Bone Mineral Density, Osteoporosis, and Fracture Risk in Adult Patients with Psoriasis or Psoriatic Arthritis: A Systematic Review and Meta-Analysis of Observational Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Investigation and Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction and Outcome of Interest
2.4. Qualitative Systematic Review
2.5. Data Synthesis and Statistical Analysis
2.6. Small-Study Effects
3. Results
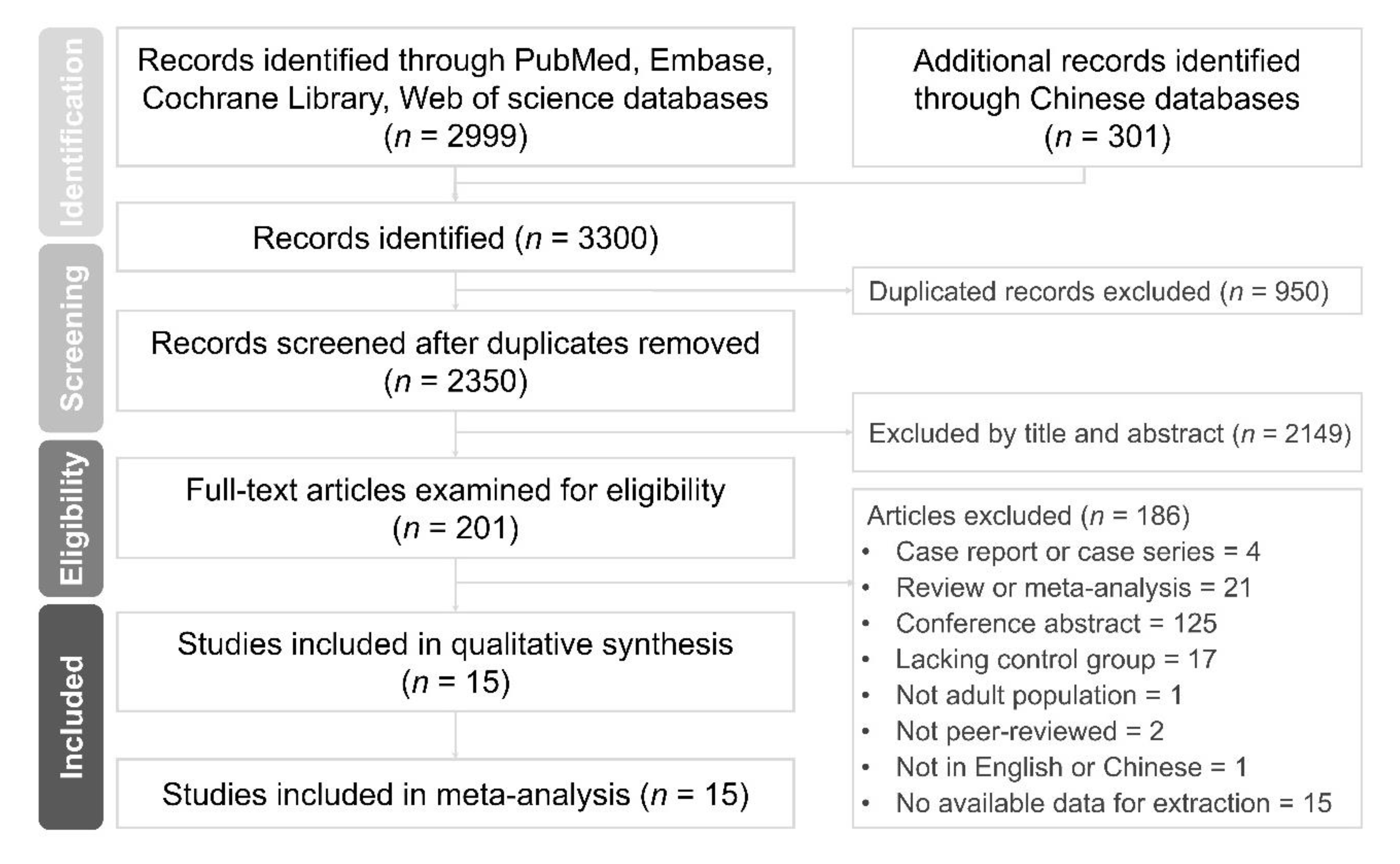
3.1. Search Results
3.2. Characteristics of Qualifying Studies
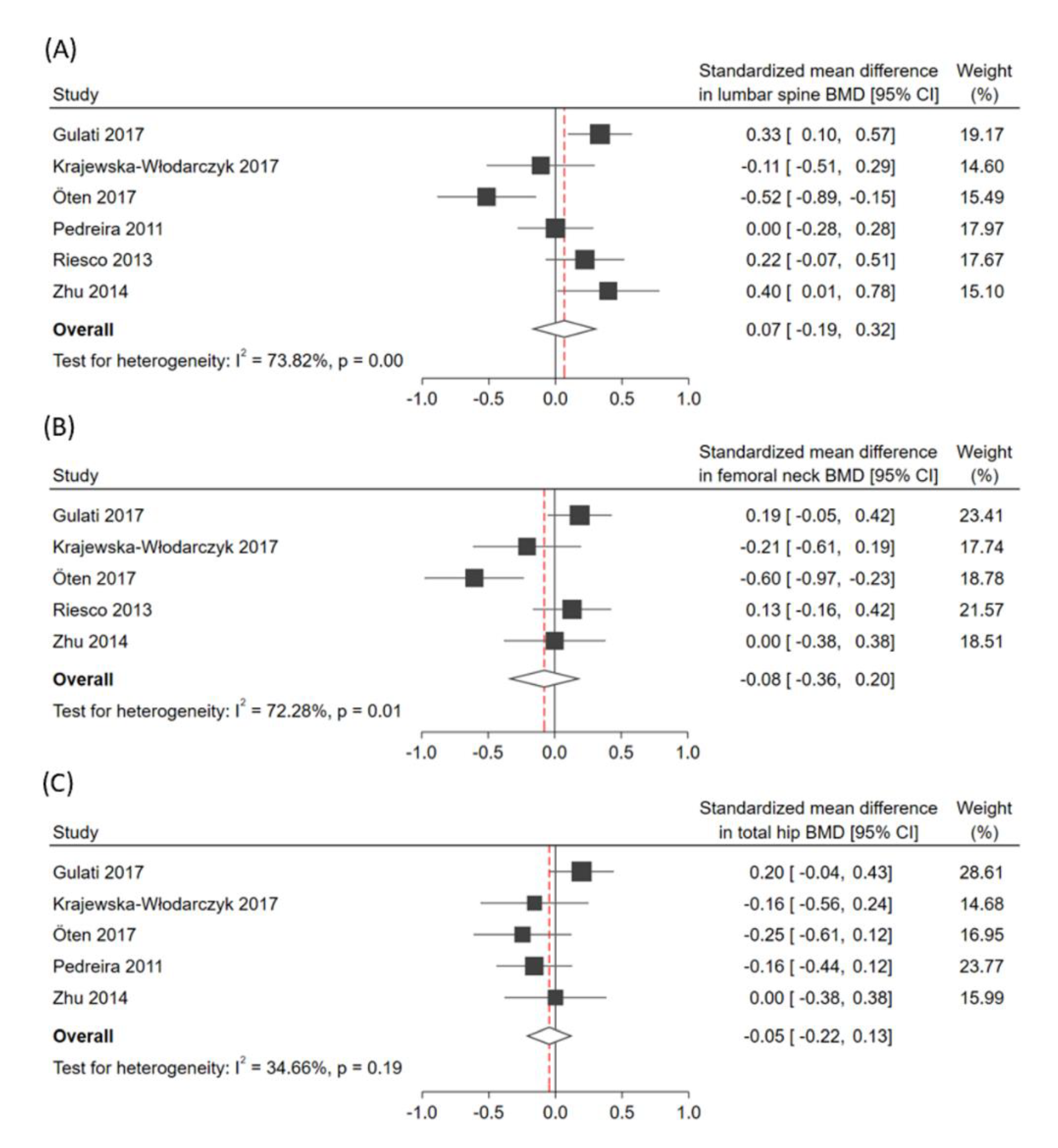
3.3. Pooled Effects of the Primary Outcome
3.4. Pooled Effects of Secondary Outcomes
3.5. Heterogeneity and Small-Study Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Bilal, J.; Malik, S.U.; Riaz, I.B.; Kurtzman, D.J. Psoriasis and Psoriatic Spectrum Disease: A Primer for the Primary Care Physician. Am. J. Med. 2018, 131, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chandran, V.; Tsoi, L.; O’Rielly, D.; Nair, R.P.; Gladman, D.; Elder, J.T.; Rahman, P. Quantifying Differences in Heritability among Psoriatic Arthritis (PsA), Cutaneous Psoriasis (PsC) and Psoriasis vulgaris (PsV). Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Stuart, P.E.; Nair, R.P.; Tsoi, L.C.; Tejasvi, T.; Das, S.; Kang, H.M.; Ellinghaus, E.; Chandran, V.; Callis-Duffin, K.; Ike, R.; et al. Genome-wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. Am. J. Hum. Genet. 2015, 97, 816–836. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Bogdanos, D.P. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun. Rev. 2017, 16, 10–15. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef]
- Okhovat, J.-P.; Ogdie, A.; Reddy, S.; Rosen, C.F.; Scher, J.U.; Merola, J.F. Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network Consortium (PPACMAN) Survey: Benefits and Challenges of Combined Rheumatology-dermatology Clinics. J. Rheumatol. 2017, 44, 693–694. [Google Scholar] [CrossRef]
- Savage, L.; Tinazzi, I.; Zabotti, A.; Laws, P.M.; Wittmann, M.; McGonagle, D. Defining Pre-Clinical Psoriatic Arthritis in an Integrated Dermato-Rheumatology Environment. J. Clin. Med. 2020, 9, 3262. [Google Scholar] [CrossRef]
- Alivernini, S.; Tolusso, B.; Petricca, L.; Bui, L.; Di Sante, G.; Peluso, G.; Benvenuto, R.; Fedele, A.L.; Federico, F.; Ferraccioli, G.; et al. Synovial features of patients with rheumatoid arthritis and psoriatic arthritis in clinical and ultrasound remission differ under anti-TNF therapy: A clue to interpret different chances of relapse after clinical remission? Ann. Rheum. Dis. 2017, 76, 1228–1236. [Google Scholar] [CrossRef]
- Sirufo, M.M.; De Pietro, F.; Bassino, E.M.; Ginaldi, L.; De Martinis, M. Osteoporosis in Skin Diseases. Int. J. Mol. Sci. 2020, 21, 4749. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Ritchlin, C. Altered Bone Remodeling in Psoriatic Disease: New Insights and Future Directions. Calcif. Tissue Int. 2018, 102, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Leonardi, C.L.; Davis, D.M.; Gelfand, J.M.; Lichten, J.; Mehta, N.N.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Elewski, B.E.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol. 2019, 80, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.D.F.S.P.D.; Rocha, B.D.O.; Duarte, G.V. Psoriasis: Classical and emerging comorbidities. An. Bras. Dermatol. 2015, 90, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Aldei, A.; Johnson, S.R.; Cheung, A.M.; Salonen, D.; Gladman, D.D. Prevalence and risk factors of low bone mineral density in psoriatic arthritis: A systematic review. Semin. Arthritis Rheum. 2016, 46, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Dreiher, J.; Weitzman, D.; Cohen, A.D. Psoriasis and Osteoporosis: A Sex-Specific Association? J. Investig. Dermatol. 2009, 129, 1643–1649. [Google Scholar] [CrossRef]
- Lajevardi, V.; Abedini, R.; Moghaddasi, M.; Nassiri, S.; Goodarzi, A. Bone mineral density is lower in male than female patients with plaque-type psoriasis in Iran. Int. J. Women’s Dermatol. 2017, 3, 201–205. [Google Scholar] [CrossRef]
- Ogdie, A.; Harter, L.; Shin, D.; Baker, J.; Takeshita, J.; Choi, H.K.; Love, T.J.; Gelfand, J.M. The risk of fracture among patients with psoriatic arthritis and psoriasis: A population-based study. Ann. Rheum. Dis. 2017, 76, 882–885. [Google Scholar] [CrossRef]
- Paskins, Z.; Whittle, R.; Sultan, A.A.; Müller, S.; Blagojevic-Bucknall, M.; Helliwell, T.; Packham, J.; Hider, S.; Roddy, E.; Mallen, C. Risk of fragility fracture among patients with late-onset psoriasis: A UK population-based study. Osteoporos. Int. 2018, 29, 1–6. [Google Scholar] [CrossRef]
- Harrison, B.J.; Hutchinson, C.E.; Adams, J.; Bruce, I.N.; Herrick, A.L. Assessing periarticular bone mineral density in patients with early psoriatic arthritis or rheumatoid arthritis. Ann. Rheum. Dis. 2002, 61, 1007–1011. [Google Scholar] [CrossRef]
- Busquets, N.; Gómez-Vaquero, C.; Rodríguez-Rodríguez, L.; Vilaseca, D.R.; Narváez, J.; Carmona, L.; Nolla, J.M. Bone mineral density status and frequency of osteoporosis and clinical fractures in 155 patients with psoriatic arthritis followed in a university hospital. Reumatol. Clin. 2014, 10, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.F.L.; Scolnik, M.; Pierini, F.S.; Zucaro, N.M.M.; Gallego, J.F.J.; Soriano, E.R. Fragility fractures in psoriatic arthritis patients: A matched retrospective cohort study. Clin. Rheumatol. 2020, 39, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Modalsli, E.; Åsvold, B.; Romundstad, P.; Langhammer, A.; Hoff, M.; Forsmo, S.; Naldi, L.; Saunes, M. Psoriasis, fracture risk and bone mineral density: The HUNT Study, Norway. Br. J. Dermatol. 2017, 176, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.P.; Shane, E.; Lyritis, G.; Skarantavos, G.; Mendelsohn, R.; Boskey, A.L. Bone Fragility and Collagen Cross-Links. J. Bone Miner. Res. 2004, 19, 2000–2004. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Buckley, L.; Humphrey, M.B. Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 2018, 379, 2547–2556. [Google Scholar] [CrossRef]
- Orsolini, G.; Fassio, A.; Rossini, M.; Adami, G.; Giollo, A.; Caimmi, C.; Idolazzi, L.; Viapiana, O.; Gatti, D. Effects of biological and targeted synthetic DMARDs on bone loss in rheumatoid arthritis. Pharmacol. Res. 2019, 147, 104354. [Google Scholar] [CrossRef]
- Kawai, V.K.; Stein, C.M.; Perrien, D.S.; Griffin, M.R. Effects of anti-tumor necrosis factor α agents on bone. Curr. Opin. Rheumatol. 2012, 24, 576–585. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 11 October 2020).
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 [Updated September 2020]; The Cochrane Collaboration: London, UK, 2020; Available online: www.training.cochrane.org/handbook (accessed on 17 October 2020).
- Dekkers, O.M.; Vandenbroucke, J.P.; Cevallos, M.; Renehan, A.G.; Altman, D.G.; Egger, M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019, 16, e1002742. [Google Scholar] [CrossRef] [PubMed]
- Metelli, S.; Chaimani, A. Challenges in meta-analyses with observational studies. Evid. Based Ment. Health 2020, 23, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Guolo, A.; Varin, C. Random-effects meta-analysis: The number of studies matters. Stat. Methods Med. Res. 2015, 26, 1500–1518. [Google Scholar] [CrossRef] [PubMed]
- Röver, C.; Knapp, G.; Friede, T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med. Res. Methodol. 2015, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Jackson, D.; Bender, R.; Kuss, O.; Langan, D.; Higgins, J.P.; Knapp, G.; Salanti, G. Methods to calculate uncertainty in the estimated overall effect size from a random-effects meta-analysis. Res. Synth. Methods 2019, 10, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid.-Based Heal. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Del Puente, A.; Esposito, A.; Costa, L.; Benigno, C.; Foglia, F.; Oriente, A.; Bottiglieri, P.; Caso, F.; Scarpa, R. Fragility Fractures in Patients with Psoriatic Arthritis. J. Rheumatol. Suppl. 2015, 93, 36–39. [Google Scholar] [CrossRef]
- Gulati, A.M.; Hoff, M.; Salvesen, Ø.; Dhainaut, A.; Semb, A.G.; Kavanaugh, A.; Haugeberg, G. Bone mineral density in patients with psoriatic arthritis: Data from the Nord-Trøndelag Health Study 3. RMD Open 2017, 3, e000413. [Google Scholar] [CrossRef]
- Haddad, A.; Ashkenazi, R.I.; Bitterman, H.; Feldhamer, I.; Greenberg-Dotan, S.; Lavi, I.; Batat, E.; Bergman, I.; Cohen, A.D.; Zisman, D. Endocrine Comorbidities in Patients with Psoriatic Arthritis: A Population-based Case-controlled Study. J. Rheumatol. 2017, 44, 786–790. [Google Scholar] [CrossRef]
- Kaine, J.; Song, X.; Kim, G.; Hur, P.; Palmer, J.B. Higher Incidence Rates of Comorbidities in Patients with Psoriatic Arthritis Compared with the General Population Using U.S. Administrative Claims Data. J. Manag. Care Spec. Pharm. 2019, 25, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W. Changes in body composition and bone mineral density n postmenopausal women with psoriatic arthritis. Reumatologia 2017, 5, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Oten, E.; Baskan, B.; Sivas, F.; Bodur, H. Relation Between Osteoporosis and Vitamin D Levels and Disease Activity in Psoriatic Arthritis. Erciyes Med. J. 2017, 39, 94–100. [Google Scholar] [CrossRef][Green Version]
- Pedreira, P.G.; Pinheiro, M.M.; Szejnfeld, V.L. Bone mineral density and body composition in postmenopausal women with psoriasis and psoriatic arthritis. Arthritis Res. 2011, 13, R16. [Google Scholar] [CrossRef]
- Riesco, M.; Manzano, F.; Font, P.; García, A.; Nolla, J.M. Osteoporosis in psoriatic arthritis: An assessment of densitometry and fragility fractures. Clin. Rheumatol. 2013, 32, 1799–1804. [Google Scholar] [CrossRef]
- Solak, B.; Dikicier, B.S.; Celik, H.D.; Erdem, T. Bone Mineral Density, 25-OH Vitamin D and Inflammation in Patients with Psoriasis. Photodermatol. Photoimmunol. Photomed. 2016, 32, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.Y.; Griffith, J.F.; Qin, L.; Hung, V.W.Y.; Fong, T.-N.; Au, S.-K.; Kwok, A.; Leung, P.-C.; Li, E.K.; Tam, L. Density, structure, and strength of the distal radius in patients with psoriatic arthritis: The role of inflammation and cardiovascular risk factors. Osteoporos. Int. 2014, 26, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Papaioannou, A.; Morin, S.; Cheung, A.M.; Atkinson, S.; Brown, J.P.; Feldman, S.; Hanley, D.A.; Hodsman, A.; Jamal, S.A.; Kaiser, S.M.; et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. Can. Med. Assoc. J. 2010, 182, 1864–1873. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Ott, S.M. Bone strength: More than just bone density. Kidney Int. 2016, 89, 16–19. [Google Scholar] [CrossRef]
- Torres-Del-Pliego, E.; Vilaplana, L.; Güerri-Fernández, R.; Diez-Perez, A. Measuring Bone Quality. Curr. Rheumatol. Rep. 2013, 15, 373. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Haschka, J.; Muschitz, C.; Kocijan, A.; Baierl, A.; Kleyer, A.; Schett, G.; Kapiotis, S.; Resch, H.; Sticherling, M.; et al. Bone microstructure and volumetric bone mineral density in patients with hyperuricemia with and without psoriasis. Osteoporos. Int. 2020, 31, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Pfeil, A.; Krojniak, L.; Renz, D.M.; Reinhardt, L.; Franz, M.; Oelzner, P.; Wolf, G.; Böttcher, J. Psoriatic arthritis is associated with bone loss of the metacarpals. Arthritis Res. 2016, 18, 248. [Google Scholar] [CrossRef] [PubMed]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2010 (GBD 2010) Results by Cause 1990–2010; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2012. [Google Scholar] [CrossRef]
- Osmancevic, A.; Landin-Wilhelmsen, K.; Larkö, O.; Mellström, D.; Wennberg, A.-M.; Hulthén, L.; Krogstad, A.-L. Risk factors for osteoporosis and bone status in postmenopausal women with psoriasis treated with UVB therapy. Acta. Derm. Venereol. 2008, 88, 240–246. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.; Sung, J. Obesity and Bone Health Revisited: A Mendelian Randomization Study for Koreans. J. Bone Miner. Res. 2019, 34, 1058–1067. [Google Scholar] [CrossRef]
- Aune, D.; Snekvik, I.; Schlesinger, S.; Norat, T.; Riboli, E.; Vatten, L.J. Body mass index, abdominal fatness, weight gain and the risk of psoriasis: A systematic review and dose–response meta-analysis of prospective studies. Eur. J. Epidemiol. 2018, 33, 1163–1178. [Google Scholar] [CrossRef]
- Atakpo, P.; Vassar, M. Publication bias in dermatology systematic reviews and meta-analyses. J. Dermatol. Sci. 2016, 82, 69–74. [Google Scholar] [CrossRef]

| Lumbar Spine | Femoral Neck | Total Hip | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subgroups | N | SMD (95%CI) | I2 (%) | N | SMD (95%CI) | I2 (%) | N | SMD (95%CI) | I2 (%) |
| Study population | |||||||||
| Psoriasis | 1 | −0.07 (−0.40 to 0.27) | NA | 0 | NA | NA | 1 | −0.24 (−0.58 to 0.09) | NA |
| Psoriatic arthritis | 6 | 0.07 (−0.19 to 0.33) | 73.5 | 5 | −0.08 (−0.36 to 0.20) | 72.3 | 5 | −0.04 (−0.22 to 0.14) | 31.0 |
| Study design | |||||||||
| Cross-sectional | 5 | 0.00 (−0.28 to 0.29) | 71.9 | 4 | −0.16 (−0.49 to 0.17) | 70.0 | 4 | −0.15 (−0.32 to 0.03) | 0 |
| Cohort | 1 | 0.33 (0.10 to 0.57) * | NA | 1 | 0.19 (−0.05 to 0.42) | NA | 1 | 0.20 (−0.04 to 0.43) | NA |
| Geographic location | |||||||||
| America | 1 | 0.00 (−0.28 to 0.28) | NA | 0 | NA | NA | 1 | −0.16 (−0.44 to 0.12) | NA |
| Asia | 2 | −0.06 (−0.96 to 0.83) | 91.3 | 2 | −0.30 (−0.90 to 0.29) | 80.1 | 2 | −0.13 (−0.39 to 0.13) | 0 |
| Europe | 3 | 0.19 (−0.04 to 0.42) | 42.8 | 3 | 0.08 (−0.12 to 0.29) | 29.5 | 2 | 0.06 (−0.28 to 0.40) | 54.9 |
| Age (years) | |||||||||
| <50 | 1 | −0.52 (−0.89 to −0.15) * | NA | 1 | −0.60 (−0.97 to −0.23) * | NA | 1 | −0.25 (−0.61 to 0.12) | NA |
| 50–59 | 3 | 0.31 (0.14 to 0.47) * | 0 | 3 | 0.13 (−0.03 to 0.30) | 0 | 2 | 0.14 (−0.06 to 0.34) | 0 |
| ≥60 | 2 | −0.04 (−0.27 to 0.19) | 0 | 1 | −0.21 (−0.61 to 0.19) | NA | 2 | −0.16 (−0.39 to 0.07) | 0 |
| BMI status | |||||||||
| Overweight | 4 | 0.23 (0.06 to 0.40) * | 26.2 | 3 | 0.13 (−0.03 to 0.30) | 0 | 3 | 0.02 (−0.20 to 0.25) | 45.4 |
| Obesity | 2 | −0.32 (−0.72 to 0.08) | 53.6 | 2 | −0.41 (−0.80 to −0.03) * | 50.6 | 2 | −0.21 (−0.48 to 0.06) | 0 |
| Disease duration | |||||||||
| ≥10 years | 5 | 0.00 (−0.28 to 0.29) | 71.9 | 4 | −0.16 (−0.49 to 0.17) | 70.0 | 4 | −0.15 (−0.32 to 0.03) | 0 |
| <10 years | 1 | 0.33 (0.10 to 0.57) * | NA | 1 | 0.19 (−0.05 to 0.42) | NA | 1 | 0.20 (−0.04 to 0.43) | NA |
| Medication use | |||||||||
| Yes | 5 | 0.10 (−0.19 to 0.38) | 77.6 | 4 | −0.05 (−0.38 to 0.28) | 77.7 | 4 | −0.03 (−0.24 to 0.18) | 47.0 |
| No | 1 | −0.11 (−0.51 to 0.29) | NA | 1 | −0.21 (−0.61 to 0.19) | NA | 1 | −0.16 (−0.56 to 0.24) | NA |
| Risk of bias | |||||||||
| NOS ≥ 7 | 2 | 0.29 (0.11 to 0.47) * | 0 | 2 | 0.16 (−0.02 to 0.35) | 0 | 1 | 0.20 (−0.04 to 0.43) | NA |
| NOS < 7 | 4 | −0.06 (−0.41 to 0.29) | 74.3 | 3 | −0.27 (−0.63 to 0.08) | 61.4 | 4 | −0.15 (−0.32 to 0.03) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-L.; Lu, J.-W.; Huang, Y.-W.; Wang, J.-H.; Su, K.-Y. Bone Mineral Density, Osteoporosis, and Fracture Risk in Adult Patients with Psoriasis or Psoriatic Arthritis: A Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Med. 2020, 9, 3712. https://doi.org/10.3390/jcm9113712
Chen T-L, Lu J-W, Huang Y-W, Wang J-H, Su K-Y. Bone Mineral Density, Osteoporosis, and Fracture Risk in Adult Patients with Psoriasis or Psoriatic Arthritis: A Systematic Review and Meta-Analysis of Observational Studies. Journal of Clinical Medicine. 2020; 9(11):3712. https://doi.org/10.3390/jcm9113712
Chicago/Turabian StyleChen, Tai-Li, Jing-Wun Lu, Yu-Wen Huang, Jen-Hung Wang, and Kuei-Ying Su. 2020. "Bone Mineral Density, Osteoporosis, and Fracture Risk in Adult Patients with Psoriasis or Psoriatic Arthritis: A Systematic Review and Meta-Analysis of Observational Studies" Journal of Clinical Medicine 9, no. 11: 3712. https://doi.org/10.3390/jcm9113712
APA StyleChen, T.-L., Lu, J.-W., Huang, Y.-W., Wang, J.-H., & Su, K.-Y. (2020). Bone Mineral Density, Osteoporosis, and Fracture Risk in Adult Patients with Psoriasis or Psoriatic Arthritis: A Systematic Review and Meta-Analysis of Observational Studies. Journal of Clinical Medicine, 9(11), 3712. https://doi.org/10.3390/jcm9113712





