The Effect of Smartphone-Based Cognitive Training on the Functional/Cognitive Markers of Schizophrenia: A One-Year Randomized Study
Abstract
1. Introduction
2. Materials and Methods
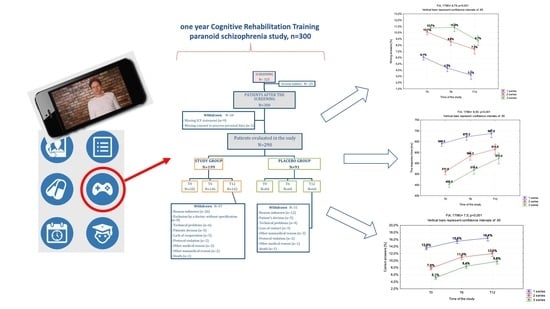
2.1. Study Design and Participants
2.2. Intervention and Implementation
2.3. Outcomes and Measures
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Patients
3.2. Conducted Cognitive Training Modules
3.3. Response Time Analysis
3.4. Correct Answers Rate
3.5. Incorrect Answers Rate
3.6. Cognitive Fatigability
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hyman, S.E. MEDICINE: What are the right targets for psychopharmacology? Science 2003, 299, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Keefe, R.S.E.; Fenton, W.S. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr. Bull. 2007, 33, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S. A Longitudinal study of symptom dimensions in schizophrenia. Arch. Gen. Psychiatry 1995, 52, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.W. A Summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr. Bull. 2005, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.R.J.; Cannon, T.D.; Gur, R.E.; Gur, R.C.; Moberg, P. Attentional dysfunctions in neuroleptic-naive and neuroleptic-withdrawn schizophrenic patients and their siblings. J. Abnorm. Psychol. 1997, 106, 203–212. [Google Scholar] [CrossRef]
- Javitt, D.C. Twenty-five years of glutamate in schizophrenia: Are we there yet? Schizophr. Bull. 2012, 38, 911–913. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. The physiological approach: Functional architecture of working memory and disordered cognition in schizophrenia. Biol. Psychiatry 1999, 46, 650–661. [Google Scholar] [CrossRef]
- Mueser, K.T.; McGurk, S.R. Schizophrenia. Lancet 2004, 363, 2063–2072. [Google Scholar] [CrossRef]
- Green, M.F.; Kern, R.S.; Braff, D.L.; Mintz, J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr. Bull. 2000, 26, 119–136. [Google Scholar] [CrossRef]
- Keefe, R.S.; Eesley, C.E.; Poe, M.P. Defining a cognitive function decrement in schizophrenia. Biol. Psychiatry 2005, 57, 688–691. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Javitt, D.C. Thinking glutamatergically: Changing concepts of schizophrenia based upon changing neurochemical models. Clin. Schizophr. Relat. Psychoses 2010, 4, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, K.-H.; Reddy, L.F.; Fiszdon, J.M. Measuring motivation in schizophrenia: Is a general state of motivation necessary for task-specific motivation? Schizophr. Res. 2014, 153, 209–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 1998, 12, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Keshavan, M. Cognitive remediation in schizophrenia. Clin. Psychopharmacol. Neurosci. 2012, 10, 125–135. [Google Scholar] [CrossRef]
- Holthausen, E.A.E.; Wiersma, D.; Cahn, W.; Kahn, R.S.; Dingemans, P.M.; Schene, A.H.; Bosch, R.J.V.D. Predictive value of cognition for different domains of outcome in recent-onset schizophrenia. Psychiatry Res. 2007, 149, 71–80. [Google Scholar] [CrossRef]
- Paz, R.D.; Tardito, S.; Atzori, M.; Tseng, K.Y. Glutamatergic dysfunction in schizophrenia: From basic neuroscience to clinical psychopharmacology. Eur. Neuropsychopharmacol. 2008, 18, 773–786. [Google Scholar] [CrossRef]
- Waszkiewicz, N. Mentally sick or not—(Bio)Markers of psychiatric disorders needed. J. Clin. Med. 2020, 9, 2375. [Google Scholar] [CrossRef]
- Mohamed, S.; Rosenheck, R.; Swartz, M.; Stroup, S.; Lieberman, J.A.; Keefe, R.S. Relationship of cognition and psychopathology to functional impairment in schizophrenia. Am. J. Psychiatry 2008, 165, 978–987. [Google Scholar] [CrossRef]
- Hill, S.K.; Bishop, J.R.; Palumbo, D.; Sweeney, J.A. Effect of second-generation antipsychotics on cognition: Current issues and future challenges. Expert Rev. Neurother. 2010, 10, 43–57. [Google Scholar] [CrossRef]
- Woodward, N.D.; Purdon, S.E.; Meltzer, H.Y.; Zald, D.H. A meta-analysis of cognitive change with haloperidol in clinical trials of atypical antipsychotics: Dose effects and comparison to practice effects. Schizophr. Res. 2007, 89, 211–224. [Google Scholar] [CrossRef]
- Mishara, A.L.; Goldberg, T.E. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: Opening a closed book. Biol. Psychiatry 2004, 55, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Barlati, S.; Deste, G.; De Peri, L.; Ariu, C.; Vita, A. Cognitive remediation in schizophrenia: Current status and future perspectives. Schizophr. Res. Treat. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Linke, M.; Jarema, M. Cognitive rehabilitation for people living with schizophrenia—The newest interventions. Psychiatr. Polska 2014, 48, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Grynszpan, O.; Perbal, S.; Pelissolo, A.; Fossati, P.; Jouvent, R.; Dubal, S.; Perez-Diaz, F. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: A meta-analytical study. Psychol. Med. 2011, 41, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.; Franck, N. Rehabilitation interventions to promote recovery from schizophrenia: A systematic review. Front. Psychiatry 2017, 8, 100. [Google Scholar] [CrossRef]
- Wykes, T.; Huddy, V.; Cellard, C.; McGurk, S.R.; Czobor, P. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. Am. J. Psychiatry 2011, 168, 472–485. [Google Scholar] [CrossRef]
- Firth, J.; Torous, J. Smartphone apps for schizophrenia: A systematic review. JMIR mHealth uHealth 2015, 3, e102. [Google Scholar] [CrossRef]
- Gay, K.; Torous, J.; Joseph, A.; Pandya, A.; Duckworth, K. Digital technology use among individuals with schizophrenia: Results of an online survey. JMIR Ment. Health 2016, 3, e15. [Google Scholar] [CrossRef]
- Ben-Zeev, D.; Kaiser, S.M.; Brenner, C.J.; Begale, M.; Duffecy, J.; Mohr, D.C. Development and usability testing of FOCUS: A smartphone system for self-management of schizophrenia. Psychiatr. Rehabil. J. 2013, 36, 289–296. [Google Scholar] [CrossRef]
- Naslund, J.A.; Marsch, L.A.; McHugo, G.J.; Bartels, S.J. Emerging mHealth and eHealth interventions for serious mental illness: A review of the literature. J. Ment. Health 2015, 24, 321–332. [Google Scholar] [CrossRef]
- Gire, N.; Farooq, S.; Naeem, F.; Duxbury, J.; McKeown, M.; Kundi, P.S.; Chaudhry, I.B.; Husain, N. mHealth based interventions for the assessment and treatment of psychotic disorders: A systematic review. mHealth 2017, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Zhang, J.; Guo, Z.; Lu, W.; Cai, J.; Shi, Z.; Zhang, C. A pilot study of iPad-assisted cognitive training for schizophrenia. Arch. Psychiatr. Nurs. 2014, 28, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Biagianti, B.; Fisher, M.; Howard, L.; Rowlands, A.; Vinogradov, S.; Woolley, J. Feasibility and preliminary efficacy of remotely delivering cognitive training to people with schizophrenia using tablets. Schizophr. Res. Cogn. 2017, 10, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.C.; Carpenter, W.T.; Kane, J.M.; Lasser, R.A.; Marder, S.R.; Weinberger, D.R. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am. J. Psychiatry 2005, 162, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Krzystanek, M.; Borkowski, M.; Skałacka, K.; Krysta, K. A telemedicine platform to improve clinical parameters in paranoid schizophrenia patients: Results of a one-year randomized study. Schizophr. Res. 2019, 204, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Dunn, O.J. Multiple comparisons among means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Krzystanek, M.; Krysta, K.; Skałacka, K. Treatment compliance in the long-term paranoid schizophrenia telemedicine study. J. Technol. Behav. Sci. 2017, 2, 84–87. [Google Scholar] [CrossRef]
- Areán, P.A.; Hallgren, K.A.; Jordan, J.T.; Gazzaley, A.; Atkins, D.C.; Heagerty, P.J.; Anguera, J.A. The use and effectiveness of mobile apps for depression: Results from a fully remote clinical trial. J. Med. Internet Res. 2016, 18, e330. [Google Scholar] [CrossRef]
- Bless, J.J.; Westerhausen, R.; Kompus, K.; Gudmundsen, M.; Hugdahl, K. Self-supervised, mobile-application based cognitive training of auditory attention: A behavioral and fMRI evaluation. Internet Interv. 2014, 1, 102–110. [Google Scholar] [CrossRef]
- Oh, S.J.; Seo, S.; Lee, J.H.; Song, M.J.; Shin, M.-S. Effects of smartphone-based memory training for older adults with subjective memory complaints: A randomized controlled trial. Aging Ment. Health 2017, 22, 526–534. [Google Scholar] [CrossRef]
- Elbogen, E.B.; Dennis, P.A.; Van Voorhees, E.E.; Blakey, S.M.; Johnson, J.L.; Johnson, S.C.; Wagner, H.R.; Hamer, R.M.; Beckham, J.C.; Manly, T.; et al. Cognitive rehabilitation with mobile technology and social support for veterans with TBI and PTSD. J. Head Trauma Rehabil. 2019, 34. [Google Scholar] [CrossRef] [PubMed]

| Variable | Study Group (n = 199) | Reference Group (n = 91) | p |
|---|---|---|---|
| Mean age, years | 32.0 (5.92) | 32.2 (6.94) | 0.8 |
| Sex—male | 114 (57.3%) | 60 (65.9%) | 0.16 |
| Race—Caucasian | 199 (100%) | 91 (100%) | 1 |
| Clinical status | |||
| Total PANSS score | 58.0 (20.3) | 59.8 (23.7) | 0.51 |
| Calgary scale | 4.0 (4.2) | 3.4 (4.1) | 0.26 |
| CGI-S scale | 2.7 (1.0) | 2.7 (1.1) | 1.0 |
| Cognitive parameters | |||
| Response time, ms | 549.7 (131.5) | 532.8 (160.7) | 0.345 |
| Correct answer rate, % | 6.4 (4.7) | 3.8 (4.1) | 0.0001 |
| Incorrect answer rate, % | 6.4 (4.6) | 4.7 (4.9) | 0.0045 |
| Fatigability, % | 85.0 (5.6) | 86.4 (6.6) | 0.0631 |
| Time Point | N | Mean (SD) | Median (IQR) | Min–Max |
|---|---|---|---|---|
| T1 | 102 | 4.6 (3.1) | 4.0 (5.0) | 1.0–17.0 |
| T2 | 95 | 4.2 (2.8) | 4.0 (5.5) | 1.0–12.0 |
| T3 | 89 | 3.8 (3.0) | 3.0 (5.0) | 1.0–18.0 |
| T4 | 74 | 4.2 (3.1) | 3.0 (4.0) | 1.0–18.0 |
| T5 | 69 | 3.8 (2.6) | 3.0 (3.0) | 1.0–10.0 |
| T6 | 72 | 3.6 (2.8) | 3.0 (4.0) | 1.0–17.0 |
| T7 | 62 | 4.4 (2.8) | 4.0 (5.0) | 1.0–12.0 |
| T8 | 68 | 4.0 (3.2) | 3.0 (6.0) | 1.0–18.0 |
| T9 | 46 | 4.1 (2.9) | 3.5 (4.0) | 1.0–15.0 |
| T10 | 50 | 4.3 (3.0) | 4.0 (3.8) | 1.0–16.0 |
| T11 | 55 | 4.0 (3.3) | 3.0 (5.5) | 1.0–18.0 |
| T12 | 50 | 3.6 (2.9) | 3.0 (4.0) | 1.0–15.0 |
| Overall | 144 | 23.6 (29.5) | 10.0 (30.0) | 1.0–186.0 |
| Variable | Time | N | Mean (SD) | Median (IQR) | Min–Max | p (T0–T12) |
|---|---|---|---|---|---|---|
| Response time, ms | ||||||
| Study group | T0 | 168 | 549.7 (131.5) | 560.7 (188.4) | 92.5–950.0 | 0.0001 |
| T12 | 63 | 627.2 (130.7) | 667.6 (177.0) | 279.6–840.6 | ||
| Reference group | T0 | 64 | 532.8 (160.7) | 508.3 (213.2) | 54.7–945.5 | 0.234 |
| T12 | 7 | 614.1 (156.4) | 707.3 (210.4) | 317.1–742.7 | ||
| Correct answers rate, % | ||||||
| Study group | T0 | 168 | 6.4 (4.7) | 5.8 (12.5) | 0.0–19.8 | 0.0001 |
| T12 | 63 | 11.2 (5.8) | 12.2 (16.7) | 0.0–20.0 | ||
| Reference group | T0 | 64 | 3.8 (4.1) | 1.8 (8.7) | 0.0–14.2 | 0.088 |
| T12 | 7 | 8.9 (6.5) | 8.0 (14.8) | 1.6–16.4 | ||
| Incorrect answers rate, % | ||||||
| Study group | T0 | 168 | 6.4 (4.6) | 6.0 (7.2) | 0.0–24.4 | 0.153 |
| T12 | 63 | 5.5 (4.1) | 4.7 (5.6) | 0.0–15.3 | ||
| Reference group | T0 | 64 | 4.7 (4.9) | 2.7 (7.8) | 0.0–17.6 | 0.643 |
| T12 | 7 | 4.1 (2.9) | 4.4 (4.7) | 0.7–8.9 | ||
| Variable | N | Mean (SD) | Median (IQR) | Min–Max | p |
|---|---|---|---|---|---|
| Response time, ms | |||||
| Study group | 187 | 594.0 (133.1) | 621.0 (194.3) | 92.0–950.0 | 0.173 |
| Reference group | 66 | 565.5 (149.0) | 591.1 (212.3) | 186.3–809.2 | |
| Correct answers rate, % | |||||
| Study group | 187 | 9.4 (5.5) | 9.3 (15.3) | 0.0–20.0 | 0.0004 |
| Reference group | 66 | 6.7 (5.0) | 6.2 (11.8) | 0.5–15.1 | |
| Incorrect answers rate, % | |||||
| Study group | 187 | 6.3 (4.5) | 5.6 (6.4) | 0.0–35.3 | 0.131 |
| Reference group | 66 | 5.4 (4.0) | 4.9 (6.1) | 0.2–14.7 | |
| Fatigability, % | |||||
| Study group | 187 | 83.0 (5.3) | 81.1 (6.4) | 52.9–96.7 | 0.0183 |
| Reference group | 66 | 85.0 (6.0) | 83.4 (10.7) | 74.2–96.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzystanek, M.; Krysta, K.; Borkowski, M.; Skałacka, K.; Przybyło, J.; Pałasz, A.; Mucic, D.; Martyniak, E.; Waszkiewicz, N. The Effect of Smartphone-Based Cognitive Training on the Functional/Cognitive Markers of Schizophrenia: A One-Year Randomized Study. J. Clin. Med. 2020, 9, 3681. https://doi.org/10.3390/jcm9113681
Krzystanek M, Krysta K, Borkowski M, Skałacka K, Przybyło J, Pałasz A, Mucic D, Martyniak E, Waszkiewicz N. The Effect of Smartphone-Based Cognitive Training on the Functional/Cognitive Markers of Schizophrenia: A One-Year Randomized Study. Journal of Clinical Medicine. 2020; 9(11):3681. https://doi.org/10.3390/jcm9113681
Chicago/Turabian StyleKrzystanek, Marek, Krzysztof Krysta, Mariusz Borkowski, Katarzyna Skałacka, Jacek Przybyło, Artur Pałasz, Davor Mucic, Ewa Martyniak, and Napoleon Waszkiewicz. 2020. "The Effect of Smartphone-Based Cognitive Training on the Functional/Cognitive Markers of Schizophrenia: A One-Year Randomized Study" Journal of Clinical Medicine 9, no. 11: 3681. https://doi.org/10.3390/jcm9113681
APA StyleKrzystanek, M., Krysta, K., Borkowski, M., Skałacka, K., Przybyło, J., Pałasz, A., Mucic, D., Martyniak, E., & Waszkiewicz, N. (2020). The Effect of Smartphone-Based Cognitive Training on the Functional/Cognitive Markers of Schizophrenia: A One-Year Randomized Study. Journal of Clinical Medicine, 9(11), 3681. https://doi.org/10.3390/jcm9113681