A Retrospective Analysis Evaluating the Outcome of Parenteral Nutrition in the Treatment of Anorexia Nervosa in Korea
Abstract
1. Introduction
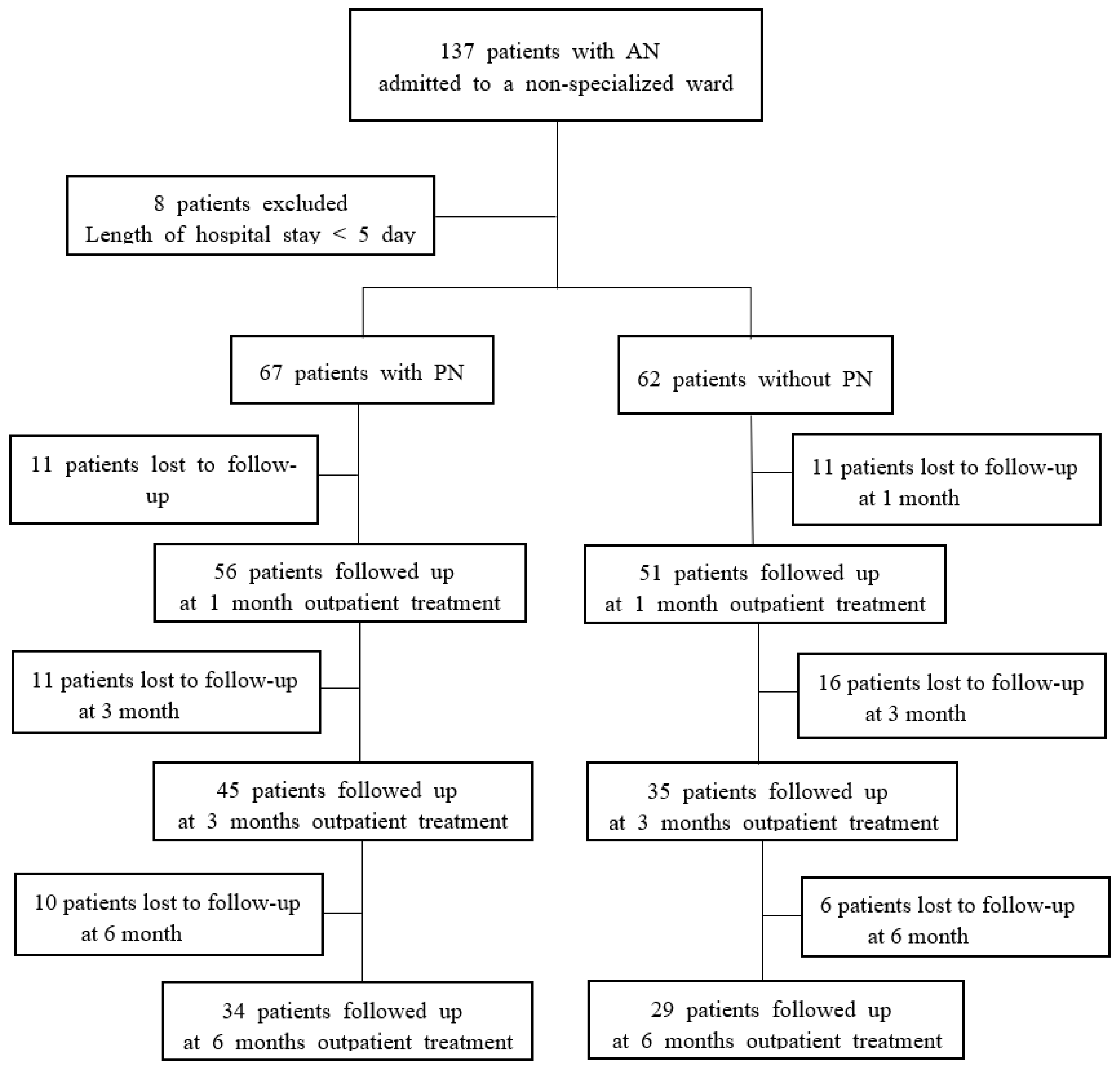
2. Methods
2.1. Participants
2.2. Treatment Protocol for Eating Disorders in the Non-Specialized Ward
2.3. Outpatient Treatment
2.4. Clinical Data Collection
2.5. Statistical Analyses
3. Results
3.1. Clinical Characteristics of Patients Who Received Supplemental Parenteral Refeeding and Patients with Oral Refeeding Alone
3.2. Nutritional Therapy in Patients Who Received Supplemental Parenteral Refeeding and Patients with Oral Refeeding Alone
3.3. Medical Findings at Admission and Discharge in Patients with Supplemental Parenteral Refeeding and Patients with Oral Refeeding Alone
3.4. Safety of and Compliance with Parenteral Nutrition
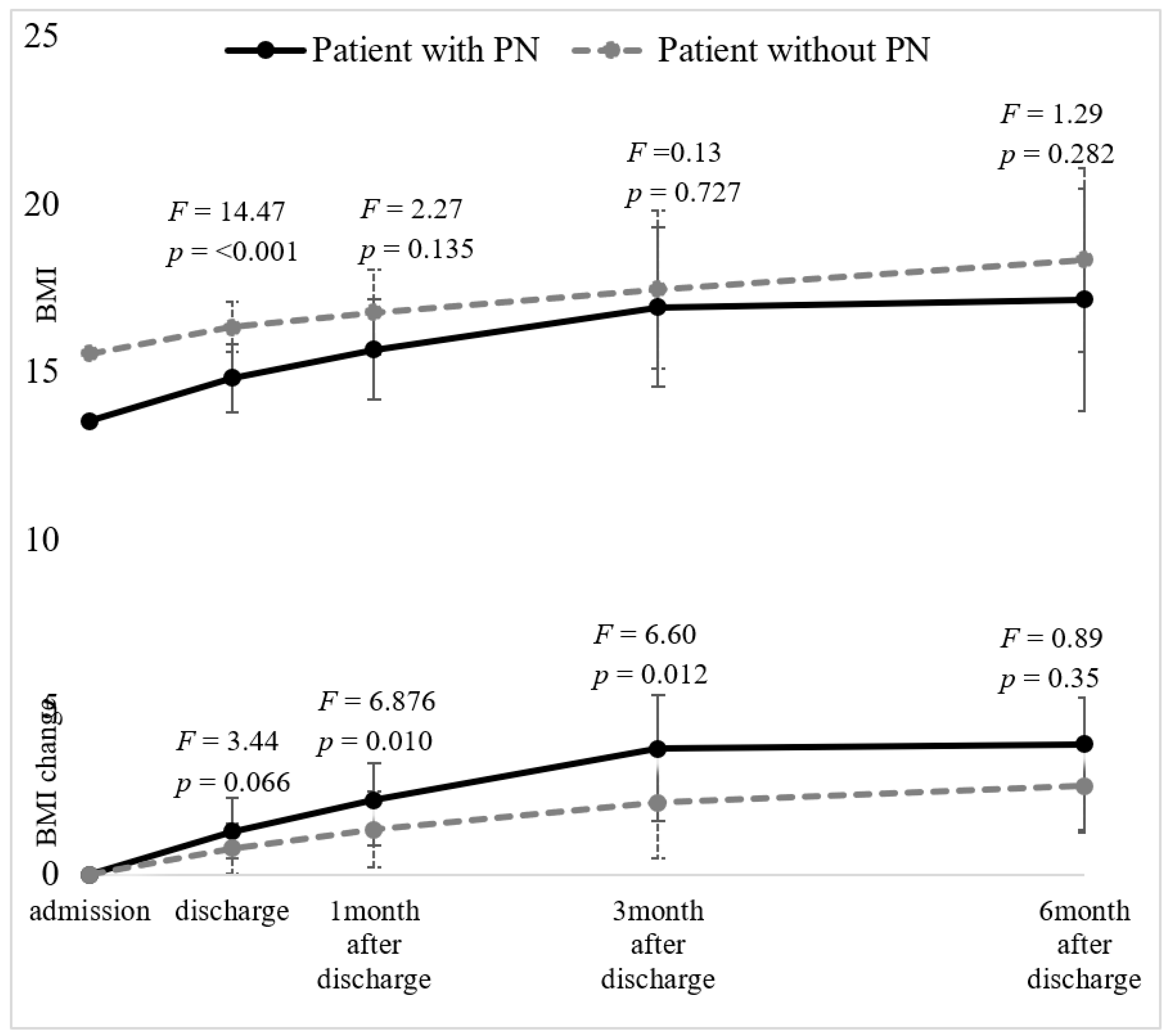
3.5. Weight Gain in Patients Who Received Supplemental Parenteral Refeeding and Patients with Oral Refeeding Alone
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating Disorders; Lancet: London, UK, 2020; pp. 899–911. [Google Scholar]
- Baran, S.A.; Weltzin, T.E.; Kaye, W.H. Low discharge weight and outcome in anorexia-nervosa. Am. J. Psychiatry 1995, 152, 1070–1072. [Google Scholar] [PubMed]
- Lock, J.; Litt, I. What predicts maintenance of weight for adolescents medically hospitalized for anorexia nervosa? Eat. Disord. 2003, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lund, B.C.; Hernandez, E.R.; Yates, W.R.; Mitchell, J.R.; McKee, P.A.; Johnson, C.L. Rate of Inpatient Weight Restoration Predicts Outcome in Anorexia Nervosa. Int. J. Eat. Disord. 2009, 42, 301–305. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Mental Health. Available online: http://pathways.nice.org.uk/pathways/eating-disorders (accessed on 12 October 2017).
- American Psychiatric Association. Treatment Recommendations for Patients With Eating Disorders. Am. J. Psychiatry 2006, 163, 5–54. [Google Scholar]
- Hay, P.; Chinn, D.; Forbes, D.; Madden, S.; Newton, R.; Sugenor, L.; Touyz, S.; Ward, W. Royal Australian and New Zealand College of Psychiatrists Clinical practice guidelines for the treatment of eating disorders. Aust. N. Z. J. Psychiatry 2014, 48, 977–1008. [Google Scholar] [CrossRef]
- OECD. Health at a Glance 2019: OECD Indicator; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Mehler, P.S.; Weiner, K.L. Use of total parenteral nutrition in the refeeding of selected patients with severe anorexia nervosa. Int. J. Eat. Disord. 2007, 40, 285–287. [Google Scholar] [CrossRef]
- Rizzo, S.M.; Douglas, J.W.; Lawrence, J.C. Enteral nutrition via nasogastric tube for refeeding patients with anorexia nervosa: A systematic review. Nutr. Clin. Pract. 2019, 34, 359–370. [Google Scholar] [CrossRef]
- Diamanti, A.; Basso, M.S.; Castro, M.; Bianco, G.; Ciacco, E.; Calce, A.; Caramadre, A.M.; Noto, C.; Gambarara, M. Clinical efficacy and safety of parenteral nutrition in adolescent girls with anorexia nervosa. J. Adolesc. Health 2008, 42, 111–118. [Google Scholar] [CrossRef]
- Michihata, N.; Matsui, H.; Fushimi, K.; Yasunaga, H. Comparison between enteral nutrition and intravenous hyperalimentation in patients with eating disorders: Results from the Japanese diagnosis procedure combination database. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2014, 19, 473–478. [Google Scholar] [CrossRef]
- Latzer, Y.; Eysen-Eylat, D.; Tabenkin, H. A case report: Treatment of severe anorexia nervosa with home total parenteral hyperalimentation. Int. J. Eat. Disord. 2000, 27, 115–118. [Google Scholar] [CrossRef]
- Tonoike, T.; Takahashi, T.; Watanabe, H.; Kimura, H.; Suwa, M.; Akahori, K.; Itakura, Y. Treatment with intravenous hyperalimentation for severely anorectic patients and its outcome. Psychiatry. Clinic. Neurosci. 2004, 58, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mehler, P.S.; Andersen, A.E. Eating Disorders: A Guide to Medical Care and Complications; The Johns Hopkins University Press: Baltimore, MD, USA, 2010. [Google Scholar]
- Gentile, M.G. Enteral Nutrition for Feeding Severely Underfed Patients with Anorexia Nervosa. Nutrients 2012, 4, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Schalla, M.A.; Stengel, A. Activity based anorexia as an animal model for anorexia nervosa—A systematic review. Front. Nutr. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Press: Washington, DC, USA, 2013. [Google Scholar]
- Madden, S.; Miskovic-Wheatley, J.; Wallis, A.; Kohn, M.; Lock, J.; Le Grange, D.; Jo, B.; Clarke, S.; Rhodes, P.; Hay, P.; et al. A randomized controlled trial of in-patient treatment for anorexia nervosa in medically unstable adolescents. Psychol. Med. 2015, 45, 415–427. [Google Scholar] [CrossRef]
- Schmidt, U.; Wade, T.D.; Treasure, J. The Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA): Development, key features, and preliminary evidence. J. Cogn. Psychother. 2014, 28, 48–71. [Google Scholar] [CrossRef]
- McIntosh, V. Specialist supportive clinical management (SSCM) for anorexia nervosa: Content analysis, change over course of therapy, and relation to outcome. J. Eat. Disord. 2015, 3, 1. [Google Scholar] [CrossRef]
- Fairburn, C.G. Cognitive Behavior Therapy and Eating Disorders; Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- Spielberger, C.D. State-trait anxiety inventory. Corsini Encycl. Psychol. 2010. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory-II. Psychol. Assess. 1996, 78, 490–498. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metabol. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Mehler, P.S.; Blalock, D.V.; Walden, K.; Kaur, S.; McBride, J.; Walsh, K.; Watts, J. Medical findings in 1026 consecutive adult inpatient-residential eating disordered patients. Int. J. Eat. Disord. 2018, 51, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Touyz, S.; Claudino, A.M.; Lujic, S.; Smith, C.A.; Madden, S. Inpatient versus outpatient care, partial hospitalisation and waiting list for people with eating disorders. Cochrane Database Syst. Rev. 2019, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Druml, C.; Ballmer, P.E.; Druml, W.; Oehmichen, F.; Shenkin, A.; Singer, P.; Soeters, P.; Weimann, A.; Bischoff, S.C. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin. Nutr. 2016, 35, 545–556. [Google Scholar] [CrossRef] [PubMed]
| Patients with PN | Patients without PN | t or χ2 | df | p | d | |||
|---|---|---|---|---|---|---|---|---|
| Variables | n | Mean (SD) | n | Mean (SD) | ||||
| Age at admission (years) | 67 | 19.04 (7.94) | 62 | 21.55 (6.64) | −1.93 | 127 | 0.055 | 0.34 |
| BMI (kg/m2) | 67 | 13.52 (1.93) | 62 | 15.55 (1.75) | −6.21 | 127 | <0.001 *** | 1.1 |
| Lowest ever BMI (kg/m2) | 67 | 13.11 (1.82) | 62 | 14.23 (1.58) | −3.71 | 127 | <0.001 *** | 0.66 |
| Highest ever BMI (kg/m2) | 67 | 19.06 (2.67) | 62 | 22.24 (3.69) | −4.36 | 127 | <0.001 *** | 0.99 |
| Duration of illness (months) | 67 | 28.48 (42.69) | 62 | 43.05 (41.94) | −1.95 | 127 | 0.053 | 0.34 |
| Number of admissions | 67 | 1.18 (.575) | 62 | 1.84 (2.29) | −2.20 | 68 | 0.031 * | 0.4 |
| Binge-eating and purging type, n (%) | 67 | 11 (16.4) | 62 | 36 (58.1) | χ2 = 24.94 | 1 | <0.001 *** | n/a |
| EDE-Q | ||||||||
| Total | 56 | 10.39 (4.64) | 48 | 11.28 (4.90) | −0.96 | 102 | 0.34 | 0.19 |
| Restraint | 56 | 3.32 (1.45) | 48 | 2.89 (1.46) | 1.51 | 102 | 0.135 | 0.3 |
| Eating concern | 56 | 2.23 (1.53) | 48 | 2.87 (1.68) | −2.02 | 102 | 0.046 * | 0.4 |
| Weight concern | 56 | 2.59 (1.53) | 48 | 2.83 (1.50) | −0.80 | 102 | 0.425 | 0.16 |
| Shape concern | 56 | 2.2 3(1.41) | 48 | 2.68 (1.57) | −1.55 | 102 | 0.124 | 0.3 |
| Psychiatric comorbid symptoms | ||||||||
| BDI | 60 | 24.50 (11.72) | 58 | 27.81 (12.78) | −1.47 | 116 | 0.145 | 0.27 |
| STAI-state | 59 | 54.92 (13.04) | 58 | 58.34 (10.98) | −1.53 | 115 | 0.128 | 0.28 |
| STAI-trait | 58 | 55.72 (13.47) | 58 | 59.19 (11.33) | −1.50 | 114 | 0.137 | 0.28 |
| Variables | Patients with PN (n = 67) | Patients without PN (n = 62) | t | df | p | d |
|---|---|---|---|---|---|---|
| At admission | ||||||
| Oral feeding (kcal/d) | 1327.61 (554.47) | 1967.90 (483.26) | −6.97 | 127 | <0.001 *** | 1.23 |
| Meal | 1194.77 (517.72) | 1706.45 (376.72) | −6.45 | 120 | <0.001 *** | 1.12 |
| Snack | 132.82 (188.60) | 261.45 (198.84) | −3.77 | 127 | <0.001 *** | 0.66 |
| PN (kcal/d) | 619.60 (265.51) | n/a | n/a | n/a | n/a | n/a |
| Duration PN (days) | 8.49 (5.62) | n/a | n/a | n/a | n/a | n/a |
| At discharge | ||||||
| Total calories (kcal/d) | 2188.20 (643.96) | 2400.00 (392.34) | −2.27 | 110 | 0.025 * | 0.39 |
| Meal | 1819.55 (511.95) | 2008.06 (260.07) | −2.67 | 99 | 0.009 ** | 0.46 |
| Snack | 380.59 (244.01) | 391.93 (179.06) | −0.30 | 127 | 0.766 | 0.05 |
| Length of stay (days) | 24.66 (11.61) | 21.65 (10.67) | 1.53 | 127 | 0.129 | 0.27 |
| n (%) | n (%) | χ2 | df | p | ||
| Refeeding edema | 12 (17.9) | 12 (19.4) | 0.04 | 1 | 0.833 | |
| Psychotherapy | 64 (95.5) | 58 (93.5) | 0.24 | 1 | 0.621 | |
| Voluntary discharge | 5 (7.5) | 10 (16.1) | 2.35 | 1 | 0.170 |
| Group | Two-Way Repeated-Measures ANOVA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with PN (n = 67) | Patients without PN (n = 62) | Main Effect | Interaction Effect | ||||||||||||
| Variables | Period | t(df) | p | Period | t(df) | p | Group | Period | F | p | |||||
| Admission | Discharge | Admission | Discharge | df | F | p | F | p | |||||||
| Systolic BP (mmHg) | 89.5 (13.0) | 89.6 (8.6) | −0.05 (66) | 0.963 | 93.4 (14.1) | 93.9 (10.0) | −0.27 (60) | 0.786 | 1.132 | 000 | 0.960 | 0.01 | 0.929 | 0.223 | 0.638 |
| Diastolic BP (mmHg) | 58.7 (10.5) | 58.8 (6.7) | −0.08 (66) | 0.937 | 60.8 (10.7) | 63.0 (9.0) | −1.41 (60) | 0.165 | 1.132 | 1.01 | 0.317 | 0.01 | 0.907 | 1.01 | 0.317 |
| HR (/min) | 68.3 (12.5) | 66.0 (12.0) | 1.10 (66) | 0.254 | 68.2 (9.2) | 65.6 (8.7) | 1.74 (60) | 0.087 | 1.132 | 0.32 | 0.573 | 0.03 | 0.863 | 0.13 | 0.719 |
| BT (°/C) | 36.5 (0.4) | 36.5 (0.3) | −0.31 (65) | 0.741 | 36.6 (0.4) | 36.5 (0.3) | 0.63 (60) | 0.533 | 1.131 | 1.58 | 0.211 | 0.47 | 0.497 | 1.56 | 0.214 |
| WBC (103/mm3) | 4.0 (1.0) | 4.5 (1.4) | −2.94 (56) | 0.004 ** | 3.96 (1.1) | 4.19 (1.2) | −1.51 (39) | 0.138 | 1.94 | 4.90 | 0.029 * | 0.75 | 0.387 | 0.47 | 0.496 |
| Hb (g/dL) | 12.3 (1.6) | 11.7 (1.2) | 3.42 (56) | 0.001 ** | 11.3 (1.5) | 11.4 (1.2) | −0.64 (39) | 0.529 | 1.94 | 9.86 | 0.002 ** | 4.25 | 0.042 * | 2.27 | 0.135 |
| PLT (103/mm3) | 219.1 (70.4) | 249.7 (66.9) | −2.42 (56) | 0.019 * | 229.9 (76.8) | 234.3 (61.8) | −0.45 (39) | 0.657 | 1.94 | 0.44 | 0.510 | 15.57 | <0.001 *** | 0.173 | 0.678 |
| Na (mEq/L) | 139.5 (3.1) | 141.0 (2.1) | −2.81 (41) | 0.008 ** | 139.7 (2.8) | 140.7 (2.2) | −0.16 (31) | 0.142 | 1.71 | 0.02 | 0.881 | 1.17 | 0.283 | 0.00 | 0.959 |
| K (mEq/L) | 4.2 (0.4) | 4.2 (0.3) | −0.35 (41) | 0.727 | 3.6 (0.7) | 4.0 (0.5) | −3.20 (31) | 0.003 ** | 1.71 | 5.95 | 0.017 * | 2.28 | 0.136 | 2.01 | 0.161 |
| Cl (mEq/L) | 104.5 (4.3) | 106.8 (5.0) | −2.45 (41) | 0.019 * | 101.3 (7.1) | 104.5 (4.8) | −2.79 (31) | 0.009 ** | 1.71 | 4.62 | 0.035 * | 0.26 | 0.614 | 0.00 | 0.976 |
| AST (U/L) | 48.6 (85.5) | 27.3 (10.3) | 1.79 (51) | 0.079 | 31.9 (19.7) | 28.5 (18.5) | 0.578 (20) | 0.570 | 1.70 | 0.04 | 0.840 | 3.61 | 0.061 | 0.00 | 0.965 |
| ALT (U/L) | 52.7 (81.3) | 33.7 (24.6) | 1.71 (51) | 0.093 | 37.4 (39.6) | 33.8 (25.8) | 0.389 (20) | 0.702 | 1.70 | 0.03 | 0.863 | 2.52 | 0.117 | 0.01 | 0.934 |
| Protein (g/dL) | 6.6 (0.7) | 6.5 (0.5) | 0.77 (47) | 0.448 | 6.2 (0.6) | 6.4 (0.4) | −0.98 (20) | 0.340 | 1.66 | 5.78 | 0.019 * | 0.04 | 0.841 | 0.95 | 0.334 |
| Albumin (g/dL) | 4.3 (0.5) | 4.2 (0.4) | 0.55 (49) | 0.585 | 4.1 (0.4) | 4.1 (0.3) | 0.00 (22) | 1.00 | 1.70 | 1.36 | 0.247 | 0.03 | 0.876 | 0.12 | 0.734 |
| Cholesterol (mg/dL) | 199.7 (59.5) | 181.2 (30.9) | 2.08 (35) | 0.045 * | 203.9 (63.6) | 181.5 (31.0) | 1.61 (15) | 0.128 | 1.49 | 0.17 | 0.684 | 0.48 | 0.490 | 0.14 | 0.706 |
| Glucose (mg/dL) | 77.3 (13.2) | 74.1 (10.5) | 1.51 (39) | 0.140 | 70.9 (12.6) | 73.1 (11.1) | −0.69 (15) | 0.502 | 1.53 | 3.15 | v082 | 1.21 | 0..276 | 0.60 | 0.442 |
| Discharge | Follow-Up at 1 Month | Follow-Up at 3 Months | Follow-Up at 6 Months | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | PN+ (n = 67) | PN− (n = 62) | F (df = 127) | p | Δη2 | PN+ (n = 56) | PN− (n = 51) | F (df=105) | p | Δη2 | PN+ (n = 45) | PN− (n =35) | F (df =78) | p | Δη2 | PN+ (n = 34) | PN− (n = 29) | F (df = 61) | p | Δη2 |
| BMI (kg/m2) | 14.79 (1.77) | 16.41 (1.68) | 14.47 | <0.001 *** | 0.096 | 15.65 (1.99) | 16.81 (1.65) | 2.27 | 0.135 | 0.021 | 16.92 (2.54) | 17.58 (2.60) | 0.12 | 0.727 | 0.002 | 17.14 (3.10) | 18.55 (2.79) | 1.29 | 0.282 | 0.040 |
| Weight gain from admission (kg) | 3.14 (2.57) | 2.19 (1.84) | 3.44 | 0.066 | 0.025 | 5.43 (3.67) | 3.56 (3.32) | 6.95 | 0.010 ** | 0.060 | 9.25 (5.93) | 5.89 (5.94) | 6.33 | 0.014 * | 0.074 | 9.67 (8.50) | 7.11 (6.65) | 0.75 | 0.388 | 0.012 |
| Rate (kg/week) | 0.88 (0.90) | 0.79 (1.02) | 0.00 | 0.961 | 0.000 | 0.52 (0.70) | 0.33 (0.72) | 2.75 | 0.100 | 0.025 | 0.42 (0.50) | 0.18 (0.54) | 3.64 | 0.060 | 0.045 | 0.12 (0.33) | 0.14 (0.30) | 0.83 | 0.365 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.-K.; Lee, Y.-K.; Na, J.C.; Kim, D.-Y.; Kim, Y.-R. A Retrospective Analysis Evaluating the Outcome of Parenteral Nutrition in the Treatment of Anorexia Nervosa in Korea. J. Clin. Med. 2020, 9, 3711. https://doi.org/10.3390/jcm9113711
Ko J-K, Lee Y-K, Na JC, Kim D-Y, Kim Y-R. A Retrospective Analysis Evaluating the Outcome of Parenteral Nutrition in the Treatment of Anorexia Nervosa in Korea. Journal of Clinical Medicine. 2020; 9(11):3711. https://doi.org/10.3390/jcm9113711
Chicago/Turabian StyleKo, Jeong-Kyung, You-Kyung Lee, Jong Chun Na, Dong-Yeon Kim, and Youl-Ri Kim. 2020. "A Retrospective Analysis Evaluating the Outcome of Parenteral Nutrition in the Treatment of Anorexia Nervosa in Korea" Journal of Clinical Medicine 9, no. 11: 3711. https://doi.org/10.3390/jcm9113711
APA StyleKo, J.-K., Lee, Y.-K., Na, J. C., Kim, D.-Y., & Kim, Y.-R. (2020). A Retrospective Analysis Evaluating the Outcome of Parenteral Nutrition in the Treatment of Anorexia Nervosa in Korea. Journal of Clinical Medicine, 9(11), 3711. https://doi.org/10.3390/jcm9113711





