Procalcitonin to Reduce Antibiotic Exposure during Acute Chest Syndrome in Adult Patients with Sickle-Cell Disease
Abstract
1. Introduction
2. Experiment Section
2.1. Study Design
2.2. Antibiotic Management
2.3. Documentation of Lung Infection
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. ACS Episodes, Lung Infections, and PCT Dosage
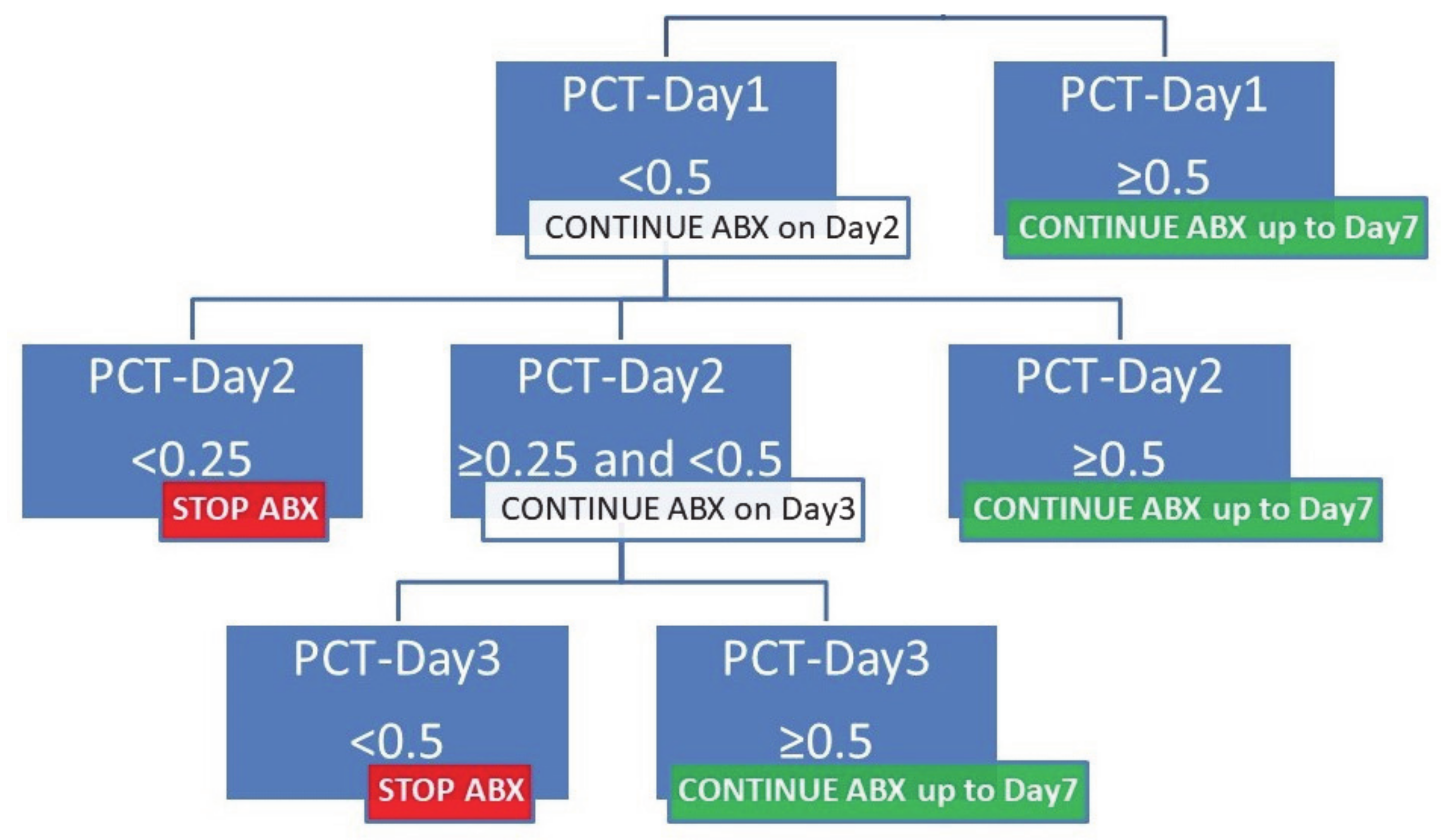
3.2. Procalcitonin Algorithm for Acute Chest Syndrome
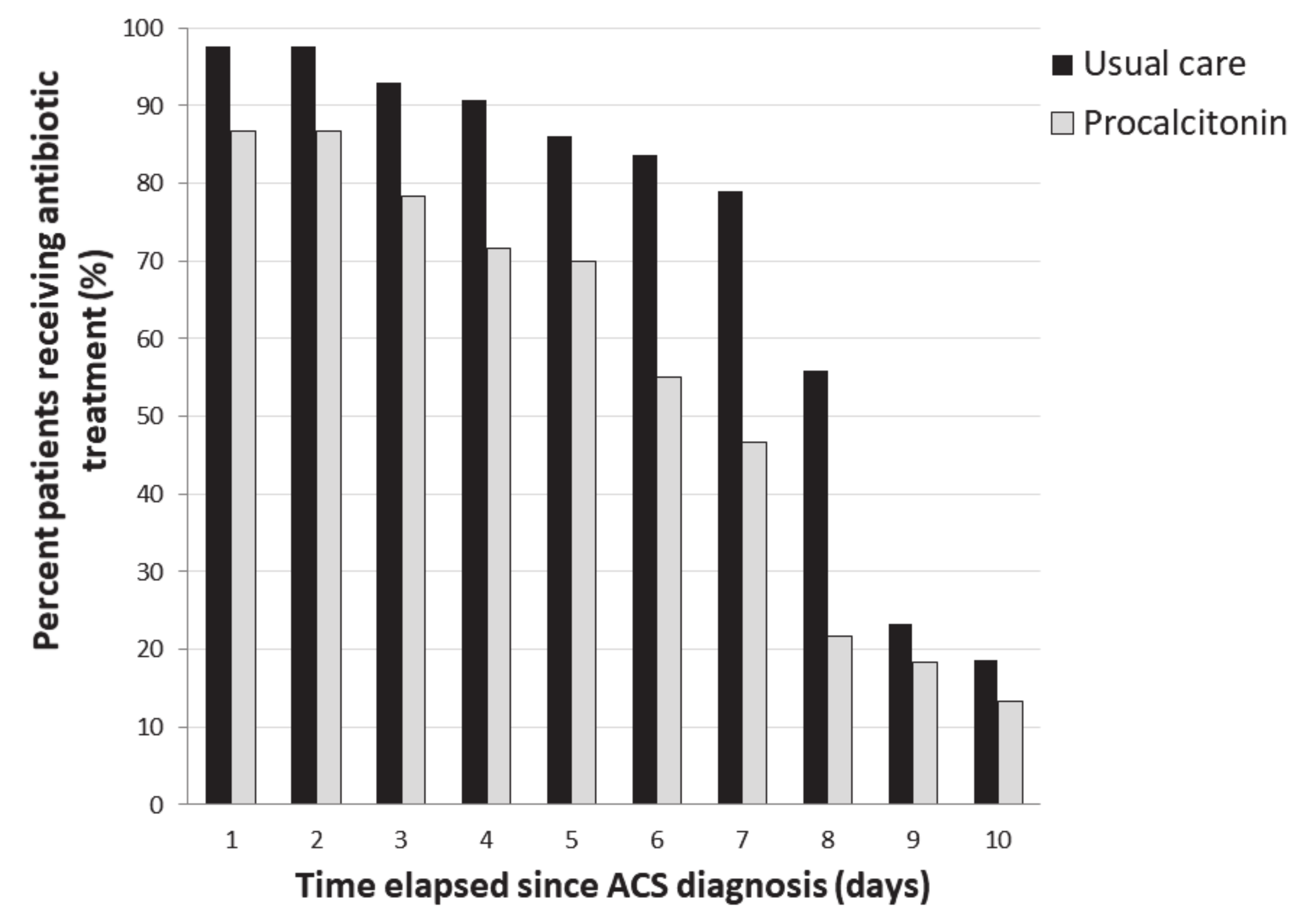
3.3. Compliance with Algorithm and Antibiotic Use
3.4. Complications
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vichinsky, E.P.; Neumayr, L.D.; Earles, A.N.; Williams, R.; Lennette, E.T.; Dean, D.; Nickerson, B.; Orringer, E.; McKie, V.; Bellevue, R.; et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N. Engl. J. Med. 2000, 342, 1855–1865. [Google Scholar] [CrossRef]
- Maitre, B.; Habibi, A.; Roudot-Thoraval, F.; Bachir, D.; Belghiti, D.D.; Galacteros, F.; Godeau, B. Acute chest syndrome in adults with sickle cell disease. Chest 2000, 117, 1386–1392. [Google Scholar] [CrossRef]
- Yawn, B.P.; Buchanan, G.R.; Afenyi-Annan, A.N.; Ballas, S.K.; Hassell, K.L.; James, A.H.; Jordan, L.; Lanzkron, S.M.; Lottenberg, R.; Savage, W.J.; et al. Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA 2014, 312, 1033–1048. [Google Scholar] [CrossRef]
- Howard, J.; Hart, N.; Roberts-Harewood, M.; Cummins, M.; Awogbade, M.; Davis, B.; BCSH Committee. Guideline on the management of acute chest syndrome in sickle cell disease. Br. J. Haematol. 2015, 169, 492–505. [Google Scholar] [CrossRef]
- Habibi, A.; Arlet, J.-B.; Stankovic, K.; Gellen-Dautremer, J.; Ribeil, J.-A.; Bartolucci, P.; Lionnet, F. Centre de référence maladies rares «syndromes drépanocytaires majeurs» [French guidelines for the management of adult sickle cell disease: 2015 update]. Rev Med. Interne 2015, 36, 5S3–5S84. [Google Scholar] [CrossRef]
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- De Jong, E.; van Oers, J.A.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; Haas, L.E.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: A randomised, controlled, open-label trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Stolz, D.; Bingisser, R.; Müller, C.; Miedinger, D.; Huber, P.R.; Zimmerli, W.; Harbarth, S.; Tamm, M.; Müller, B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: A randomized trial. Am. J. Respir. Crit. Care Med. 2006, 174, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Ghafuri, D.L.; Glassberg, J.; Kassim, A.A.; Rodeghier, M.; DeBaun, M.R. Rapidly progressive acute chest syndrome in individuals with sickle cell anemia: A distinct acute chest syndrome phenotype. Am. J. Hematol. 2016, 91, 1185–1190. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Breiman, R.F.; Mandell, L.A.; File, T.M. Community-Acquired Pneumonia in Adults: Guidelines for Management. Clin. Infect. Dis. 1998, 26, 811–838. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Patel, D.K.; Mohapatra, M.K.; Thomas, A.G.; Patel, S.; Purohit, P. Procalcitonin as a biomarker of bacterial infection in sickle cell vaso-occlusive crisis. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014018. [Google Scholar] [CrossRef] [PubMed]
- Stankovic Stojanovic, K.; Steichen, O.; Lionnet, F.; Bachmeyer, C.; Lecomte, I.; Avellino, V.; Grateau, G.; Girot, R.; Lefevre, G. Is procalcitonin a marker of invasive bacterial infection in acute sickle-cell vaso-occlusive crisis? Infection 2011, 39, 41–45. [Google Scholar] [CrossRef]
- Scott, L.K.; Grier, L.R.; Arnold, T.C.; Conrad, S.A. Serum procalcitonin concentration as a negative predictor of serious bacterial infection in acute sickle cell pain crisis. Med. Sci. Monit. 2003, 9, CR426–CR431. [Google Scholar]
- Rincón-López, E.M.; Navarro Gómez, M.L.; Hernández-Sampelayo Matos, T.; Saavedra-Lozano, J.; Aguilar de la Red, Y.; Hernández Rupérez, B.; Cela de Julián, E.; RETRO-DREP Study Group. Low-risk factors for severe bacterial infection and acute chest syndrome in children with sickle cell disease. Pediatr. Blood Cancer 2019, 66, e27667. [Google Scholar] [CrossRef]
- Schuetz, P.; Christ-Crain, M.; Thomann, R.; Falconnier, C.; Wolbers, M.; Widmer, I.; Neidert, S.; Fricker, T.; Blum, C.; Schild, U.; et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA 2009, 302, 1059–1066. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Jaccard-Stolz, D.; Bingisser, R.; Gencay, M.M.; Huber, P.R.; Tamm, M.; Müller, B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet 2004, 363, 600–607. [Google Scholar] [CrossRef]
- Stolz, D.; Christ-Crain, M.; Bingisser, R.; Leuppi, J.; Miedinger, D.; Müller, C.; Huber, P.; Müller, B.; Tamm, M. Antibiotic treatment of exacerbations of COPD: A randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007, 131, 9–19. [Google Scholar] [CrossRef]
- Briel, M.; Schuetz, P.; Mueller, B.; Young, J.; Schild, U.; Nusbaumer, C.; Périat, P.; Bucher, H.C.; Christ-Crain, M. Procalcitonin-guided antibiotic use vs. a standard approach for acute respiratory tract infections in primary care. Arch. Intern. Med. 2008, 168, 2000–2007. [Google Scholar] [CrossRef]
- Ugajin, M.; Yamaki, K.; Hirasawa, N.; Yagi, T. Predictive values of semi-quantitative procalcitonin test and common biomarkers for the clinical outcomes of community-acquired pneumonia. Respir. Care 2014, 59, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.; Bigham, M.T.; Giuliano, J.S. Use of procalcitonin for the prediction and treatment of acute bacterial infection in children. Curr. Opin. Pediatr. 2014, 26, 292–298. [Google Scholar] [CrossRef]
- Dayie, N.T.K.D.; Tetteh-Ocloo, G.; Labi, A.-K.; Olayemi, E.; Slotved, H.-C.; Lartey, M.; Donkor, E.S. Pneumococcal carriage among sickle cell disease patients in Accra, Ghana: Risk factors, serotypes and antibiotic resistance. PLoS ONE 2018, 13, e0206728. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, J.; Hulin, A.; Habibi, A.; Mekontso Dessap, A.; Razazi, K. Profound underdosing of β-lactams in patients with sickle-cell disease. J. Antimicrob. Chemother. 2018. [Google Scholar] [CrossRef] [PubMed]
| Pathogen | Proven Infections | Possible Infections | Procalcitonin Levels, µg/L |
|---|---|---|---|
| Median, SD, (Range) | |||
| Staphylococcus aureus | 2 | 4 * | Day 1: 0.73, 23.64, (0.1–59) |
| Day 2: 2.38, 7.79, (0.1–16.7) | |||
| Day 3: 0.18, 8.29, (0.1–14.5) | |||
| Streptococcus pneumoniae | 1 | Day 1: 0.2, Day 2: 0.2, Day 3: 0.2 | |
| Enterobacter aerogenes | 1 | Day 1: 0.21, Day 2: 1.07, Day 3: 75.9 | |
| Mycoplasma pneumoniae | 1 | 2 # | Day 1: 0.1, Day 2: 8.88, Day 3: 3.39, 4.5, (0.2–6.57) |
| Legionella pneumophila | 1 | Day 1: 0.2; Day 3: 0.1 | |
| Influenza virus | 1 | Day 3: <0.1 |
| Parameter | All Episodes | Study Period | p-Value | |
|---|---|---|---|---|
| Control | Intervention | |||
| (n = 103) | (n = 43) | (n = 60) | ||
| ACS-associated treatments | ||||
| Exchange transfusion | 28 (27) | 19 (44) | 9 (15) | 0.001 |
| Exchange transfusion before Day 3 | 25 (24) | 18 (42) | 7 (12) | <0.001 |
| Red-blood-cell transfusion | 26 (25) | 15 (35) | 11 (18) | 0.06 |
| Red-blood-cell transfusion before Day 3 | 24 (23) | 13 (30) | 11 (18) | 0.2 |
| Phlebotomy | 7 (7) | 3 (7) | 4 (7) | >0.99 |
| Exchange and single transfusion | 47 (46) | 29 (67) | 18 (30) | <0.001 |
| Exchange and single transfusion < Day 3 | 45 (44) | 29 (67) | 16 (27) | <0.001 |
| Mechanical ventilation | 3 (3) | 2 (5) | 1 (2) | 0.6 |
| Antibiotics duration, days | ||||
| Total antibiotics duration | 7 (5–8) | 8 (7,8) | 6 (3–7) | <0.001 |
| Beta-lactam duration | 6 (2–7) | 7 (2–8) | 5 (2–7) | 0.05 |
| Macrolide duration | 0 (0–6) | 5 (0–7) | 0 (0–3) | 0.001 |
| Antibiotics-free day at Day 21, days | ||||
| Total antibiotics-free days | 14 (13–16) | 13 (13–14) | 15 (14–18) | 0.001 |
| Beta-lactam-free days | 15 (13–19) | 14 (13–19) | 16 (14–19) | 0.05 |
| Macrolide-free days | 21 (15–21) | 16 (14–21) | 21 (18–21) | 0.001 |
| Three days or fewer of antibiotics | 22 (22) | 4 (9) | 18 (30) | 0.01 |
| Microbiological diagnostic criteria | 13 (13) | 4 (9) | 9 (15) | 0.39 |
| Proved infection | 7 (7) | 1 (2) | 6 (10) | 0.23 |
| Partial microbiological diagnostic criteria | 6 (6) | 3 (3) | 3 (3) | 0.27 |
| Antibiotics reintroduction | 5 (5) | 0 (0) | 5 (8) | 0.07 |
| Infection relapse | 0 (0) | 0 (0) | 0 (0) | |
| Pulmonary reinfection | 0 (0) | 0 (0) | 0 (0) | |
| Extrapulmonary infection | 2 (2) | 1 (2) | 1 (2) | >0.99 |
| Clostridium infection | 0 (0) | 0 (0) | 0 (0) | |
| ICU admission | 47 (46) | 25 (58) | 22 (37) | 0.03 |
| Length of hospitalization, days | 7.0 (6.0–12.0) | 7 (5.0–11.0) | 8.0 (6.0–12.0) | 0.3 |
| Rehospitalization | 5 (5) | 1 (2) | 4 (7) | 0.3 |
| Inhospital death | 1 (1) | 0 (0) | 1 (2) | >0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razazi, K.; Gendreau, S.; Cuquemelle, E.; Khellaf, M.; Guillaud, C.; Godeau, B.; Melica, G.; Moutereau, S.; Gomart, C.; Fourati, S.; et al. Procalcitonin to Reduce Antibiotic Exposure during Acute Chest Syndrome in Adult Patients with Sickle-Cell Disease. J. Clin. Med. 2020, 9, 3718. https://doi.org/10.3390/jcm9113718
Razazi K, Gendreau S, Cuquemelle E, Khellaf M, Guillaud C, Godeau B, Melica G, Moutereau S, Gomart C, Fourati S, et al. Procalcitonin to Reduce Antibiotic Exposure during Acute Chest Syndrome in Adult Patients with Sickle-Cell Disease. Journal of Clinical Medicine. 2020; 9(11):3718. https://doi.org/10.3390/jcm9113718
Chicago/Turabian StyleRazazi, Keyvan, Ségolène Gendreau, Elise Cuquemelle, Mehdi Khellaf, Constance Guillaud, Bertrand Godeau, Giovanna Melica, Stéphane Moutereau, Camille Gomart, Slim Fourati, and et al. 2020. "Procalcitonin to Reduce Antibiotic Exposure during Acute Chest Syndrome in Adult Patients with Sickle-Cell Disease" Journal of Clinical Medicine 9, no. 11: 3718. https://doi.org/10.3390/jcm9113718
APA StyleRazazi, K., Gendreau, S., Cuquemelle, E., Khellaf, M., Guillaud, C., Godeau, B., Melica, G., Moutereau, S., Gomart, C., Fourati, S., De Prost, N., Carteaux, G., Brun-Buisson, C., Bartolucci, P., Habibi, A., & Mekontso Dessap, A. (2020). Procalcitonin to Reduce Antibiotic Exposure during Acute Chest Syndrome in Adult Patients with Sickle-Cell Disease. Journal of Clinical Medicine, 9(11), 3718. https://doi.org/10.3390/jcm9113718





