Global Asthma Network Phase I Surveillance: Geographical Coverage and Response Rates
Abstract
1. Introduction
- (1)
- Globally, the burden of asthma is changing in adults and children;
- (2)
- There is large variation in the diagnosis of asthma;
- (3)
- In many locations, asthma is under-diagnosed and its management is suboptimal; and
- (4)
- There are potentially modifiable risk factors for asthma.
- (1)
- To conduct asthma surveillance around the world in two age groups of school pupils, and their parents, measuring prevalence, severity, management and risk factors, following the methods of ISAAC Phase III;
- (2)
- To examine time trends in prevalence, severity, management and risk factors from centres which completed ISAAC Phase III; and
- (3)
- To evaluate the appropriateness of asthma management, especially access to quality-assured essential asthma medicines, as defined by WHO [9].
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Global Asthma Network. Available online: http://www.globalasthmanetwork.org (accessed on 17 November 2020).
- Ellwood, P.; Asher, M.I.; Billo, N.E.; Bissell, K.; Chiang, C.Y.; Ellwood, E.M.; El-Sony, A.; García-Marcos, L.; Mallol, J.; Marks, G.B.; et al. The Global Asthma Network rationale and methods for Phase I global surveillance: Prevalence, severity, management and risk factors. Eur. Respir. J. 2017, 49, 1601635. [Google Scholar] [CrossRef]
- The Global Asthma Report 2011; The International Union Against Tuberculosis and Lung Disease: Paris, France, 2011; ISBN 978-2-914365-83-3.
- Lai, C.K.W.; Beasley, R.; Crane, J.; Foliaki, S.; Shah, J.; Weiland, S. ISAAC Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Aït-Khaled, N.; Beasley, R.; Mallol, J.; Keil, U.; Mitchell, E.; Robertson, C.; ISAAC Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007, 62, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Sembajwe, G.; Cifuentes, M.; Tak, S.W.; Kriebel, D.; Gore, R.; Punnett, L. National income, self-reported wheezing and asthma diagnosis from the World Health Survey. Eur. Respir. J. 2010, 35, 279–286. [Google Scholar] [CrossRef] [PubMed]
- The Global Asthma Report 2018; Global Asthma Network: Auckland, New Zealand, 2018; ISBN 978-0-473-46523-0/978-0-473-46524-7.
- Asher, I.; Garcia-Marcos, L.; Pearce, N.; Strachan, D. Trends in worldwide asthma prevalence. Eur. Respir. J. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- WHO Model Lists of Essential Medicines. Available online: https://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 17 November 2020).
- The Global Asthma Report 2014; Global Asthma Network: Auckland, New Zealand, 2014; pp. 44–57. ISBN 978-0-473-29125-9/978-0-473-29126-6.
- Asher, I.; Haahtela, T.; Selroos, O.; Ellwood, P.; Ellwood, E.; The Global Asthma Study Network Group. Global Asthma Network survey suggests more national asthma strategies could reduce burden of asthma. Allergol. Immunopathol. 2017, 45, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bissell, K.; Ellwood, P.; Ellwood, E.; Chiang, C.Y.; Marks, G.B.; El Sony, A.; Asher, I.; Billo, N.; Perrin, C.; The Global Asthma Network Study Group. Essential medicines at the national level: The Global Asthma Network’s essential asthma medicines survey 2014. Int. J. Environ. Res. Public Health 2019, 16, 605. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, P.; Ellwood, E.; Asher, I. Asthma management guidelines and strategies—Who has them? Am. J. Respir. Crit. Care Med. 2014, 189, A104. [Google Scholar]
- Ellwood, P.; Asher, M.I.; Ellwood, E.; Global Asthma Network Steering Group. Manual for Global Surveillance: Prevalence, Severity and Risk Factors; Global Asthma Network Data Centre: Auckland, New Zealand, 2015; ISBN 978-0-473-31442-2. [Google Scholar]
- Ellwood, P.; Williams, H.; Ait-Khaled, N.; Bjorksten, B.; Robertson, C.; Group IPIS. Translation of questions: The International Study of Asthma and Allergies in Childhood (ISAAC) experience. Int. J. Tuberc. Lung Dis. 2009, 13, 1174–1182. [Google Scholar] [PubMed]
- Crane, J.; Mallol, J.; Beasley, R.; Stewart, A.; Asher, M.I. Agreement between written and video questions for comparing asthma symptoms in ISAAC. Eur. Respir. J. 2003, 21, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Burney, P.G.J.; Luczynska, C.; Chinn, S.; Jarvis, D. The European Community Respiratory Health Survey. Eur. Respir. J. 1994, 7, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; the ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Sole, D.; Rosario Filho, N.; Sarinho, E.C.; Silva, A.R.; Britto, M.; Riedi, C.; Cardozo, C.; Camelo-Nunes, I.C.; De Andrade, D.; Mallol, J. Prevalence of asthma and related symptoms in adolescents: Findings from 3 surveys. J. Investig. Allergol. Clin. Immunol. 2015, 25, 73–74. [Google Scholar] [PubMed]
- Mallol, J.; Aguirre, V.; Mallol-Simmonds, M.; Matamala-Bezmalinovic, A.; Calderon-Rodriguez, L.; Osses-Vergara, F. Changes in the prevalence of asthma and related risk factors in adolescents: Three surveys between 1994 and 2015. Allergol. Immunopathol. 2019, 47, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chinratanapisit, S.; Suratannon, N.; Pacharn, P.; Sritipsukho, P.; Vichyanond, P. Prevalence and severity of asthma, rhinoconjunctivitis and eczema in children from the Bangkok area: The Global Asthma Network (GAN) Phase, I. Asian Pac. J. Allergy Immunol. 2019, 37, 226–231. [Google Scholar] [PubMed]
- Del-Rio-Navarro, B.E.; Navarrete Rodríguez, E.M.; Berber, A.; Reyes-Noriega, N.; García-Marcos Álvarez, L.; Grupo GAN México; Grupo ISAAC México. The burden of asthma in an inner-city area: A historical review 10 years after ISAAC. World Allergy Org. J. 2020, 13, 100092. [Google Scholar] [CrossRef] [PubMed]
- Del-Rio-Navarro, B.E.; Berber, A.; Noriega, N.R.; Navarrete Rodríguez, E.M.; García Almaráz, R.; Mérida Palacio, J.V.; Ellwood, P.; García-Marcos, L. What are the time trends in the prevalence of asthma symptoms in Mexico? Allergol. Immunopathol. 2020, in press. [Google Scholar]
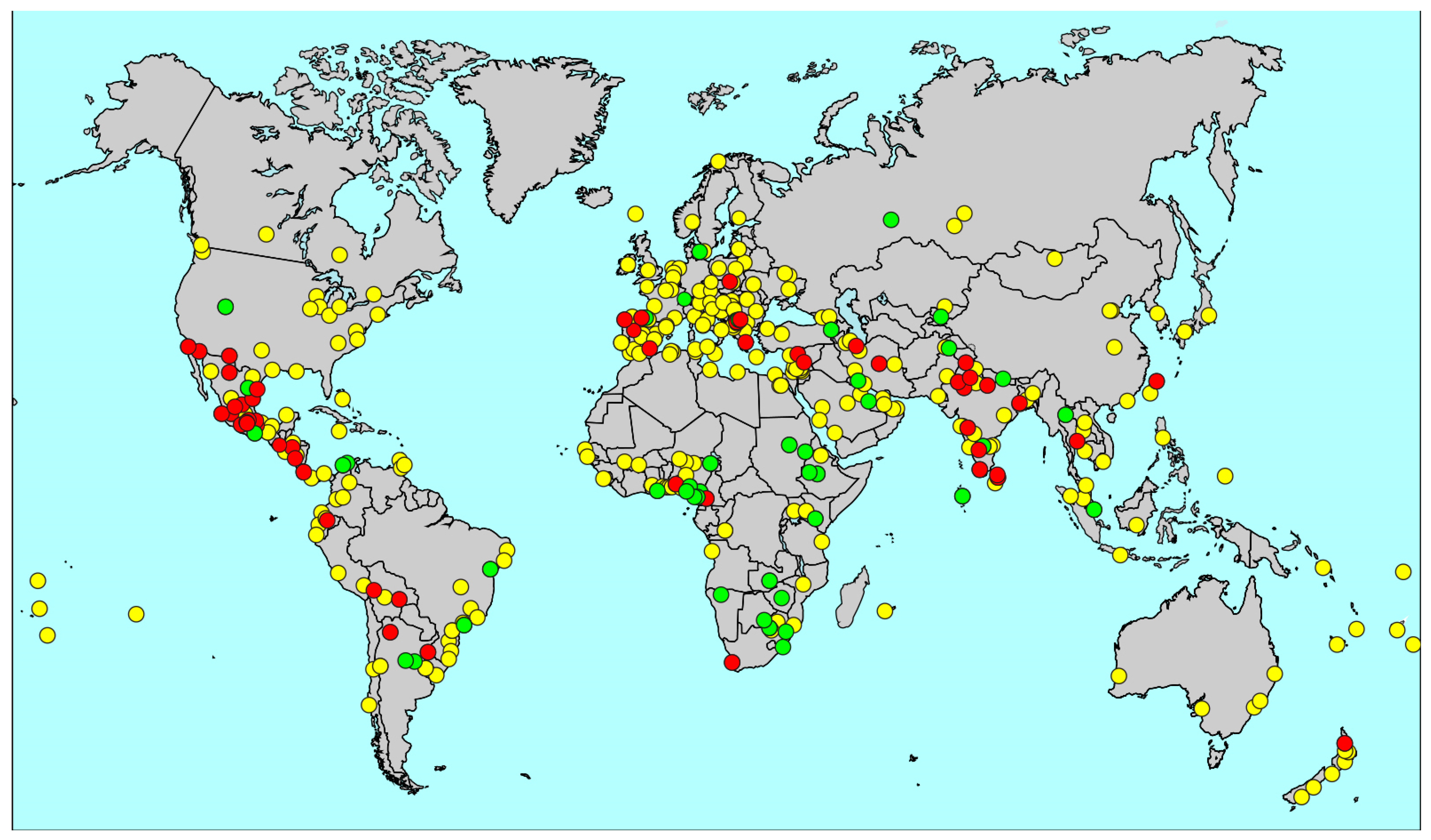
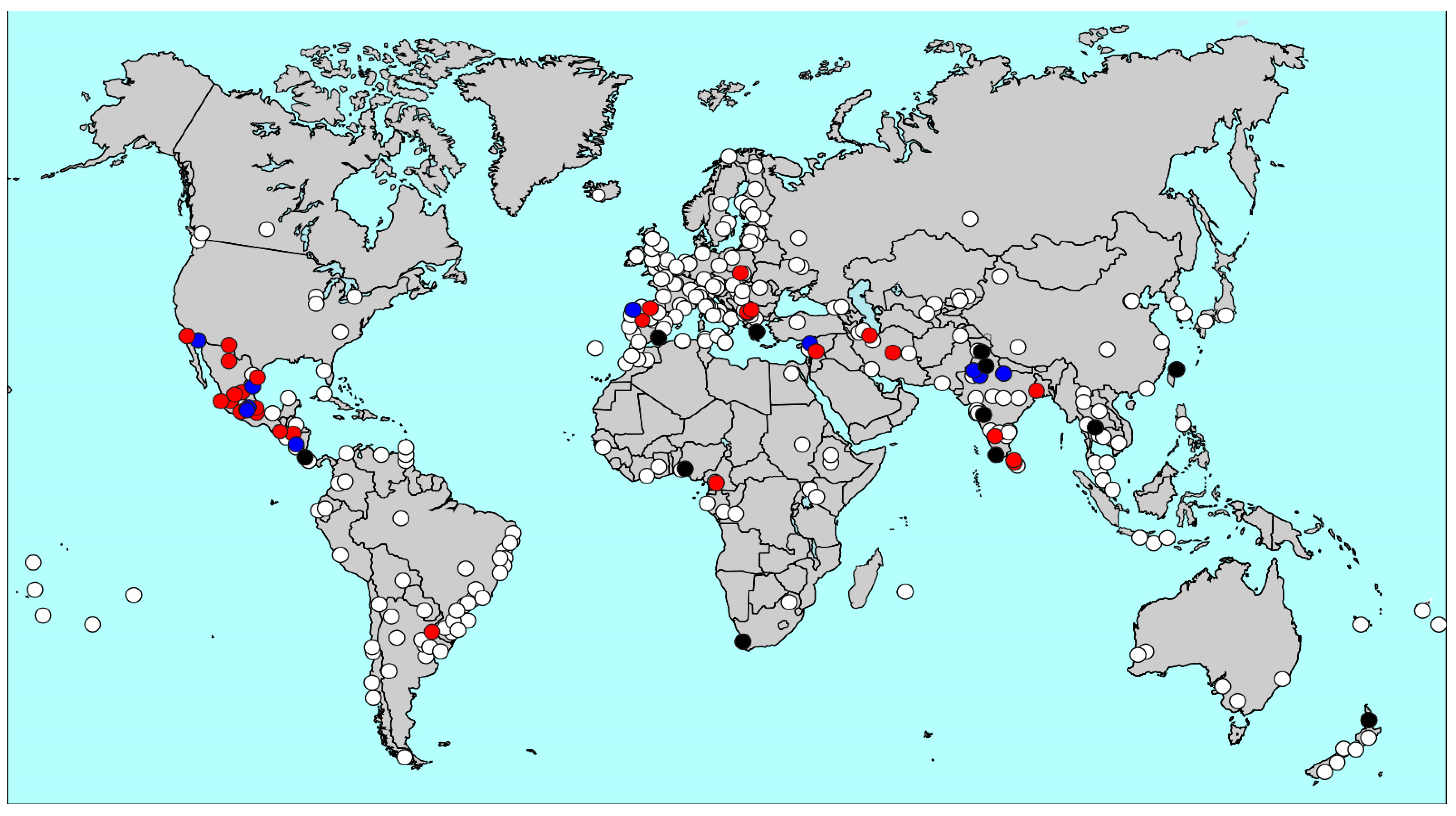
| GAN Phase I Centres | ISAAC Phase III Centres * | ISAAC Phase I Centres * | |||||
|---|---|---|---|---|---|---|---|
| Questionnaire Module | 6–7 | 13–14 | Adults | 6–7 | 13–14 | 6–7 | 13–14 |
| Symptoms: | |||||||
| Asthma (written) | 41 | 53 | 40 | 144 {17} | 233 {21} | 91 {9} | 155 {12} |
| Asthma (video) | NA | 29 | NA | NA | 139 {8} | NA | 99 {3} |
| Rhinoconjunctivitis | 41 | 53 | NA | 144 {17} | 233 {21} | 91 {9} | 155 {12} |
| Eczema | 41 | 53 | 40 | 142 {17} | 231 {21} | 91 {9} | 155 {12} |
| Risk factors: | |||||||
| ISAAC Phase 3 questions | 40 | 52 | 38 | 75 {17} | 122 {21} | ND | ND |
| Active smoking | NA | 52 | 38 | ND | ND | ND | ND |
| Perinatal questions | 39 | NA | NA | ND | ND | ND | ND |
| Indoor environment | 39 | NA | 38 | ND | ND | ND | ND |
| Asthma-related: | |||||||
| Management (now) | 41 | 53 | 40 | ND | ND | ND | ND |
| Management (infancy) | 39 | NA | NA | ND | ND | ND | ND |
| School absence | 41 | 53 | 40 | ND | ND | ND | ND |
| Work absence | NA | NA | 40 | ND | ND | ND | ND |
| Hospitalisation | 41 | 53 | 40 | ND | ND | ND | ND |
| 6–7-Year-Olds | 13–14-Year-Olds | Adults | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Risk Factors | Management | Symptoms | Risk Factors | Management | Symptoms | Risk Factors | Management | ||||||||||
| Centre Name | P | S | P | S | P | S | P | S | P | S | P | S | P | S | P | S | P | S |
| Yaounde | 722 | 27 | 722 | 27 | 703 | 27 | 1066 | 22 | 1066 | 22 | 1056 | 22 | 860 | 32 | 832 | 32 | 824 | 32 |
| Ibadan | 0 | 0 | 0 | 0 | 0 | 0 | 2897 | 23 | 2894 | 23 | 2810 | 23 | 2321 | 23 | 2321 | 23 | 2217 | 23 |
| Cape Town | 0 | 0 | 0 | 0 | 0 | 0 | 3979 | 29 | 3976 | 29 | 3879 | 29 | 0 | 0 | 0 | 0 | 0 | 0 |
| Taipei | 3036 | 25 | 3036 | 25 | 3034 | 25 | 3474 | 24 | 3465 | 24 | 3464 | 24 | 9689 | 49 | 9673 | 49 | 9594 | 49 |
| Bangkok | 3067 | 7 | 3067 | 7 | 3063 | 7 | 3206 | 6 | 3201 | 6 | 3084 | 6 | 5418 | 13 | 5416 | 13 | 5311 | 13 |
| Yazd | 0 | 0 | 0 | 0 | 0 | 0 | 5141 | 48 | 5141 | 48 | 5141 | 48 | 0 | 0 | 0 | 0 | 0 | 0 |
| Karaj | 572 | 39 | 0 | 0 | 572 | 39 | 754 | 42 | 0 | 0 | 754 | 42 | 1175 | 75 | 0 | 0 | 1175 | 75 |
| Lattakia | 1115 | 9 | 1078 | 9 | 1111 | 9 | 1215 | 10 | 1214 | 10 | 1203 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Damascus | 0 | 0 | 0 | 0 | 0 | 0 | 1100 | 11 | 1100 | 11 | 1100 | 11 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kottayam | 2099 | 50 | 2099 | 50 | 2085 | 50 | 2090 | 20 | 2088 | 20 | 2050 | 20 | 6940 | 69 | 6937 | 69 | 6743 | 69 |
| New Delhi | 2516 | 54 | 2516 | 54 | 2516 | 54 | 3024 | 59 | 3024 | 59 | 3024 | 59 | 9449 | 113 | 9449 | 113 | 9010 | 113 |
| Chandigarh | 2473 | 57 | 2473 | 57 | 2473 | 57 | 3000 | 54 | 3000 | 54 | 2999 | 54 | 10,386 | 111 | 10,386 | 111 | 10,384 | 111 |
| Bikaner | 2600 | 45 | 2600 | 45 | 2551 | 45 | 2702 | 33 | 2702 | 33 | 2702 | 33 | 10,495 | 78 | 10,495 | 78 | 10,473 | 78 |
| Jaipur | 2296 | 44 | 2296 | 44 | 2250 | 44 | 3060 | 57 | 3057 | 57 | 2977 | 57 | 8933 | 101 | 8902 | 101 | 8524 | 101 |
| iLucknow | 2969 | 32 | 2969 | 32 | 2931 | 32 | 2968 | 31 | 2969 | 31 | 2933 | 31 | 11,820 | 63 | 11,786 | 63 | 11,405 | 63 |
| Kolkata | 0 | 0 | 0 | 0 | 0 | 0 | 2998 | 37 | 2998 | 37 | 2886 | 37 | 7823 | 91 | 7818 | 91 | 7547 | 91 |
| Pune | 2404 | 26 | 2404 | 26 | 2403 | 26 | 3030 | 34 | 3030 | 34 | 3021 | 34 | 8000 | 60 | 7994 | 60 | 7909 | 60 |
| Mysuru (Mysore) | 2730 | 30 | 2730 | 30 | 2730 | 30 | 3051 | 29 | 3051 | 29 | 3051 | 29 | 11,178 | 59 | 11,178 | 59 | 11,177 | 59 |
| Peradeniya | 1492 | 12 | 1492 | 12 | 1455 | 12 | 1696 | 11 | 1696 | 11 | 1547 | 11 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anuradhapura | 2180 | 10 | 2180 | 10 | 2120 | 10 | 2986 | 10 | 2989 | 10 | 2638 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Uruguaiana | 0 | 0 | 0 | 0 | 0 | 0 | 1058 | 17 | 1052 | 17 | 1057 | 17 | 896 | 17 | 896 | 17 | 884 | 17 |
| Costa Rica | 1936 | 34 | 1936 | 34 | 1936 | 34 | 1338 | 33 | 1338 | 33 | 1316 | 33 | 3272 | 67 | 3272 | 67 | 3102 | 67 |
| Guatemala City | 1072 | 39 | 1072 | 39 | 1071 | 39 | 1420 | 42 | 1408 | 42 | 1400 | 42 | 1078 | 30 | 1078 | 30 | 1055 | 29 |
| Tegucigalpa | 361 | 22 | 361 | 22 | 359 | 22 | 1431 | 65 | 1431 | 65 | 1415 | 65 | 254 | 10 | 254 | 10 | 252 | 10 |
| Mexico City North | 2515 | 58 | 2515 | 58 | 2498 | 58 | 3375 | 9 | 3370 | 9 | 3375 | 9 | 5231 | 66 | 5219 | 66 | 5104 | 66 |
| Guadalajara | 2082 | 21 | 2082 | 21 | 2075 | 21 | 2519 | 13 | 2518 | 13 | 2516 | 13 | 489 | 20 | 487 | 20 | 483 | 20 |
| Mexicali | 2001 | 37 | 2001 | 37 | 1999 | 37 | 2479 | 20 | 2464 | 20 | 2469 | 20 | 2436 | 41 | 2427 | 41 | 2427 | 41 |
| Ciudad Victoria | 2444 | 20 | 2444 | 20 | 2439 | 20 | 2468 | 8 | 2465 | 8 | 2467 | 8 | 6239 | 28 | 6202 | 28 | 6149 | 28 |
| San Luis Potosí | 2108 | 28 | 2108 | 28 | 2108 | 28 | 2580 | 19 | 2580 | 19 | 2579 | 19 | 2835 | 27 | 2833 | 27 | 2801 | 27 |
| Tijuana | 2082 | 47 | 2082 | 47 | 2072 | 47 | 2601 | 13 | 2595 | 13 | 2577 | 13 | 1397 | 26 | 1395 | 26 | 1376 | 26 |
| Toluca Urban | 2712 | 21 | 2712 | 21 | 2702 | 21 | 2650 | 6 | 2642 | 6 | 2643 | 6 | 6162 | 27 | 6122 | 27 | 6072 | 27 |
| Toluca Rural | 2975 | 17 | 2976 | 17 | 2974 | 17 | 3122 | 16 | 3114 | 16 | 3091 | 16 | 7587 | 33 | 7583 | 33 | 7470 | 33 |
| Chihuahua | 1969 | 33 | 1969 | 33 | 1962 | 33 | 2180 | 7 | 2103 | 7 | 2161 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ciudad Juárez | 2117 | 39 | 2118 | 39 | 2114 | 39 | 2443 | 16 | 2439 | 16 | 2426 | 16 | 2610 | 37 | 2598 | 37 | 2601 | 37 |
| Michoácan | 2166 | 39 | 2166 | 39 | 2156 | 39 | 2504 | 14 | 2502 | 14 | 2503 | 14 | 2232 | 39 | 2232 | 39 | 2206 | 39 |
| Xalapa | 3716 | 83 | 3717 | 83 | 3712 | 83 | 3339 | 21 | 3335 | 21 | 3327 | 21 | 0 | 0 | 0 | 0 | 0 | 0 |
| Còrdoba | 2746 | 60 | 2746 | 60 | 2738 | 60 | 2991 | 25 | 2980 | 25 | 2989 | 25 | 2839 | 35 | 2832 | 35 | 2829 | 35 |
| Puerto Vallarta | 2241 | 46 | 2241 | 46 | 2238 | 46 | 2439 | 15 | 2439 | 15 | 2428 | 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aguascalientes | 3175 | 19 | 3176 | 19 | 3165 | 19 | 3336 | 19 | 3334 | 19 | 3331 | 19 | 2907 | 33 | 2898 | 33 | 2861 | 33 |
| Matamoros | 806 | 24 | 806 | 24 | 799 | 24 | 2892 | 12 | 2882 | 12 | 2865 | 12 | 1315 | 24 | 1306 | 24 | 1298 | 24 |
| Managua | 3162 | 59 | 3162 | 59 | 3127 | 59 | 3131 | 50 | 3126 | 50 | 2973 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prishtina | 0 | 0 | 0 | 0 | 0 | 0 | 1054 | 14 | 1056 | 14 | 1052 | 14 | 2006 | 14 | 2006 | 14 | 1977 | 14 |
| Gjakova | 0 | 0 | 0 | 0 | 0 | 0 | 676 | 5 | 676 | 5 | 676 | 5 | 1352 | 5 | 1352 | 5 | 1350 | 5 |
| Prizren | 0 | 0 | 0 | 0 | 0 | 0 | 1427 | 10 | 1427 | 10 | 1427 | 10 | 2712 | 10 | 0 | 0 | 2699 | 10 |
| Gjilan | 0 | 0 | 0 | 0 | 0 | 0 | 1200 | 6 | 1200 | 6 | 1200 | 6 | 1835 | 6 | 1835 | 6 | 1834 | 6 |
| Ferizaj | 0 | 0 | 0 | 0 | 0 | 0 | 890 | 9 | 890 | 9 | 885 | 9 | 1371 | 9 | 1372 | 9 | 1328 | 9 |
| Katowice | 1462 | 36 | 1462 | 36 | 1460 | 36 | 3185 | 29 | 3184 | 29 | 3180 | 29 | 2220 | 35 | 2219 | 35 | 2201 | 35 |
| Auckland | 1538 | 22 | 1538 | 22 | 1538 | 22 | 1885 | 7 | 1885 | 7 | 1860 | 7 | 3002 | 29 | 2994 | 29 | 2986 | 29 |
| Athens | 0 | 0 | 0 | 0 | 0 | 0 | 1934 | 20 | 1934 | 20 | 1934 | 20 | 1897 | 20 | 1897 | 20 | 1897 | 20 |
| Cartagena | 3509 | 61 | 3509 | 61 | 3496 | 61 | 3436 | 26 | 3430 | 26 | 3428 | 26 | 6961 | 60 | 6956 | 60 | 6832 | 60 |
| Salamanca | 2388 | 51 | 2388 | 51 | 2387 | 51 | 3485 | 31 | 3484 | 31 | 3481 | 31 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cantabria | 2841 | 75 | 2841 | 75 | 2836 | 75 | 4381 | 47 | 4372 | 47 | 4374 | 47 | 0 | 0 | 0 | 0 | 0 | 0 |
| A Coruña | 3407 | 48 | 3407 | 48 | 3407 | 48 | 3462 | 26 | 3461 | 26 | 3455 | 26 | 0 | 0 | 0 | 0 | 0 | 0 |
| All centres | 91,802 | 1506 | 91,197 | 1467 | 91,365 | 1506 | 13,2748 | 1260 | 131,777 | 1218 | 131,179 | 1260 | 177,622 | 1685 | 173,452 | 1600 | 174,367 | 1684 |
| GAN Phase I | ISAAC Phase III | ISAAC Phase I | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survey | Response (%) | Survey | Response (%) | Survey | Response (%) | ||||||
| Country | Centre Name | Years | 6–7 | 13–14 | Adult * | Years | 6–7 | 13–14 | Years | 6–7 | 13–14 |
| Cameroon | Yaounde | 2018–19 | 53.8 | 99.9 | 34.6 a | ||||||
| Nigeria | Ibadan | 2018 | - | 85.0 | 79.5 c | 2001–02 | 99.7 | 1995 | 76.4 | ||
| South Africa | Cape Town | 2017 | - | 84.4 | d | 2002 | 83.4 | 1995 | 82.8 | ||
| Taiwan | Taipei | 2016–17 | 76.3 | 93.0 | 84.5 a | 2001–02 | 96.8 | 95.9 | 1995 | 92.2 | 93.2 |
| Thailand | Bangkok | 2017–18 | 86.3 | 97.9 | 86.1 a | 2001 | 72.8 | 93.8 | 1995–96 | 90.8 | 74.8 |
| Iran | Yazd | 2020 | - | 71.3 | d | ||||||
| Iran | Karaj | 2019–20 | 72.0 | 71.9 | 88.6 a | ||||||
| Syrian Arab Republic | Lattakia | 2019 | 93.0 | 99.6 | d | 2001–03 | 99.1 | 99.8 | |||
| Syrian Arab Republic | Damascus | 2018 | - | 91.7 | d | ||||||
| India | Kottayam | 2017–18 | 68.4 | 85.3 | 97.5 a | 2001–03 | 96.4 | 98.5 | 1994–95 | 78.1 | 90.7 |
| India | New Delhi | 2017–18 | 80.9 | 100.0 | 85.7 a | 2001–02 | 82.4 | 86.7 | 1994–95 | 99.2 | 100 |
| India | Chandigarh | 2017–18 | 100.0 | 100.0 | 95.5 a | 2001–02 | 99.4 | 1994–95 | 94.0 | 97.4 | |
| India | Bikaner | 2017–18 | 86.7 | 90.1 | 99.8 a | 2001 | 95.4 | ||||
| India | Jaipur | 2017–18 | 75.8 | 98.7 | 84.4 a | 2001 | 75.4 | 87.4 | |||
| India | Lucknow | 2017 | 91.3 | 94.0 | 99.7 a | 2001–02 | 85.7 | 75.0 | |||
| India | Kolkata | 2017–18 | - | 99.9 | 80.2 c | ||||||
| India | Pune | 2017–18 | 79.8 | 99.6 | 81.4 a | 2001–02 | 90.4 | 70.8 | 1994–95 | 99.6 | 99.8 |
| India | Mysuru (Mysore) | 2017–18 | 90.9 | 99.5 | 97.4 a | ||||||
| Sri Lanka | Peradeniya | 2018 | 74.6 | 80.8 | d | ||||||
| Sri Lanka | Anuradhapura | 2018 | 72.7 | 85.4 | d | ||||||
| Brazil | Uruguaiana | 2016–18 | - | 88.2 | 76.7 c | ||||||
| Costa Rica | Costa Rica | 2017–18 | 64.5 | 66.9 | e | 2001–02 | 80.9 | 69.6 | 1994–95 | 84.1 | 91.4 |
| Guatemala | Guatemala City | 2018 | 32.2 | 40.6 | e | ||||||
| Honduras | Tegucigalpa | 2017–18 | 76.5 | 98.0 | e | ||||||
| Mexico | Mexico City North | 2015–16 | 86.7 | 93.8 | 55.9 a | 2002–03 | 91.6 | 99.8 | |||
| Mexico | Guadalajara | 2016 | 83.3 | 90.0 | 12.1 b | ||||||
| Mexico | Mexicali | 2015–16 | 77.0 | 83.7 | 32.7 a | 2002–03 | 74.3 | 93.6 | |||
| Mexico | Ciudad Victoria | 2015–16 | 81.5 | 82.3 | 78.6 a | 2003 | 73.1 | 79.5 | |||
| Mexico | San Luis Potosí | 2015–16 | 99.4 | 97.3 | 36.7 a | ||||||
| Mexico | Tijuana | 2015–16 | 83.3 | 86.7 | 41.4 b | ||||||
| Mexico | Toluca Urban | 2015–16 | 95.7 | 98.1 | 65.5 a | ||||||
| Mexico | Toluca Rural | 2015–16 | 93.0 | 94.6 | 69.1 a | 2002 | 89.5 | 86.1 | |||
| Mexico | Chihuahua | 2015–16 | 87.5 | 87.2 | d | ||||||
| Mexico | Ciudad Juárez | 2016–17 | 84.7 | 88.8 | 36.7 a | ||||||
| Mexico | Michoacán | 2016 | 90.3 | 92.7 | 75.8 b | ||||||
| Mexico | Xalapa | 2016–17 | 92.9 | 90.2 | d | ||||||
| Mexico | Còrdoba | 2016 | 91.5 | 93.5 | 30.2 a | ||||||
| Mexico | Puerto Vallarta | 2015–17 | 93.4 | 90.3 | d | ||||||
| Mexico | Aguascalientes | 2015–16 | 90.7 | 95.3 | 44.0 a | ||||||
| Mexico | Matamoros | 2015–17 | 80.6 | 93.3 | 93.7 b | ||||||
| Nicaragua | Managua | 2018 | 87.9 | 90.5 | d | 2002 | 96.0 | 94.5 | |||
| Kosovo | Prishtina | 2017 | - | 99.9 | 99.9 c | ||||||
| Kosovo | Gjakova | 2018 | - | 90.1 | 100.0 c | ||||||
| Kosovo | Prizren | 2017 | - | 89.0 | 99.7 c | ||||||
| Kosovo | Gjilan | 2017 | - | 80.0 | 81.5 c | ||||||
| Kosovo | Ferizaj | 2017 | - | 99.9 | 85.1 c | ||||||
| Poland | Katowice | 2017–18 | 36.8 | 79.1 | 85.6 b | ||||||
| New Zealand | Auckland | 2018–19 | 63.7 | 85.5 | 51.3 a | 2001 | 84.6 | 92.3 | 1992–93 | 90.2 | 94.6 |
| Greece | Athens | 2020 | - | 75.5 | 99.9 c | 1994–95 | 87.0 | ||||
| Spain | Cartagena | 2015–16 | 65.9 | 73.8 | 61.5 a | 2001–02 | 72.3 | 79.6 | 1993 | 68.5 | 95.1 |
| Spain | Salamanca | 2017–18 | 73.7 | 95.0 | d | ||||||
| Spain | Cantabria | 2017–18 | 56.2 | 77.4 | d | ||||||
| Spain | A Coruña | 2018–19 | 71.0 | 92.1 | d | 2003 | 73.8 | 93.6 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellwood, P.; Ellwood, E.; Rutter, C.; Perez-Fernandez, V.; Morales, E.; García-Marcos, L.; Pearce, N.; Asher, M.I.; Strachan, D.; on behalf of the GAN Phase I Study Group. Global Asthma Network Phase I Surveillance: Geographical Coverage and Response Rates. J. Clin. Med. 2020, 9, 3688. https://doi.org/10.3390/jcm9113688
Ellwood P, Ellwood E, Rutter C, Perez-Fernandez V, Morales E, García-Marcos L, Pearce N, Asher MI, Strachan D, on behalf of the GAN Phase I Study Group. Global Asthma Network Phase I Surveillance: Geographical Coverage and Response Rates. Journal of Clinical Medicine. 2020; 9(11):3688. https://doi.org/10.3390/jcm9113688
Chicago/Turabian StyleEllwood, Philippa, Eamon Ellwood, Charlotte Rutter, Virginia Perez-Fernandez, Eva Morales, Luis García-Marcos, Neil Pearce, M Innes Asher, David Strachan, and on behalf of the GAN Phase I Study Group. 2020. "Global Asthma Network Phase I Surveillance: Geographical Coverage and Response Rates" Journal of Clinical Medicine 9, no. 11: 3688. https://doi.org/10.3390/jcm9113688
APA StyleEllwood, P., Ellwood, E., Rutter, C., Perez-Fernandez, V., Morales, E., García-Marcos, L., Pearce, N., Asher, M. I., Strachan, D., & on behalf of the GAN Phase I Study Group. (2020). Global Asthma Network Phase I Surveillance: Geographical Coverage and Response Rates. Journal of Clinical Medicine, 9(11), 3688. https://doi.org/10.3390/jcm9113688






