, I.; Agertoft, L.; et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J. Allergy Clin. Immunol. 2018, 141, 529–538. [Google Scholar] [CrossRef]
- Abramson, M.; Puy, R.M.; Weiner, J.M. Injection allergen immunotherapy for asthma. Cochrane Database Syst. Rev. 2010, CD001186. [Google Scholar] [CrossRef]
- Suárez-Fueyo, A.; Ramos, T.; Galán, A.; Jimeno, L.; Wurtzen, P.A.; Marin, A.; De Frutos, C.; Blanco, C.; Carrera, A.C.; Barber, D.; et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J. Allergy Clin. Immunol. 2014, 133, 130–138. [Google Scholar] [CrossRef]
- Varona, R.; Ramos, T.; Escribese, M.M.; Jimeno, L.; Galán, A.; Würtzen, P.A.; Vega, F.; Marín, A.; Martín, S.; Carrera, A.C.; et al. Persistent regulatory T-cell response 2 years after 3 years of grass tablet SLIT: Links to reduced eosinophil counts, sIgE levels, and clinical benefit. Allergy 2019, 74, 349–360. [Google Scholar] [CrossRef]
- Nolte, H.; Maloney, J.; Nelson, H.S.; Bernstein, D.I.; Lu, S.; Li, Z.; Kaur, A.; Zieglmayer, P.; Zieglmayer, R.; Lemell, P.; et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J. Allergy Clin. Immunol. 2015, 135, 1494–1501. [Google Scholar] [CrossRef]
- Mosbech, H.; Deckelmann, R.; De Blay, F.; Pastorello, E.A.; Trebas-Pietras, E.; Andres, L.P.; Malcus, I.; Ljørring, C.; Canonica, G.W. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2014, 134, 568–575. [Google Scholar] [CrossRef]
- Virchow, J.C.; Backer, V.; Kuna, P.; Prieto, L.; Nolte, H.; Villesen, H.H.; Ljørring, C.; Riis, B.; De Blay, F. Efficacy of a House Dust Mite Sublingual Allergen Immunotherapy Tablet in Adults With Allergic Asthma. JAMA 2016, 315, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Guía Española Para el Manejo del Asma (GEMA) Versión 5.0. Available online: http://gemasma.com/ (accessed on 25 October 2020).
- Agache, I.; Lau, S.; Akdis, C.A.; Smolinska, S.; Bonini, M.; Cavkaytar, O.; Flood, B.; Gajdanowicz, P.; Izuhara, K.; Kalayci, O.; et al. EAACI Guidelines on Allergen Immunotherapy: House dust mite-driven allergic asthma. Allergy 2019, 74, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Corren, J.; Creticos, P.; De Blay, F.; Gevaert, P.; Hellings, P.; Kowal, K.; Le Gall, M.; Nenasheva, N.; Passalacqua, G.; et al. A 300 IR sublingual tablet is an effective, safe treatment for house dust mite-induced allergic rhinitis: An international, double-blind, placebo-controlled, randomized phase III clinical trial. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Akitsu, K.; Kubota, K. Effect of Sublingual Immunotherapy on Airway Inflammation and Airway Wall Thickness in Allergic Asthma. J. Allergy Clin. Immunol. Pr. 2019, 7, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Akitsu, K.; Kubota, K.; Ohtawa, J. Association between biomarkers and house dust mite sublingual immunotherapy in allergic asthma. Clin. Exp. Allergy 2020, 50, 1035–1043. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Ariza, A.; Testera-Montes, A.; Rondón, C.; Campo, P. Allergen Immunotherapy for Local Respiratory Allergy. Curr. Allergy Asthma Rep. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Rondón, C.; Campo, P.; Salas, M.; Aranda, A.; Molina, A.; González, M.; Galindo, L.; Mayorga, C.; Torres, M.J.; Blanca, M. Efficacy and safety ofD. pteronyssinusimmunotherapy in local allergic rhinitis: A double-blind placebo-controlled clinical trial. Allergy 2016, 71, 1057–1061. [Google Scholar] [CrossRef]
- Rondon, C.; Blanca-Lopez, N.; Campo, P.; Mayorga, C.; Jurado-Escobar, R.; Torres, M.J.; Canto, G.; Blanca, M. Specific immunotherapy in local allergic rhinitis: A randomized, double-blind placebo-controlled trial with Phleum pratense subcutaneous allergen immunotherapy. Allergy 2018, 73, 905–915. [Google Scholar] [CrossRef]
- Bożek, A.; Kołodziejczyk, K.; Jarzab, B. Efficacy and safety of birch pollen immunotherapy for local allergic rhinitis. Ann. Allergy Asthma Immunol. 2018, 120, 53–58. [Google Scholar] [CrossRef]
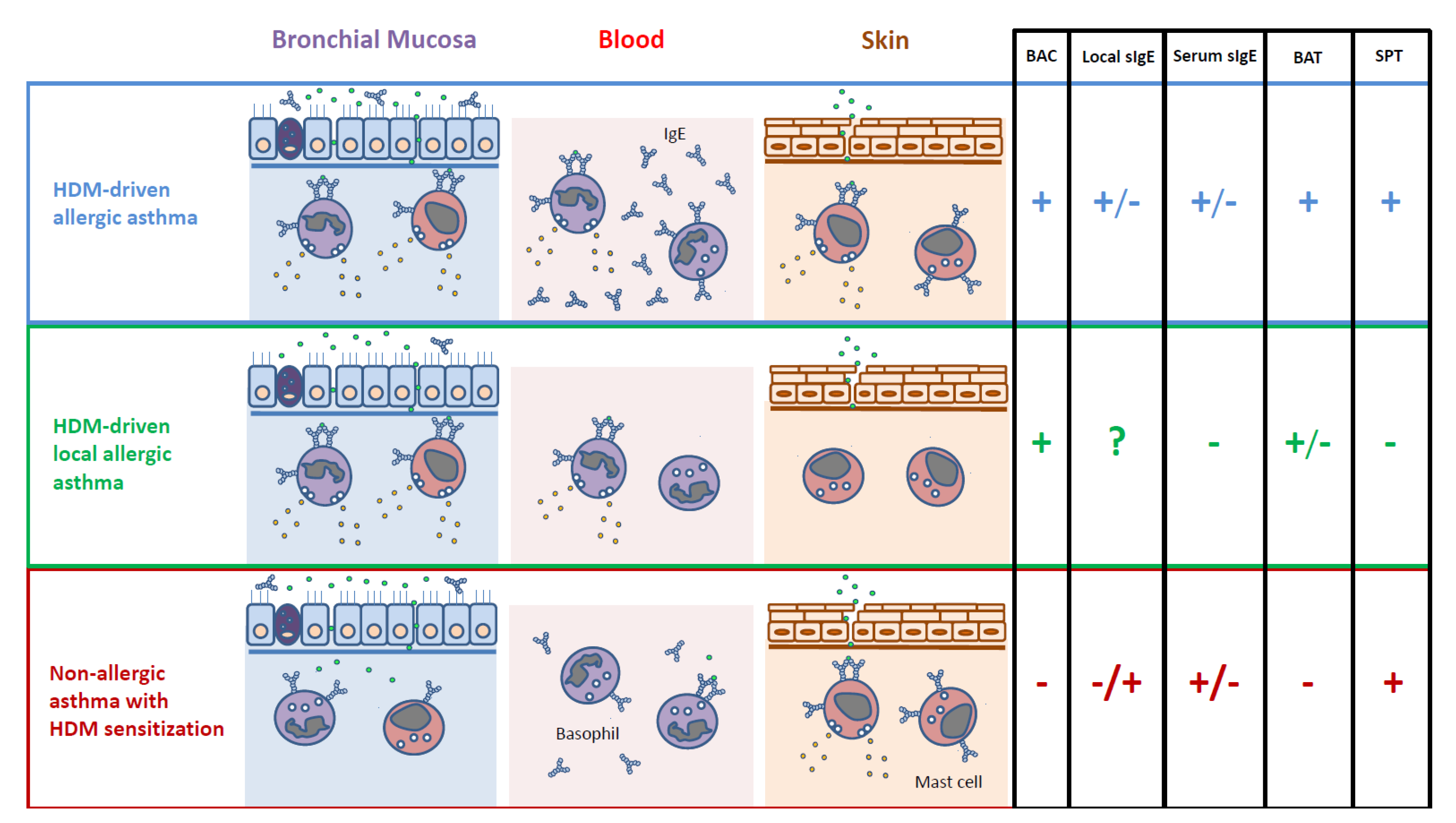
| HDM-Driven Allergic Asthma | HDM-Driven Local Allergic Asthma | Non-Allergic Asthma with HDM Sensitization | |
|---|---|---|---|
| Nasal affection | Virtually always | Always | Common, but not always |
| Nasal counterpart | Allergic rhinitis | Local allergic rhinitis | Non-allergic rhinitis |
| Atopy | Present | Absent | Present |
| Family history of allergy | Frequent | Frequent | Infrequent |
| Allergic triggers | House dust mites. Others possible. | House dust mites. Others possible. | None |
| Severity | Mild to severe | Only demonstrated in mild to moderate cases | Mild to severe |
| Age of onset | Early (childhood/adolescence) | Probably early (childhood/adolescence) | Later than allergic phenotypes |
| Natural evolution | Progressive worsening and onset of new systemic sensitizations | Progressive worsening and onset of new local sensitizations | Stable severity since onset in most cases |
| Eosinophilia | Yes | Yes | Sometimes |
| Bronchial sIgE | Frequent | Unknown | Possible |
| BAC needed for diagnosis | Sometimes | Always | Sometimes |
| Indication of ICS | Yes | Yes | Yes |
| Effect of ICS | Beneficial | Beneficial | Variable |
| Indication of omalizumab * | Yes | No | Theoretically not, but often prescribed ** |
| Effect of omalizumab | Beneficial | Probably beneficial | Not beneficial in most cases |
| Indication of reslizumab mepolizumab, benralizumab and dupilumab * | In most cases | Potential, but the phenotype is not identified yet among severe asthmatics. | Variable |
| Effect of reslizumab, mepolizumab, benralizumab and dupilumab | Beneficial in most cases | Potentially beneficial, but the phenotype is not identified yet among severe asthmatics. | Variable |
| Indication of AIT | Yes | No | No |
| Effect of AIT | Beneficial | Probably beneficial | Not beneficial |
| Nasal Allergen Challenge | Bronchial Allergen Challenge | |
|---|---|---|
| Standardized for clinical use | Yes | No |
| Need to withdraw ICS | No | Yes |
| Minimum FEV1 required | Flexible as long as the bronchial disease is sufficiently controlled | 70% |
| Primary diagnostic use | Allergic rhinitis, local allergic rhinitis and dual allergic rhinitis [53] | Allergic asthma and local allergic asthma |
| Recommended monitoring system | Symptoms score (subjective) and objective measurement of the nasal patency (e.g., by acoustic rhinometry) | Bronchial obstruction by spirometry. Possible: symptom score, AHR (e.g., by methacholine challenge) and inflammation (e.g., FeNO) |
| Cutoff points for positivity | More defined | Less defined |
| Safety in asthma patients | High | Moderate |
| Reproducibility | High [54,55] | High |
| Length of the procedure including observation period | 30 min to 1 h | From 7 to 24 h |
| Sample collection in connection to the procedure | Nasal lavage or secretions. Mucosal scraping, brushing or biopsy. FnNO. | Induced sputum, BAL. Mucosal brushing or biopsy. FeNO. |
| Capacity of phenotyping the united airways disease | Variable (depends on the phenotype) | Unknown |
| Small Drugs | Biologicals | Allergen Immunotherapy | |
|---|---|---|---|
| Molecular weight (kDa) | 0.9 | 150 | 5–50 |
| Structure | Chemical compound | Monoclonal antibody (immunoglobulin) | Protein |
| Production mode | Chemical synthesis | Genetic engineering and cell culture | Purification of native extract |
| Site of action | Extra or intracellular | Extracellular | Extra and intracellular |
| Administration route | Inhaled or oral | Subcutaneous or intravenous | Subcutaneous or sublingual |
| Half-life | Hours | Weeks | Weeks |
| Dose interval | Maximum 24 h | 2–4 weeks | 24 h to 4–6 weeks |
| Precision medicine | Pharmacogenomics | Immunology and metabolomics | Molecular allergology |
| Specificity | Low/medium/high | High | Very high |
| Sustained effect after discontinuation | No | No | Yes |
| Disease-modifying effect | No | No | Yes |
| Administration period | Indefinite | Indefinite | 3 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eguiluz-Gracia, I.; Palomares, F.; Salas, M.; Testera-Montes, A.; Ariza, A.; Davila, I.; Bartra, J.; Mayorga, C.; Torres, M.J.; Rondon, C. Precision Medicine in House Dust Mite-Driven Allergic Asthma. J. Clin. Med. 2020, 9, 3827. https://doi.org/10.3390/jcm9123827
Eguiluz-Gracia I, Palomares F, Salas M, Testera-Montes A, Ariza A, Davila I, Bartra J, Mayorga C, Torres MJ, Rondon C. Precision Medicine in House Dust Mite-Driven Allergic Asthma. Journal of Clinical Medicine. 2020; 9(12):3827. https://doi.org/10.3390/jcm9123827
Chicago/Turabian StyleEguiluz-Gracia, Ibon, Francisca Palomares, Maria Salas, Almudena Testera-Montes, Adriana Ariza, Ignacio Davila, Joan Bartra, Cristobalina Mayorga, Maria Jose Torres, and Carmen Rondon. 2020. "Precision Medicine in House Dust Mite-Driven Allergic Asthma" Journal of Clinical Medicine 9, no. 12: 3827. https://doi.org/10.3390/jcm9123827
APA StyleEguiluz-Gracia, I., Palomares, F., Salas, M., Testera-Montes, A., Ariza, A., Davila, I., Bartra, J., Mayorga, C., Torres, M. J., & Rondon, C. (2020). Precision Medicine in House Dust Mite-Driven Allergic Asthma. Journal of Clinical Medicine, 9(12), 3827. https://doi.org/10.3390/jcm9123827






