Arginine and Arginine/ADMA Ratio Predict 90-Day Mortality in Patients with Out-of-Hospital Cardiac Arrest—Results from the Prospective, Observational COMMUNICATE Trial
Abstract
1. Introduction
2. Methods
2.1. Study Setting Population
2.2. Data Collection
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
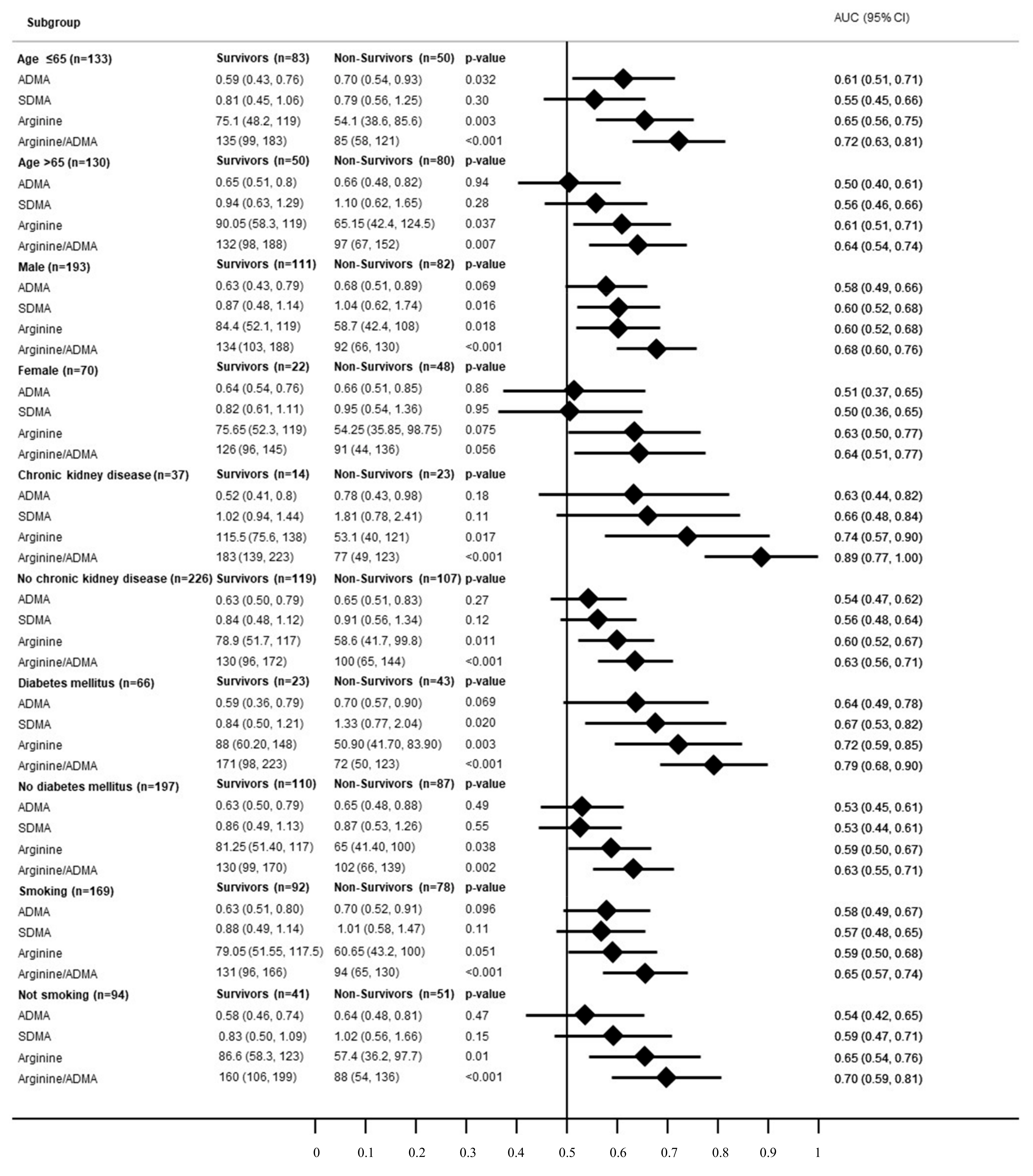
3.2. Subgroup Analysis
3.3. Secondary Endpoints
3.3.1. In-Hospital Mortality
3.3.2. Neurological Outcome (CPC) at Hospital Discharge
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Primary Endpoint: 90-Day Mortality | ||||
|---|---|---|---|---|
| (C) Adjusted for age, gender and cardiovascular risk factors | (D) Adjusted for age, gender and laboratory values (PCT, Lc, CRP) | |||
| OR or Coefficient (95% CI) | p-value | OR or Coefficient (95% CI) | p-value | |
| ADMA | 2.12 (0.94, 4.78) | 0.072 | 3.07 (1.19, 7.90) | 0.02 |
| ADMA log | 1.56 (0.83, 2.92) | 0.167 | 2.03 (1.00, 4.10) | 0.049 |
| ADMA per decile | 1.05 (0.96, 1.16) | 0.280 | 1.09 (0.99, 1.20) | 0.09 |
| SDMA | 0.93 (0.74, 1.17) | 0.552 | 0.97 (0.76, 1.20) | 0.79 |
| SDMA log | 1.14 (0.81, 1.61) | 0.459 | 1.24 (0.85, 1.80) | 0.26 |
| SDMA per decile | 1.05 (0.96, 1.16) | 0.277 | 1.08 (0.97, 1.00) | 0.16 |
| Arginin | 0.53 (0.30, 0.93) | 0.028 | 0.78 (0.43, 1.42) | 0.43 |
| Arginin log | 0.49 (0.31, 0.77) | 0.002 | 0.62 (0.39, 0.98) | 0.04 |
| Arginin per decile | 0.86 (0.78, 0.95) | 0.002 | 0.91 (0.82, 1.00) | 0.09 |
| Ratio | 0.65 (0.47, 0.90) | 0.010 | 0.75 (0.55, 1.03) | 0.08 |
| Ratio log | 0.42 (0.26, 0.66) | <0.001 | 0.49 (0.31, 0.70) | <0.01 |
| Ratio per decile | 0.81 (0.73, 0.90) | <0.001 | 0.85 (0.76, 0.90) | <0.01 |
| Secondary endpoint: In-hospital mortality | ||||
| (C) Adjusted for age, gender and cardiovascular risk factors | (D) Adjusted for age, gender and laboratory values (PCT, Lc, CRP) | |||
| OR or Coefficient (95% CI) | p-Value | OR or Coefficient (95% CI) | p-Value | |
| ADMA | 2.06 (0.93, 4.57) | 0.074 | 2.75 (1.07, 7.08) | 0.04 |
| ADMA log | 1.54 (0.83, 2.88) | 0.174 | 1.89 (0.92, 3.88) | 0.08 |
| ADMA per decile | 1.05 (0.96, 1.15) | 0.285 | 1.08 (0.97, 1.21) | 0.14 |
| SDMA | 0.94 (0.76, 1.17) | 0.59 | 0.91 (0.69, 1.2) | 0.48 |
| SDMA log | 1.03 (0.74, 1.45) | 0.849 | 1.04 (0.72, 1.52) | 0.83 |
| SDMA per decile | 1.02 (0.93, 1.12) | 0.676 | 1.02 (0.92, 1.13) | 0.72 |
| Arginin | 0.67 (0.39, 1.16) | 0.157 | 1.11 (0.6, 2.04) | 0.74 |
| Arginin log | 0.60 (0.40, 0.89) | 0.012 | 0.80 (0.51, 1.26) | 0.34 |
| Arginin per decile | 0.89 (0.81, 0.98) | 0.018 | 0.97 (0.87, 1.08) | 0.56 |
| Ratio | 0.75 (0.56, 1.02) | 0.066 | 0.90 (0.67, 1.21) | 0.49 |
| Ratio log | 0.52 (0.35, 0.78) | <0.001 | 0.66 (0.43, 1.01) | 0.06 |
| Ratio per decile | 0.86 (0.78, 0.94) | <0.001 | 0.92 (0.82, 1.02) | 0.11 |
| Secondary endpoint: hospital discharge CPC | ||||
| (C) Adjusted for age, gender and cardiovascular risk factors | (D) Adjusted for age, gender and laboratory values (PCT, Lc, CRP) | |||
| OR or Coefficient (95% CI) | p-Value | OR or Coefficient (95% CI) | p-Value | |
| ADMA | 1.66 (0.76, 3.64) | 0.203 | 2.02 (0.78, 5.25) | 0.15 |
| ADMA log | 1.23 (0.67, 2.28) | 0.506 | 1.38 (0.67, 2.84) | 0.38 |
| ADMA per decile | 1.02 (0.93, 1.12) | 0.727 | 1.03 (0.92, 1.14) | 0.65 |
| SDMA | 0.91 (0.73, 1.13) | 0.380 | 0.89 (0.67, 1.17) | 0.39 |
| SDMA log | 0.86 (0.62, 1.20) | 0.371 | 0.87 (0.60, 1.25) | 0.45 |
| SDMA per decile | 0.99 (0.90, 1.09) | 0.859 | 0.99 (0.89, 1.11) | 0.90 |
| Arginin | 0.51 (0.29, 0.89) | 0.017 | 0.78 (0.42, 1.43) | 0.42 |
| Arginin log | 0.48 (0.30, 0.75) | 0.001 | 0.63 (0.39, 1.01) | 0.06 |
| Arginin per decile | 0.85 (0.78, 0.94) | 0.001 | 0.91 (0.82, 1.02) | 0.10 |
| Ratio | 0.70 (0.52, 0.95) | <0.001 | 0.83 (0.61, 1.12) | 0.23 |
| Ratio log | 0.46 (0.29, 0.71) | <0.001 | 0.58 (0.37, 0.92) | 0.02 |
| Ratio per decile | 0.83 (0.76, 0.92) | <0.001 | 0.89 (0.80, 0.99) | 0.03 |
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Young, G.B. Clinical practice. Neurologic prognosis after cardiac arrest. N. Engl. J. Med. 2009, 361, 605–611. [Google Scholar] [CrossRef]
- Kantae, V.; Krekels, E.H.J.; Van Esdonk, M.J.; Lindenburg, P.; Harms, A.C.; Knibbe, C.A.J.; van der Graaf, P.H.; Hankemeier, T. Integration of pharmacometabolomics with pharmacokinetics and pharmacodynamics: Towards personalized drug therapy: An addition to the topical collection “Recent Advances in Pharmacometabolomics: Enabling Tools for Precision Medicine”. Metabolomics 2017, 13. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Nickler, M.; Ottiger, M.; Steuer, C.; Huber, A.; Anderson, J.B.; Muller, B.; Schuetz, P. Systematic review regarding metabolic profiling for improved pathophysiological understanding of disease and outcome prediction in respiratory infections. Respir. Res. 2015, 16, 125. [Google Scholar] [CrossRef]
- Abela, R.; Alarcon, A.; Alex, J.; Arrell, C.; Arsov, V.; Bettoni, S.; Bopp, M.; Bostedt, C.; Braun, H.H.; Calvi, M.; et al. The SwissFEL soft X-ray free-electron laser beamline: Athos. J. Synchrotron Radiat. 2019, 26, 1073–1084. [Google Scholar] [CrossRef]
- Scott, J.A.; Duongh, M.; Young, A.W.; Subbarao, P.; Gauvreau, G.M.; Grasemann, H. Asymmetric dimethylarginine in chronic obstructive pulmonary disease (ADMA in COPD). Int. J. Mol. Sci. 2014, 15, 6062–6071. [Google Scholar] [CrossRef]
- Shivkar, R.R.; Abhang, S.A. Ratio of Serum Asymmetric Dimethyl Arginine (ADMA)/ Nitric Oxide in Coronary Artery Disease patients. J. Clin. Diagn. Res. JCDR 2014, 8, CC04–CC06. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Rizzo, V.; Bianchi, A.; di Pasqua, L.G.; Berardo, C.; Richelmi, P.; Vairetti, M. Changes in ADMA/DDAH pathway after hepatic ischemia/reperfusion injury in rats: The role of bile. Biomed. Res. Int. 2014, 2014, 627434. [Google Scholar] [CrossRef]
- Cook, J.P. Does ADMA Cause Endothelial Dysfunktion? Arterioscler Thromb. Vasc. Biol. 2000, 20, 2032–2037. [Google Scholar] [CrossRef]
- Jacobi, J.; Tsao, P.S. Asymmetrical dimethylarginine in renal disease: Limits of variation or variation limits? A systematic review. Am. J. Nephrol. 2008, 28, 224–237. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Boger, R.H.; Bode-Boger, S.M.; Froelich, J.C.; Haller, H.; Ritz, E.; Fliser, D. Marked Increase of Asymmetric Dimethylarginine in Patients with Incipient Primary Chronic renal disease. J. Am. Soc. Nephrol. 2002, 13, 170–176. [Google Scholar]
- Mokhaneli, M.C.; Fourie, C.M.T.; Botha-Le Roux, S.; Boger, R.H.; Schwedhelm, E.; Mels, C.M.C. Asymmetric dimethylarginine and L-homoarginine prospectively relate to carotid wall thickness in a South African cohort. Amino Acids 2020, 52, 965–973. [Google Scholar] [CrossRef]
- Brinkmann, S.J.; Worner, E.A.; Buijs, N.; Richir, M.; Cynober, L.; van Leeuwen, P.A.; Couderc, R. The Arginine/ADMA Ratio is Related to the Prevention of Atherosclerotic Plaques in Hypercholesterolemic Rabbits When Giving a Combined Therapy with Atorvastatine and Arginine. Int. J. Mol. Sci. 2015, 16, 12230–12242. [Google Scholar] [CrossRef]
- Notsu, Y.; Yano, S.; Shibata, H.; Nagai, A.; Nabika, T. Plasma arginine/ADMA ratio as a sensitive risk marker for atherosclerosis: Shimane CoHRE study. Atherosclerosis 2015, 239, 61–66. [Google Scholar] [CrossRef]
- Boger, R. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc. Res. 2003, 59, 824–833. [Google Scholar] [CrossRef]
- Yu, E.; Ruiz-Canela, M.; Hu, F.B.; Clish, C.B.; Corella, D.; Salas-Salvado, J.; Hruby, A.; Fito, M.; Liang, L.; Toledo, E.; et al. Plasma Arginine/Asymmetric Dimethylarginine Ratio and Incidence of Cardiovascular Events: A Case-Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 1879–1888. [Google Scholar] [CrossRef]
- Bode-Boger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J. Am. Soc. Nephrol. 2006, 17, 1128–1134. [Google Scholar] [CrossRef]
- Schnabel, R.; Blankenberg, S.; Lubos, E.; Lackner, K.J.; Rupprecht, H.J.; Espinola-Klein, C.; Jachmann, N.; Post, F.; Peetz, D.; Bickel, C.; et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: Results from the AtheroGene Study. Circ. Res. 2005, 97, e53–e59. [Google Scholar] [CrossRef]
- Maarsingh, H.; Pera, T.; Meurs, H. Arginase and pulmonary diseases. Naunyn Schmiedebergs Arch. Pharm. 2008, 378, 171–184. [Google Scholar] [CrossRef]
- Vogeli, A.; Ottiger, M.; Meier, M.A.; Steuer, C.; Bernasconi, L.; Kulkarni, P.; Huber, A.; Christ-Crain, M.; Henzen, C.; Hoess, C.; et al. Admission levels of asymmetric and symmetric dimethylarginine predict long-term outcome in patients with community-acquired pneumonia. Respir. Res. 2017, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Coskun, C.N.; Usanmaz, S.E.; Savci, V.; Demirel-Yilmaz, E. Time-Dependent Production of Endothelium-Related Biomarkers is Affected Differently in Hemorrhagic and Septic Shocks. Inflammation 2018, 41, 33–41. [Google Scholar] [CrossRef]
- Koch, A.; Weiskirchen, R.; Kunze, J.; Duckers, H.; Bruensing, J.; Buendgens, L.; Matthes, M.; Luedde, T.; Trautwein, C.; Tacke, F. Elevated asymmetric dimethylarginine levels predict short- and long-term mortality risk in critically ill patients. J. Crit. Care 2013, 28, 947–953. [Google Scholar] [CrossRef]
- Davis, J.S.; Darcy, C.J.; Yeo, T.W.; Jones, C.; McNeil, Y.R.; Stephens, D.P.; Celermajer, D.S.; Anstey, N.M. Asymmetric dimethylarginine, endothelial nitric oxide bioavailability and mortality in sepsis. PLoS ONE 2011, 6, e17260. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Wang, Z.; Koeth, R.; Levison, B.; DelFraino, B.; Dzavik, V.; Griffith, O.W.; Hathaway, D.; Panza, J.A.; Nissen, S.E.; et al. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation 2007, 116, 2315–2324. [Google Scholar] [CrossRef]
- O’Dwyer, M.J.; Dempsey, F.; Crowley, V.; Kelleher, D.P.; McManus, R.; Ryan, T. Septic shock is correlated with asymmetrical dimethyl arginine levels, which may be influenced by polymorphism in dimethylarginine dimethylaminohydrolase II gene: A prospactive observational study. Crit. Care 2006. [Google Scholar] [CrossRef]
- Isenschmid, C.; Kalt, J.; Gamp, M.; Tondorf, T.; Becker, C.; Tisljar, K.; Locher, S.; Schuetz, P.; Marsch, S.; Hunziker, S. Routine blood markers from different biological pathways improve early risk stratification in cardiac arrest patients: Results from the prospective, observational COMMUNICATE study. Resuscitation 2018, 130, 138–145. [Google Scholar] [CrossRef]
- Isenschmid, C.; Luescher, T.; Rasiah, R.; Kalt, J.; Tondorf, T.; Gamp, M.; Becker, C.; Tisljar, K.; Sutter, R.; Schuetz, P.; et al. Performance of clinical risk scores to predict mortality and neurological outcome in cardiac arrest patients. Resuscitation 2019, 136, 21–29. [Google Scholar] [CrossRef]
- Metzger, K.; Gamp, M.; Tondorf, T.; Hochstrasser, S.; Becker, C.; Luescher, T.; Rasiah, R.; Boerlin, A.; Tisljar, K.; Emsden, C.; et al. Depression and anxiety in relatives of out-of-hospital cardiac arrest patients: Results of a prospective observational study. J. Crit. Care 2019, 51, 57–63. [Google Scholar] [CrossRef]
- Hochstrasser, S.R.; Metzger, K.; Vincent, A.M.; Becker, C.; Keller, A.K.J.; Beck, K.; Perrig, S.; Tisljar, K.; Sutter, R.; Schuetz, P.; et al. Trimethylamine-N-oxide (TMAO) predicts short- and long-term mortality and poor neurological outcome in out-of-hospital cardiac arrest patients. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Herzog, N.; Laager, R.; Thommen, E.; Widmer, M.; Vincent, A.M.; Keller, A.; Becker, C.; Beck, K.; Perrig, S.; Bernasconi, L.; et al. Association of Taurine with In-Hospital Mortality in Patients after Out-of-Hospital Cardiac Arrest: Results from the Prospective, Observational COMMUNICATE Study. J. Clin. Med. 2020, 9, 1405. [Google Scholar] [CrossRef] [PubMed]
- Widmer, M.; Thommen, E.B.; Becker, C.; Beck, K.; Vincent, A.M.; Perrig, S.; Keller, A.; Bernasconi, L.; Neyer, P.; Marsch, S.; et al. Association of acyl carnitines and mortality in out-of-hospital-cardiac-arrest patients: Results of a prospective observational study. J. Crit. Care 2020, 58, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Boerlin, A.; Luescher, T.; Becker, C.; Perrig, S.; Thommen, E.; Widmer, M.; Beck, K.; Vincent, A.; Tisljar, K.; Bernasconi, L.; et al. Low Plasma Sphingomyelin Levels Show a Weak Association with Poor Neurological Outcome in Cardiac Arrest Patients: Results from the Prospective, Observational COMMUNICATE Trial. J. Clin. Med. 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Luescher, T.; Mueller, J.; Isenschmid, C.; Kalt, J.; Rasiah, R.; Tondorf, T.; Gamp, M.; Becker, C.; Sutter, R.; Tisljar, K.; et al. Neuron-specific enolase (NSE) improves clinical risk scores for prediction of neurological outcome and death in cardiac arrest patients: Results from a prospective trial. Resuscitation 2019, 142, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef]
- Weinberger, K.M. Metabolomics in diagnosing metabolic diseases. Ther. Umsch. 2008, 65, 487–491. [Google Scholar] [CrossRef]
- Yet, I.; Menni, C.; Shin, S.Y.; Mangino, M.; Soranzo, N.; Adamski, J.; Suhre, K.; Spector, T.D.; Kastenmuller, G.; Bell, J.T. Genetic Influences on Metabolite Levels: A Comparison across Metabolomic Platforms. PLoS ONE 2016, 11, e0153672. [Google Scholar] [CrossRef]
- Illig, T.; Gieger, C.; Zhai, G.; Romisch-Margl, W.; Wang-Sattler, R.; Prehn, C.; Altmaier, E.; Kastenmuller, G.; Kato, B.S.; Mewes, H.W.; et al. A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 2010, 42, 137–141. [Google Scholar] [CrossRef]
- Siskos, A.P.; Jain, P.; Romisch-Margl, W.; Bennett, M.; Achaintre, D.; Asad, Y.; Marney, L.; Richardson, L.; Koulman, A.; Griffin, J.L.; et al. Interlaboratory Reproducibility of a Targeted Metabolomics Platform for Analysis of Human Serum and Plasma. Anal. Chem. 2017, 89, 656–665. [Google Scholar] [CrossRef]
- Adrie, C.; Cariou, A.; Mourvillier, B.; Laurent, I.; Dabbane, H.; Hantala, F.; Rhaoui, A.; Thuong, M.; Monchi, M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: The OHCA score. Eur. Heart J. 2006, 27, 2840–2845. [Google Scholar] [CrossRef]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage. Lancet 1975, 1, 480–484. [Google Scholar] [CrossRef]
- Gore, M.O.; Luneburg, N.; Schwedhelm, E.; Ayers, C.R.; Anderssohn, M.; Khera, A.; Atzler, D.; de Lemos, J.A.; Grant, P.J.; McGuire, D.K.; et al. Symmetrical dimethylarginine predicts mortality in the general population: Observations from the Dallas heart study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Ozlu, M.F.; Akgul, E.O.; Kanat, S.; Cayci, T.; Turak, O.; Yaman, H.; Sokmen, E.; Ozcan, F.; Maden, O.; et al. Elevated plasma asymmetric dimethylarginine level in acute myocardial infarction patients as a predictor of poor prognosis and angiographic impaired reperfusion. Atherosclerosis 2011, 219, 304–310. [Google Scholar] [CrossRef]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef]
- Pizzarelli, F.; Maas, R.; Dattolo, P.; Tripepi, G.; Michelassi, S.; D’Arrigo, G.; Mieth, M.; Bandinelli, S.; Ferrucci, L.; Zoccali, C. Asymmetric dimethylarginine predicts survival in the elderly. Age 2013, 35, 2465–2475. [Google Scholar] [CrossRef]
- Darcy, C.J.; Woodberry, T.; Davis, J.S.; Piera, K.A.; McNeil, Y.R.; Chen, Y.; Yeo, T.W.; Weinberg, J.B.; Anstey, N.M. Increased plasma arginase activity in human sepsis: Association with increased circulating neutrophils. Clin. Chem. Lab. Med. 2014, 52, 573–581. [Google Scholar] [CrossRef]
- Davis, J.S.; Anstey, N.M. Is plasma arginine concentration decreased in patients with sepsis? A systematic review and meta-analysis. Crit. Care Med. 2011, 39, 380–385. [Google Scholar] [CrossRef]
- Patel, J.J.; Miller, K.R.; Rosenthal, C.; Rosenthal, M.D. When Is It Appropriate to Use Arginine in Critical Illness? Nutr. Clin. Pr. 2016, 31, 438–444. [Google Scholar] [CrossRef]
- Hochman, J.S. Cardiogenic shock complicating acute myocardial infarction: Expanding the paradigm. Circulation 2003, 107, 2998–3002. [Google Scholar] [CrossRef]
- Donnino, M.W.; Andersen, L.W.; Giberson, T.; Gaieski, D.F.; Abella, B.S.; Peberdy, M.A.; Rittenberger, J.C.; Callaway, C.W.; Ornato, J.; Clore, J.; et al. Initial lactate and lactate change in post-cardiac arrest: A multicenter validation study. Crit. Care Med. 2014, 42, 1804–1811. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Boger, R.H. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat. Rev. Nephrol. 2011, 7, 275–285. [Google Scholar] [CrossRef]
- Baranyi, A.; Amouzadeh-Ghadikolai, O.; Rothenhausler, H.B.; Theokas, S.; Robier, C.; Baranyi, M.; Koppitz, M.; Reicht, G.; Hlade, P.; Meinitzer, A. Nitric Oxide-Related Biological Pathways in Patients with Major Depression. PLoS ONE 2015, 10, e0143397. [Google Scholar] [CrossRef]
- Faraci, F.M.; Brian, J.E. Nitric oxide and the cerebral circulation. Stroke 1994, 25, 692–703. [Google Scholar] [CrossRef]
- Schulze, F.; Carter, A.M.; Schwedhelm, E.; Ajjan, R.; Maas, R.; von Holten, R.A.; Atzler, D.; Grant, P.J.; Boger, R.H. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis 2010, 208, 518–523. [Google Scholar] [CrossRef]
- Tipton, K.D. Gender differences in protein metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 493–498. [Google Scholar] [CrossRef]
- Forte, P.; Kneale, B.J.; Milne, E.; Chowienczyk, P.J.; Johnston, A.; Benjamin, N.; Ritter, J.M. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension 1998, 32, 730–734. [Google Scholar] [CrossRef]
- Kumar, A.N.; Kalyankar, G.D. Effect of steroid hormones on age dependent changes in rat arginase isoenzymes. Exp. Gerontol. 1984, 19, 191–198. [Google Scholar] [CrossRef]
- Weiner, C.P.; Lizasoain, I.; Baylis, S.A.; Knowles, R.G.; Charles, I.G.; Moncada, S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc. Natl. Acad. Sci. USA 1994, 91, 5212–5216. [Google Scholar] [CrossRef]
| Factor | All | Survivors | Non-Survivors | p-Value |
|---|---|---|---|---|
| Number (n) | 263 | 133 | 130 | |
| Sociodemographics | ||||
| Age, median (IQR) | 65 (57, 74) | 62 (53, 73) | 69 (61, 78) | <0.001 |
| Male sex, n (%) | 193 (73.4%) | 111 (83.5%) | 82 (63.1%) | <0.001 |
| Cause of cardiac arrest | ||||
| Coronary artery disease, n (%) | 130 (49.6%) | 79 (59.8%) | 51 (39.2%) | <0.001 |
| Arrhythmia, n (%) | 55 (21.0%) | 28 (21.2%) | 27 (20.8%) | 0.93 |
| Other / unknown, n (%) | 77 (29.4%) | 25 (18.9%) | 52 (40.0%) | <0.001 |
| Comorbidities | ||||
| Chronic kidney disease, n (%) | 37 (14.1%) | 14 (10.6%) | 23 (17.7%) | 0.10 |
| Chronic obstructive pulmonary disease, n (%) | 19 (7.3%) | 7 (5.3%) | 12 (9.2%) | 0.22 |
| Congestive heart failure, n (%) | 38 (14.5%) | 16 (12.1%) | 22 (16.9%) | 0.27 |
| Coronary artery disease, n (%) | 180 (68.7%) | 101 (76.5%) | 79 (60.8%) | <0.01 |
| Cardiovascular risk factors | ||||
| Positive smoking history, n (%) | 132 (61.1%) | 78 (66.7%) | 54 (54.5%) | 0.07 |
| Hypertension, n (%) | 139 (53.1%) | 70 (53.0%) | 69 (53.1%) | 0.99 |
| Positive family history, n (%) | 70 (35.2%) | 40 (37.4%) | 30 (32.6%) | 0.48 |
| Hyperlipidemia, n (%) | 94 (42.5%) | 51 (44.7%) | 43 (40.2%) | 0.49 |
| Diabetes mellitus, n (%) | 66 (25.2%) | 23 (17.4%) | 43 (33.1%) | <0.01 |
| Resuscitation Information | ||||
| Time to ROSC (min), median (IQR) | 20.5 (12, 32) | 15 (10, 25) | 29 (19, 40) | <0.001 |
| Bystander CPR, n (%) | 170 (64.9%) | 99 (74.4%) | 71 (55.0%) | <0.01 |
| Initial shockable rhythm, n (%) | 159 (63.3%) | 104 (81.9%) | 55 (44.4%) | <0.001 |
| Use of epinephrine during CPR, n (%) | 159 (63.3%) | 61 (47.7%) | 98 (79.7%) | <0.001 |
| Initial ICU treatment and status | ||||
| Targeted temperature management (TTM), n (%) | 165 (63.0%) | 78 (59.1%) | 87 (66.9%) | 0.19 |
| Intubation, n (%) | 225 (85.9%) | 99 (75.0%) | 126 (96.9%) | <0.001 |
| Vasoactive drugs, n (%) | 186 (71.0%) | 83 (62.9%) | 103 (79.2%) | <0.01 |
| Mean arterial pressure (mmHg), median (IQR) | 82 (70, 93) | 84 (71.5, 93) | 80 (70, 93) | 0.19 |
| Systolic blood pressure (mmHg), median (IQR) | 116 (101, 130) | 117 (101, 128.5) | 116 (101, 130) | 0.82 |
| Diastolic blood pressure (mmHg), median (IQR) | 67 (55, 77) | 70 (58, 79) | 64.5 (51, 77) | 0.04 |
| Heartrate (bpm), median (IQR) | 85 (74, 99) | 81 (69.5, 94.5) | 88.5 (76, 102) | 0.02 |
| Respiratory rate, median (IQR) | 17 (14, 20) | 17 (14, 20) | 16 (14, 20) | 0.90 |
| Initial laboratory parameters | ||||
| pH, median (IQR) | 7.27 (7.2, 7.3) | 7.28 (7.2, 7.3) | 7.24 (7.1, 7.3) | 0.01 |
| Lactate (mmol/l), median (IQR) | 6.2 (3.6, 9) | 4.8 (2.8, 6.9) | 7.6 (5.2, 10) | <0.001 |
| Creatinine (µmol/l), median (IQR) | 99 (78, 122) | 92.5 (78, 108) | 109 (76, 144) | <0.01 |
| PCT (ng/mL), median (IQR) | 0.52 (0.15, 2.14) | 0.19 (0.1, 0.84) | 1.26 (0.4, 3.91) | <0.001 |
| WBC (g/l), median (IQR) | 14 (10.3, 18.9) | 13 (9.64, 16.2) | 15.4 (11, 20.3) | <0.01 |
| CRP (mg/dl), median (IQR) | 5.9 (1.5, 20.7) | 3 (1.25, 10.8) | 12.2 (2.6, 33.3) | <0.001 |
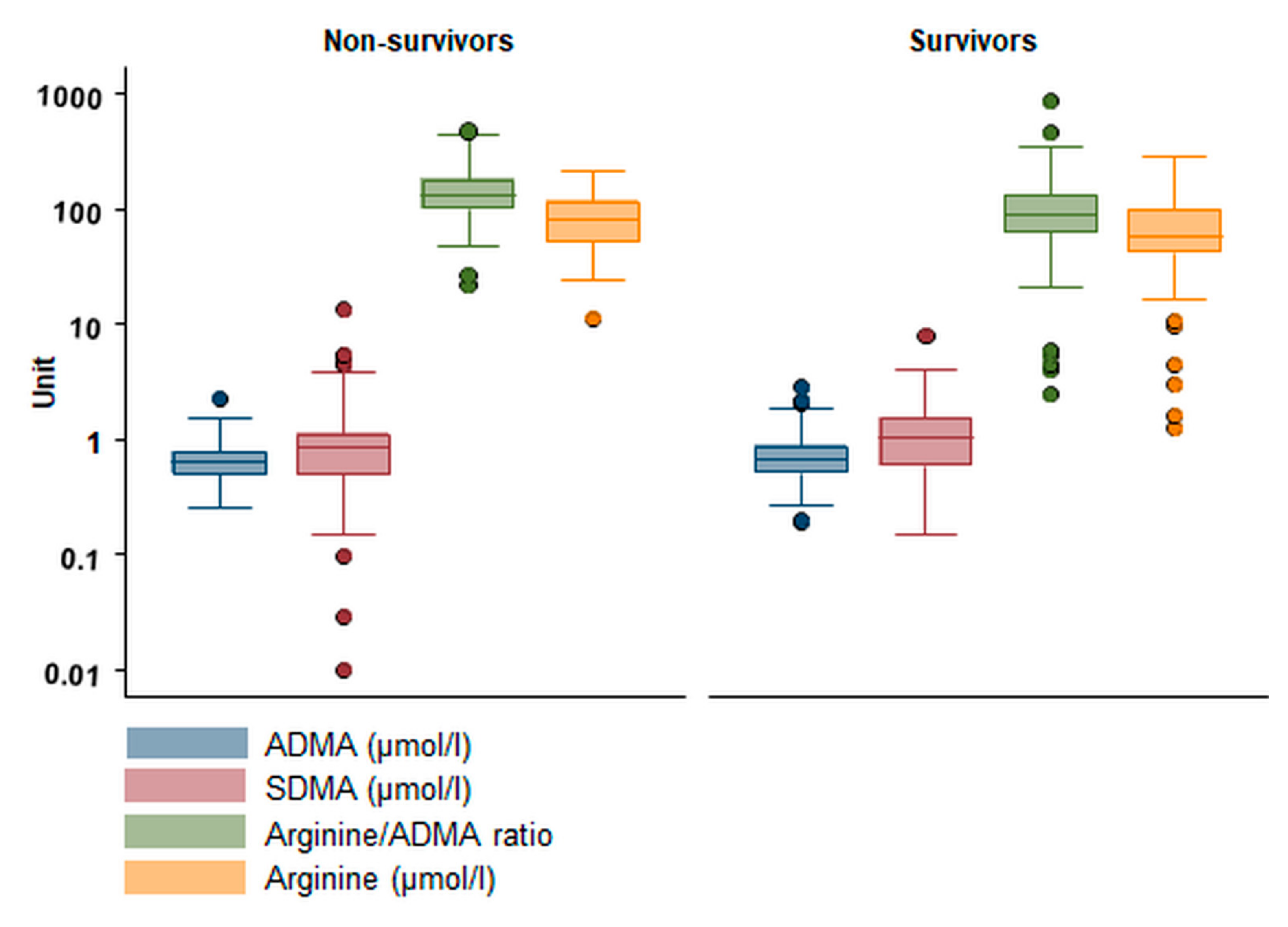
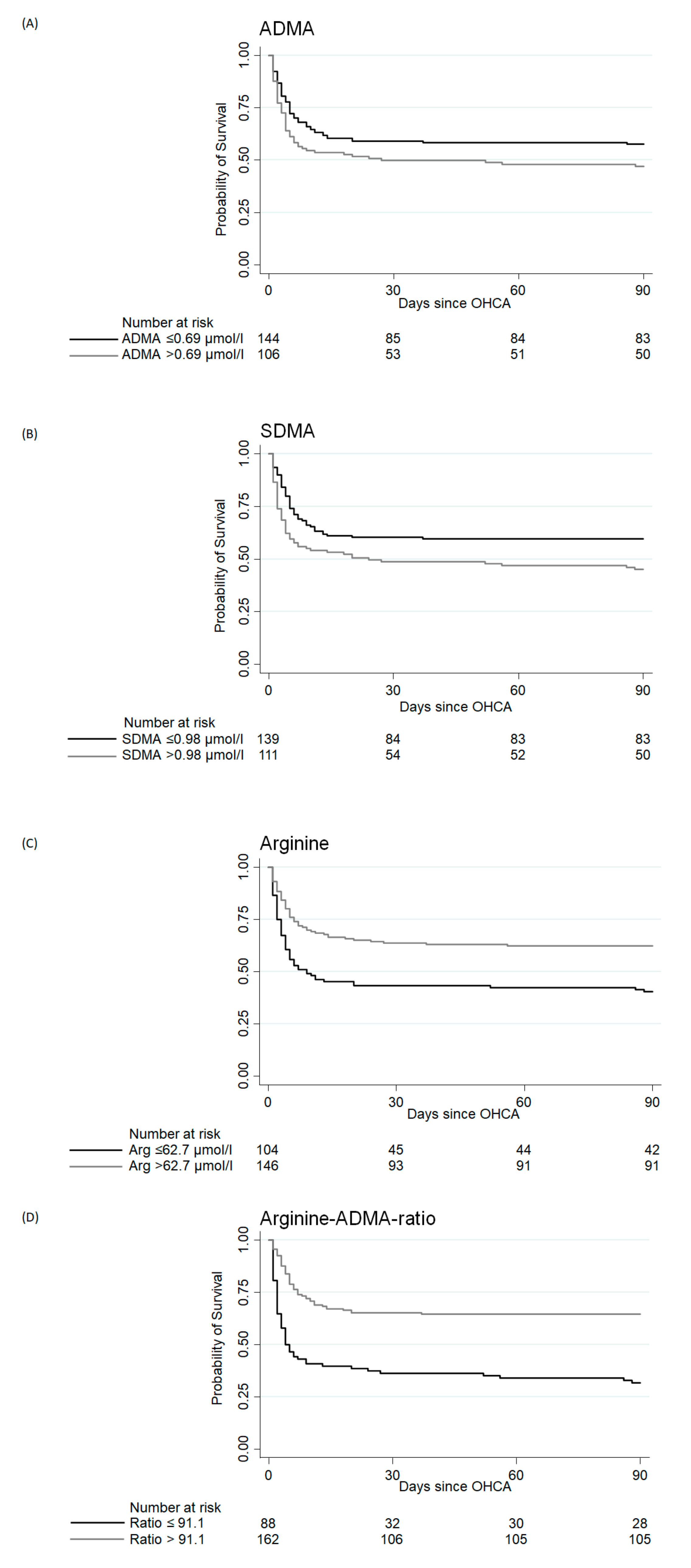
| Primary Endpoint: 90-Day Mortality | ||||||
|---|---|---|---|---|---|---|
| Survivors (n = 133) | Non-Survivors (n = 130) | p-Value | OR or Coefficient (95% CI) | p-Value | AUC | |
| ADMA (µmol/L) | 0.63 (0.50, 0.79) | 0.67 (0.51, 0.88) | 0.09 | 2.35 (1.08, 5.11) | 0.03 | 0.56 |
| ADMA log | 1.75 (0.98, 3.11) | 0.06 | ||||
| ADMA per decile | 1.07 (0.99, 1.17) | 0.10 | ||||
| SDMA (µmol/L) | 0.85 (0.50, 1.13) | 1.02 (0.58, 1.56) | 0.03 | 1.06 (0.87, 1.29) | 0.59 | 0.58 |
| SDMA log | 1.34 (0.99, 1.82) | 0.06 | ||||
| SDMA per decile | 1.10 (1.01, 1.20) | 0.03 | ||||
| Arginine (µmol/L) | 82 (52.3, 119) | 58.1 (41.7, 99.8) | <0.01 | 0.57 (0.34, 0.96) | 0.03 | 0.61 |
| Arginine log | 0.51 (0.34, 0.76) | <0.01 | ||||
| Arginine per decile | 0.87 (0.80, 0.95) | <0.01 | ||||
| Ratio | 134 (99, 183) | 91 (64, 134) | <0.001 | 0.64 (0.47, 0.88) | <0.01 | 0.67 |
| Ratio log | 0.40 (0.26, 0.61) | <0.001 | ||||
| Ratio per decile | 0.80 (0.73, 0.88) | <0.001 | ||||
| Secondary endpoint: in-hospital mortality | ||||||
| Survivors (n = 145) | Non-Survivors (n = 118) | p-Value | OR or Coefficient (95% CI) | p-Value | AUC | |
| ADMA (µmol/L) | 0.63 (0.50, 0.79) | 0.67 (0.50, 0.88) | 0.14 | 2.13 (1.01, 4.50) | 0.047 | 0.55 |
| ADMA log | 1.64 (0.92, 2.91) | 0.09 | ||||
| ADMA per decile | 1.06 (0.98, 1.16) | 0.16 | ||||
| SDMA (µmol/L) | 0.89 (0.51, 1.15) | 0.97 (0.55, 1.56) | 0.18 | 1.03 (0.85, 1.25) | 0.76 | 0.55 |
| SDMA log | 1.20 (0.89, 1.63) | 0.23 | ||||
| SDMA per decile | 1.06 (0.98, 1.16) | 0.15 | ||||
| Arginine (µmol/L) | 77.5 (51.4, 117) | 58.2 (41.8, 110) | 0.02 | 0.71 (0.43, 1.18) | 0.19 | 0.59 |
| Arginine log | 0.60 (0.42, 0.87) | <0.01 | ||||
| Arginine per decile | 0.90 (0.83, 0.98) | 0.02 | ||||
| Ratio | 130 (95, 181) | 92 (65, 138) | <0.001 | 0.75 (0.55, 1.01) | 0.06 | 0.63 |
| Ratio log | 0.50 (0.34, 0.74) | <0.001 | ||||
| Ratio per decile | 0.85 (0.78, 0.93) | <0.001 | ||||
| Secondary endpoint: Neurological outcome (CPC) at hospital discharge | ||||||
| Good Neurological Outcome (n = 122) | Poor Neurological Outcome (n = 141) | p-Value | OR or Coefficient (95%CI) | p-Value | AUC | |
| ADMA (µmol/L) | 0.65 (0.51, 0.80) | 0.65 (0.49, 0.83) | 0.53 | 1.73 (0.82, 3.64) | 0.15 | 0.52 |
| ADMA log | 1.33 (0.75, 2.33) | 0.33 | ||||
| ADMA per decile | 1.03 (0.94, 1.12) | 0.52 | ||||
| SDMA (µmol/L) | 0.91 (0.52, 1.18) | 0.91 (0.54, 1.46) | 0.54 | 0.99 (0.82, 1.21) | 0.96 | 0.52 |
| SDMA log | 1.02 (0.76, 1.37) | 0.89 | ||||
| SDMA per decile | 1.03 (0.95, 1.12) | 0.45 | ||||
| Arginine (µmol/L) | 83.2 (52.5, 119) | 58.6 (41.8, 108) | <0.01 | 0.57 (0.34, 0.96) | 0.03 | 0.61 |
| Arginine log | 0.51 (0.34, 0.77) | <0.01 | ||||
| Arginine per decile | 0.87 (0.80, 0.95) | <0.01 | ||||
| Ratio | 131 (98, 184) | 100 (64, 139) | <0.001 | 0.73 (0.54, 0.97) | 0.03 | 0.65 |
| Ratio log | 0.47 (0.31, 0.70) | <0.001 | ||||
| Ratio per decile | 0.83 (0.76, 0.91) | <0.001 | ||||
| Primary Endpoint: 90-Day Mortality | ||||
|---|---|---|---|---|
| (A) Adjusted for Age, Sex and Comorbidities | (B) Adjusted for Age, Sex and Resuscitation Circumstances | |||
| OR or Coefficient (95% CI) | p-Value | OR or Coefficient (95% CI) | p-Value | |
| ADMA | 2.44 (1.09, 5.45) | 0.03 | 3.58 (1.25, 10.24) | 0.017 |
| ADMA log | 1.72 (0.93, 3.21) | 0.09 | 2.36 (1.10, 5.08) | 0.028 |
| ADMA per decile | 1.07 (0.97, 1.17) | 0.17 | 1.11 (0.99, 1.24) | 0.07 |
| SDMA | 0.99 (0.80, 1.22) | 0.90 | 1.01 (0.80, 1.28) | 0.912 |
| SDMA log | 1.17 (0.84, 1.65) | 0.36 | 1.47 (1.00, 2.15) | 0.048 |
| SDMA per decile | 1.07 (0.97, 1.17) | 0.18 | 1.14 (1.02, 1.28) | 0.018 |
| Arginine | 0.54 (0.31, 0.94) | 0.03 | 0.45 (0.24, 0.85) | 0.014 |
| Arginine log | 0.47 (0.31, 0.73) | <0.01 | 0.45 (0.27, 0.76) | <0.01 |
| Arginine per decile | 0.85 (0.77, 0.94) | <0.01 | 0.88 (0.80, 0.98) | <0.01 |
| Ratio | 0.64 (0.46, 0.88) | <0.01 | 0.55 (0.39, 0.78) | 0.001 |
| Ratio log | 0.40 (0.25, 0.62) | <0.001 | 0.32 (0.18, 0.54) | <0.001 |
| Ratio per decile | 0.80 (0.72, 0.88) | <0.001 | 0.77 (0.69, 0.87) | <0.001 |
| Secondary endpoint: In-hospital mortality | ||||
| (A) Adjusted for age, sex and comorbidities | (B) Adjusted for age, sex and resuscitation circumstances | |||
| OR or Coefficient (95% CI) | p-Value | OR or Coefficient (95% CI) | p-Value | |
| ADMA | 1.98 (0.90, 4.36) | 0.09 | 3.00 (1.06, 8.52) | 0.039 |
| ADMA log | 1.46 (0.78, 2.72) | 0.23 | 2.17 (1.00, 4.69) | 0.049 |
| ADMA per decile | 1.04 (0.94, 1.14) | 0.45 | 1.09 (0.98, 1.22) | 0.113 |
| SDMA | 1.00 (0.81, 1.24) | 0.97 | 0.97 (0.78, 1.22) | 0.81 |
| SDMA log | 1.10 (0.78, 1.54) | 0.59 | 1.26 (0.87, 1.83) | 0.224 |
| SDMA per decile | 1.04 (0.94, 1.14) | 0.44 | 1.09 (0.97, 1.21) | 0.136 |
| Arginine | 0.63 (0.36, 1.09) | 0.10 | 0.62 (0.33, 1.17) | 0.142 |
| Arginine log | 0.55 (0.36, 0.83) | <0.01 | 0.60 (0.37, 0.96) | 0.034 |
| Arginine per decile | 0.88 (0.80, 0.96) | <0.01 | 0.90 (0.81, 1.00) | 0.057 |
| Ratio | 0.72 (0.53, 0.98) | 0.03 | 0.67 (0.48, 0.92) | 0.013 |
| Ratio log | 0.50 (0.33, 0.75) | <0.001 | 0.46 (0.28, 0.76) | 0.002 |
| Ratio per decile | 0.85 (0.77, 0.93) | <0.001 | 0.83 (0.75, 0.93) | 0.001 |
| Secondary endpoint: Neurological Outcome (CPC) at hospital discharge | ||||
| (A) Adjusted for age, sex and comorbidities | (B) Adjusted for age, sex and resuscitation circumstances | |||
| OR or Coefficient (95% CI) | p-Value | OR or Coefficient (95% CI) | p-Value | |
| ADMA | 1.64 (0.76, 3.55) | 0.21 | 2.42 (0.82, 7.16) | 0.109 |
| ADMA log | 1.18 (0.64, 2.18) | 0.59 | 1.61 (0.72, 3.57) | 0.246 |
| ADMA per decile | 1.01 (0.92, 1.10) | 0.91 | 1.04 (0.93, 1.17) | 0.491 |
| SDMA | 0.91 (0.74, 1.13) | 0.41 | 0.93 (0.74, 1.17) | 0.524 |
| SDMA log | 0.84 (0.60, 1.16) | 0.29 | 1.01 (0.68, 1.50) | 0.953 |
| SDMA per decile | 0.99 (0.90, 1.08) | 0.77 | 1.05 (0.93, 1.17) | 0.451 |
| Arginine | 0.53 (0.31, 0.93) | 0.03 | 0.43 (0.21, 0.86) | 0.018 |
| Arginine log | 0.48 (0.31, 0.75) | <0.01 | 0.44 (0.25, 0.77) | 0.004 |
| Arginine per decile | 0.85 (0.77, 0.94) | <0.01 | 0.85 (0.75, 0.96) | 0.007 |
| Ratio | 0.72 (0.54, 0.97) | 0.03 | 0.58 (0.41, 0.81) | 0.001 |
| Ratio log | 0.47 (0.31, 0.72) | <0.01 | 0.36 (0.20, 0.63) | <0.001 |
| Ratio per decile | 0.83 (0.76, 0.92) | <0.001 | 0.81 (0.71, 0.91) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, A.; Becker, C.; Nienhaus, K.; Beck, K.; Vincent, A.; Sutter, R.; Tisljar, K.; Schuetz, P.; Bernasconi, L.; Neyer, P.; et al. Arginine and Arginine/ADMA Ratio Predict 90-Day Mortality in Patients with Out-of-Hospital Cardiac Arrest—Results from the Prospective, Observational COMMUNICATE Trial. J. Clin. Med. 2020, 9, 3815. https://doi.org/10.3390/jcm9123815
Keller A, Becker C, Nienhaus K, Beck K, Vincent A, Sutter R, Tisljar K, Schuetz P, Bernasconi L, Neyer P, et al. Arginine and Arginine/ADMA Ratio Predict 90-Day Mortality in Patients with Out-of-Hospital Cardiac Arrest—Results from the Prospective, Observational COMMUNICATE Trial. Journal of Clinical Medicine. 2020; 9(12):3815. https://doi.org/10.3390/jcm9123815
Chicago/Turabian StyleKeller, Annalena, Christoph Becker, Katharina Nienhaus, Katharina Beck, Alessia Vincent, Raoul Sutter, Kai Tisljar, Philipp Schuetz, Luca Bernasconi, Peter Neyer, and et al. 2020. "Arginine and Arginine/ADMA Ratio Predict 90-Day Mortality in Patients with Out-of-Hospital Cardiac Arrest—Results from the Prospective, Observational COMMUNICATE Trial" Journal of Clinical Medicine 9, no. 12: 3815. https://doi.org/10.3390/jcm9123815
APA StyleKeller, A., Becker, C., Nienhaus, K., Beck, K., Vincent, A., Sutter, R., Tisljar, K., Schuetz, P., Bernasconi, L., Neyer, P., Pargger, H., Marsch, S., & Hunziker, S. (2020). Arginine and Arginine/ADMA Ratio Predict 90-Day Mortality in Patients with Out-of-Hospital Cardiac Arrest—Results from the Prospective, Observational COMMUNICATE Trial. Journal of Clinical Medicine, 9(12), 3815. https://doi.org/10.3390/jcm9123815





