MicroRNA-Based Fingerprinting of Cervical Lesions and Cancer
Abstract
1. Introduction
2. Pathogenesis of Cervical Cancer and Its Precursor Stages
2.1. Histological Subtypes
2.2. HPV as an Infectious Agent
2.2.1. Characteristics
2.2.2. Viral Integration into Host Genome and Cell Cycle Affecting
3. miRNA and Cervical Lesions and Cancer
3.1. miRNA Biogenesis, Function, and Expression—Modulating Factors
3.2. Aberrant Expression of miRNA in Cervical Neoplasia and Cancer
3.2.1. Dynamics of miRNA Expression Observed in Cervical Lesions and Cancer
3.2.2. Differential Expression of miRNA between HPV-Positive and -Negative Cases
3.2.3. Variability in CC Histological Subtypes
3.2.4. Prognostic Value—The Risk of Metastization
3.2.5. Prognostic Value—Susceptibility to Conventional Therapy
3.3. miRNAs as Biomarkers
Standard Testing versus miRNAs as Cervical Pathology Biomarkers—Diagnostic Accuracy
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andor, N.; Graham, T.A.; Jansen, M.; Xia, L.C.; Aktipis, C.A.; Petritsch, C.; Ji, H.P.; Maley, C.C. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat. Med. 2016, 22, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M.; Faupel-Badger, J.M. The hallmarks of premalignant conditions: A molecular basis for cancer prevention. Semin. Oncol. 2015, 43, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Haedicke, J.; Iftner, T. Human papillomaviruses and cancer. Radiother. Oncol. 2013, 108, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Senkomago, V.; Henley, S.J.; Thomas, C.C.; Mix, J.M.; Markowitz, L.E.; Saraiya, M. Human Papillomavirus-attributable cancers-United States, 2012–2016. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 724–728. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; De Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. J. Natl. Cancer Inst. 2015, 107, djv086. [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Hausen, H.Z. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 2000, 92, 690–698. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.P.; Carrillo-Beltrán, D.; Aedo-Aguilera, V.; Calaf, G.M.; León, O.; Maldonado, E.; Tapia, J.C.; Boccardo, E.; Ozbun, M.A.; Aguayo, F. Tobacco exposure enhances human Papillomavirus 16 oncogene expression via EGFR/PI3K/Akt/c-jun signaling pathway in cervical cancer cells. Front. Microbiol. 2018, 9, 3022. [Google Scholar] [CrossRef] [PubMed]
- Tamarelle, J.; Thiébaut, A.; De Barbeyrac, B.; Bébéar, C.; Ravel, J.; Delarocque-Astagneau, E. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Force, U.P.S.T.; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; et al. Screening for cervical cancer. JAMA 2018, 320, 674–686. [Google Scholar] [CrossRef]
- Castanon, A.; Landy, R.; Sasieni, P.D. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int. J. Cancer 2016, 139, 1040–1045. [Google Scholar] [CrossRef]
- Zheng, T.; Holford, T.R.; Ma, Z.; Chen, Y.; Liu, W.; Ward, B.A.; Weiderpass, E. The continuing increase in adenocarcinoma of the uterine cervix: A birth cohort phenomenon. Int. J. Epidemiol. 1996, 25, 252–258. [Google Scholar] [CrossRef]
- Galic, V.; Herzog, T.J.; Lewin, S.N.; Neugut, A.I.; Burke, W.M.; Lu, Y.-S.; Hershman, D.L.; Wright, J.D. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol. Oncol. 2012, 125, 287–291. [Google Scholar] [CrossRef]
- IHGS International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nat. Cell Biol. 2004, 431, 931–945. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yao, F.; Xiao, Z.; Sun, Y.; Ma, L. MicroRNAs and metastasis: Small RNAs play big roles. Cancer Metastasis Rev. 2017, 37, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenetics 2019, 11, 1–24. [Google Scholar] [CrossRef]
- Pardini, B.; De Maria, D.; Francavilla, A.; Di Gaetano, C.; Ronco, G.; Naccarati, A. MicroRNAs as markers of progression in cervical cancer: A systematic review. BMC Cancer 2018, 18, 1–17. [Google Scholar] [CrossRef]
- Stuebs, F.A.; Schulmeyer, C.E.; Mehlhorn, G.; Gass, P.; Kehl, S.; Renner, S.K.; Renner, S.P.; Geppert, C.; Adler, W.; Hartmann, A.; et al. Accuracy of colposcopy-directed biopsy in detecting early cervical neoplasia: A retrospective study. Arch. Gynecol. Obstet. 2018, 299, 525–532. [Google Scholar] [CrossRef]
- Herfs, M.; Yamamoto, Y.; Laury, A.; Wang, X.; Nucci, M.R.; McLaughlin-Drubin, M.E.; Münger, K.; Feldman, S.; McKeon, F.D.; Xian, W.; et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl. Acad. Sci. 2012, 109, 10516–10521. [Google Scholar] [CrossRef]
- Mirkovic, J.; Howitt, B.E.; Roncarati, P.; Demoulin, S.; Suarez-Carmona, M.; Hubert, P.; McKeon, F.D.; Xian, W.; Philippe, D.; Delvenne, P.; et al. Carcinogenic HPV infection in the cervical squamo-columnar junction. J. Pathol. 2015, 236, 265–271. [Google Scholar] [CrossRef]
- Doorbar, J.; Griffin, H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. 2019, 7, 176–179. [Google Scholar] [CrossRef]
- Waxman, A.G.; Chelmow, D.; Darragh, T.M.; Lawson, H.; Moscicki, A.-B. Revised terminology for cervical histopathology and its implications for management of high-grade squamous intraepithelial lesions of the cervix. Obstet. Gynecol. 2012, 120, 1465–1471. [Google Scholar] [CrossRef]
- Frappart, L.; Fontaniere, B.; Lucas, E.; Sankaranarayanan, R. Histopathology of the uterine cervix-digital atlas. In WHO Histological Classification of Tumours of the Uterine Cervix; International Agency for Research on Cancer: Lyon, France, 2004; ISBN 978-92-832-2424-2. [Google Scholar]
- Pirog, E.C.; Kleter, B.; Olgac, S.; Bobkiewicz, P.; Lindeman, J.; Quint, W.G.V.; Richart, R.M.; Isacson, C. Prevalence of human Papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am. J. Pathol. 2000, 157, 1055–1062. [Google Scholar] [CrossRef]
- Molijn, A.; Jenkins, D.; Chen, W.; Zhang, X.; Pirog, E.; Enqi, W.; Liu, B.; Schmidt, J.; Cui, J.; Qiao, Y.-L.; et al. The complex relationship between human papillomavirus and cervical adenocarcinoma. Int. J. Cancer 2015, 138, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Kim, K.T.; Kim, Y.N.; Lee, K.B.; Sung, M.S.; Kim, K.T.; Jeong, D.H. Cervical adenocarcinoma has a poorer prognosis and a higher propensity for distant recurrence than squamous cell carcinoma. Int. J. Gynecol. Cancer 2017, 27, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nat. Cell Biol. 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Litwin, T.R.; Clarke, M.A.; Dean, M.; Wentzensen, N. Somatic host cell alterations in HPV carcinogenesis. Viruses 2017, 9, 206. [Google Scholar] [CrossRef]
- Roberts, S.A.; Lawrence, M.S.; Klimczak, L.J.; Grimm, S.A.; Fargo, D.; Stojanov, P.; Kiezun, A.; Kryukov, G.V.; Carter, S.L.; Saksena, G.; et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat. Genet. 2013, 45, 970–976. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, W.; Bao, J.; Kuhs, K.L.; Cai, Q.; Long, J.; Shu, X.-O.; Zheng, W.; Guo, X. Integrative genomic analyses of APOBEC-mutational signature, expression and germline deletion of APOBEC3 genes, and immunogenicity in multiple cancer types. BMC Med Genom. 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Bernard, H.-U.; Burk, R.D.; Chen, Z.; Van Doorslaer, K.; Hausen, H.Z.; De Villiers, E.-M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virol. 2010, 401, 70–79. [Google Scholar] [CrossRef]
- IARC monographs on the evaluation of carcinogenic risks to humans. In Human Papillomaviruses; International Agency for Research on Cancer IARC: Lyon, France, 2007; Volume 90, ISBN 978-92-832-1290-4.
- Shannon, B.; Yi, T.J.; Perusini, S.; Gajer, P.; Ma, B.; Humphrys, M.S.; Thomas-Pavanel, J.; Chieza, L.; Janakiram, P.; Saunders, M.; et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol. 2017, 10, 1310–1319. [Google Scholar] [CrossRef]
- Radley, D.; Saah, A.; Stanley, M. Persistent infection with human papillomavirus 16 or 18 is strongly linked with high-grade cervical disease. Hum. Vaccines Immunother. 2016, 12, 768–772. [Google Scholar] [CrossRef]
- Narisawa-Saito, M.; Kiyono, T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007, 98, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Pirog, E.C.; on behalf of the RIS HPV TT Study Group; Lloveras, B.; Molijn, A.C.; Tous, S.; Guimerà, N.; Alejo, M.; Clavero, O.; Klaustermeier, J.; Jenkins, D. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod. Pathol. 2014, 27, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.D.; Harari, A.; Chen, Z. Human papillomavirus genome variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Kukimoto, I.; Muramatsu, M. Genetic variations of human Papillomavirus type 16: Implications for cervical carcinogenesis. Jpn. J. Infect. Dis. 2015, 68, 169–175. [Google Scholar] [CrossRef]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; Del Pino, M.; et al. HPV-negative tumors of the uterine cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef]
- Manawapat-Klopfer, A.; Wang, L.; Haedicke-Jarboui, J.; Stubenrauch, F.; Munk, C.; Thomsen, L.T.; Martus, P.; Kjaer, S.K.; Iftner, T. HPV16 viral load and physical state measurement as a potential immediate triage strategy for HR-HPV-infected women: A study in 644 women with single HPV16 infections. Am. J. Cancer Res. 2018, 8, 715–722. [Google Scholar]
- Shukla, S.; Mahata, S.; Shishodia, G.; Pande, S.; Verma, G.; Hedau, S.; Bhambhani, S.; Kumari, A.; Batra, S.; Basir, S.F.; et al. Physical state & copy number of high risk human papillomavirus type 16 DNA in progression of cervical cancer. Indian J. Med Res. 2014, 139, 531–543. [Google Scholar]
- Kim, J.; Kim, B.K.; Jeon, D.-S.; Lee, C.H.; Roh, J.-W.; Kim, J.-Y.; Park, S.-Y. Type-Specific Viral Load and Physical State of HPV Type 16, 18, and 58 as Diagnostic biomarkers for high-grade squamous intraepithelial lesions or cervical cancer. Cancer Res. Treat. 2019, 52, 396–405. [Google Scholar] [CrossRef]
- Moody, C.A. Impact of replication stress in human Papillomavirus pathogenesis. J. Virol. 2018, 93, 01012. [Google Scholar] [CrossRef]
- Nilsson, K.; Wu, C.; Schwartz, S. Role of the DNA damage response in human Papillomavirus RNA splicing and polyadenylation. Int. J. Mol. Sci. 2018, 19, 1735. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Gao, W.; Wang, L.; Pan, Y.; Gao, Y.; Lu, Z.; Ke, Y. Genome-wide profiling of the human papillomavirus DNA integration in cervical intraepithelial neoplasia and normal cervical epithelium by HPV capture technology. Sci. Rep. 2016, 6, 35427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-W.; Fang, F.; Guo, Y.; Zhu, T.-L.; Yu, Y.-Y.; Kong, F.-F.; Han, L.-F.; Chen, D.-S.; Li, F. HPV16 integration probably contributes to cervical oncogenesis through interrupting tumor suppressor genes and inducing chromosome instability. J. Exp. Clin. Cancer Res. 2016, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Khajavi, M.; Connolly, A.M.; Towne, C.F.; Batish, S.D.; Lupski, J.R. The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat. Genet. 2009, 41, 849–853. [Google Scholar] [CrossRef]
- Li, W.; Qi, Y.; Cui, X.; Huo, Q.; Zhu, L.; Zhang, A.; Tan, M.; Hong, Q.; Yang, Y.; Zhang, H.; et al. Characteristic of HPV integration in the genome and transcriptome of cervical cancer tissues. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Lambert, P.F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: Implications for cervical carcinogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 1654–1658. [Google Scholar] [CrossRef]
- Ehrig, F.; Häfner, N.; Driesch, C.; Christiansen, I.K.; Beer, K.; Schmitz, M.; Runnebaum, I.B.; Dürst, M. Differences in stability of viral and viral-cellular fusion transcripts in HPV-induced cervical cancers. Int. J. Mol. Sci. 2019, 21, 112. [Google Scholar] [CrossRef]
- Warburton, A.; Redmond, C.J.; Dooley, K.E.; Fu, H.; Gillison, M.L.; Akagi, K.; Symer, D.E.; Aladjem, M.I.; McBride, A.A. HPV integration hijacks and multimerizes a cellular enhancer to generate a viral-cellular super-enhancer that drives high viral oncogene expression. PLoS Genet. 2018, 14, e1007179. [Google Scholar] [CrossRef]
- Cao, C.; Hong, P.; Huang, X.; Lin, D.; Cao, G.; Wang, L.; Feng, B.; Wu, P.; Shen, H.; Xu, Q.; et al. HPV-CCDC106 integration alters local chromosome architecture and hijacks an enhancer by 3D genome structure remodeling in cervical cancer. J. Genet. Genom. 2020, S1673852720300928. [Google Scholar] [CrossRef]
- Arfi, A.; Hequet, D.; Bataillon, G.; Tran-Perennou, C.; Farkhondeh, F.; Sastre-Garau, X.; Fourchotte, V.; Rouzier, R.; Laas, E.; Pouget, N.; et al. HPV DNA integration site as proof of the origin of ovarian metastasis from endocervical adenocarcinoma: Three case reports. BMC Cancer 2019, 19, 375. [Google Scholar] [CrossRef]
- Lace, M.J.; Isacson, C.; Anson, J.R.; Lörincz, A.T.; Wilczynski, S.P.; Haugen, T.H.; Turek, L.P. Upstream regulatory region alterations found in human Papillomavirus type 16 (HPV-16) isolates from cervical carcinomas increase transcription, ori function, and HPV immortalization capacity in culture. J. Virol. 2009, 83, 7457–7466. [Google Scholar] [CrossRef]
- Mandal, P.; Saha, S.S.; Sen, S.; Bhattacharya, A.; Bhattacharya, N.P.; Bucha, S.; Sinha, M.; Chowdhury, R.R.; Mondal, N.R.; Chakravarty, B.; et al. Cervical cancer subtypes harbouring integrated and/or episomal HPV16 portray distinct molecular phenotypes based on transcriptome profiling of mRNAs and miRNAs. Cell Death Discov. 2019, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.; Das, D.; Basu, K.; Tirkey, M.; Datta, C.; Chatterjee, U. Role of p16/Ki-67 Dual immunostaining in detection of cervical cancer precursors. J. Cytol. 2018, 35, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, function and role in cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Piwecka, M.; Rolle, K.; Belter, A.; Barciszewska, A.M.; Żywicki, M.; Michalak, M.; Nowak, S.; Naskręt-Barciszewska, M.Z.; Barciszewski, J. Comprehensive analysis of microRNA expression profile in malignant glioma tissues. Mol. Oncol. 2015, 9, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sánchez, M.; Rodríguez-Vicente, A.E.; Hernández, J.-A.; Lumbreras, E.; Sarasquete, M.E.; Martín, A.-A.; Benito, R.; Vicente-Gutierrez, C.; Robledo, C.; Heras, N.D.L.; et al. MiRNA expression profile of chronic lymphocytic leukemia patients with 13q deletion. Leuk. Res. 2016, 46, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Ahmad, A.; Zubair, H.; Miree, O.; Singh, S.; Rocconi, R.P.; Scalici, J.; Singh, A.P. MicroRNAs in gynecological cancers: Small molecules with big implications. Cancer Lett. 2017, 407, 123–138. [Google Scholar] [CrossRef]
- Biamonte, F.; Zolea, F.; Santamaria, G.; Battaglia, A.M.; Cuda, G.; Costanzo, F. Human haematological and epithelial tumor-derived cell lines express distinct patterns of onco-microRNAs. Cell. Mol. Biol. 2017, 63, 75. [Google Scholar] [CrossRef]
- Wang, K.; Chen, M.; Wu, W. Analysis of microRNA (miRNA) expression profiles reveals 11 key biomarkers associated with non-small cell lung cancer. World J. Surg. Oncol. 2017, 15, 1–10. [Google Scholar] [CrossRef][Green Version]
- Reddy, K.B. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015, 15, 1–6. [Google Scholar] [CrossRef]
- Barbu, M.G.; Condrat, C.E.; Thompson, D.C.; Bugnar, O.L.; Cretoiu, D.; Toader, O.D.; Suciu, N.; Voinea, S.C. MicroRNA involvement in signaling pathways during viral infection. Front. Cell Dev. Biol. 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or tumor suppressor? The duplicity of MicroRNAs in cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.P.; Saghafinia, S.; Hanahan, D. A set of microRNAs coordinately controls tumorigenesis, invasion, and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 24184–24195. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A.; Schilling, D.; Bastian, S.; Sponagel, J.; Kuryshev, V.; Sültmann, H.; Scheffner, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015, 11, e1004712. [Google Scholar] [CrossRef]
- Bhome, R.; Del Vecchio, F.; Lee, G.-H.; Bullock, M.D.; Primrose, J.N.; Sayan, A.E.; Mirnezami, A.H. Exosomal microRNAs (exomiRs): Small molecules with a big role in cancer. Cancer Lett. 2018, 420, 228–235. [Google Scholar] [CrossRef]
- Lopez-Serra, P.; Esteller, M. DNA methylation-associated silencing of tumor-suppressor microRNAs in cancer. Oncogene 2011, 31, 1609–1622. [Google Scholar] [CrossRef]
- Sharma, G.; Dua, P.; Agarwal, S.M. A Comprehensive review of dysregulated miRNAs involved in cervical cancer. Curr. Genom. 2014, 15, 310–323. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nat. Cell Biol. 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Kulcheski, F.R.; Christoff, A.P.; Margis, R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J. Biotechnol. 2016, 238, 42–51. [Google Scholar] [CrossRef]
- Tam, C.; Wong, J.H.; Tsui, S.K.W.; Zuo, T.; Chan, T.-F.; Ng, T.B. LncRNAs with miRNAs in regulation of gastric, liver, and colorectal cancers: Updates in recent years. Appl. Microbiol. Biotechnol. 2019, 103, 4649–4677. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, L.-G.; Wang, P.; Li, J.; Tian, F.; Chu, Z.-P.; Kang, S. The expression and significance of lncRNA HOST2 and microRNA let-7b in HPV-positive cervical cancer tissues and cell lines. Eur. Rev. Med Pharmacol. Sci. 2019, 23, 2380–2390. [Google Scholar] [PubMed]
- He, H.; Liu, X.; Liu, Y.; Zhang, M.; Lai, Y.; Hao, Y.; Wang, Q.; Shi, D.; Wang, N.; Luo, X.-G.; et al. Human Papillomavirus E6/E7 and long noncoding RNA TMPOP2 mutually upregulated gene expression in cervical cancer cells. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Wang, X.; Tang, S.; Le, S.-Y.; Lu, R.; Rader, J.S.; Meyers, C.; Zheng, Z.-M. Aberrant expression of oncogenic and tumor-suppressive MicroRNAs in cervical cancer is required for cancer cell growth. PLoS ONE 2008, 3, e2557. [Google Scholar] [CrossRef] [PubMed]
- Wilting, S.M.; Snijders, P.J.F.; Verlaat, W.; Jaspers, A.; Van de Wiel, M.A.; Van Wieringen, W.N.; Meijer, G.A.; Kenter, G.G.; Yi, Y.; Le Sage, C.; et al. Altered microRNA expression associated with chromosomal changes contributes to cervical carcinogenesis. Oncogene 2013, 32, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.-K.; Li, Y.; Hafner, M.; Banerjee, N.S.; Tang, S.; Briskin, D.; Meyers, C.; Chow, L.T.; Xie, X.; et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc. Natl. Acad. Sci. USA 2014, 111, 4262–4267. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.; Marques, J.P.; Soares, A.R.; Carreto, L.; Santos, M.A.S. MicroRNA expression variability in human cervical tissues. PLoS ONE 2010, 5, e11780. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Xu, J.; Ye, F.; Shen, Y.; Zhou, J.; Lu, W.; Wan, X.; Ma, D.; Xie, X. Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV-related target genes for miR-29. J. Pathol. 2011, 224, 484–495. [Google Scholar] [CrossRef]
- Zeng, K.; Zheng, W.; Mo, X.; Liu, F.; Li, M.; Liu, Z.; Zhang, W.; Hu, X.-X. Dysregulated microRNAs involved in the progression of cervical neoplasm. Arch. Gynecol. Obstet. 2015, 292, 905–913. [Google Scholar] [CrossRef]
- Jiménez-Wences, H.; Martínez-Carrillo, D.N.; Peralta-Zaragoza, O.; Campos-Viguri, G.E.; Hernández-Sotelo, D.; Jiménez-López, M.A.; Muñoz-Camacho, J.G.; Garzón-Barrientos, V.H.; Illades-Aguiar, B.; Fernández-Tilapa, G. Methylation and expression of miRNAs in precancerous lesions and cervical cancer with HPV16 infection. Oncol. Rep. 2016, 35, 2297–2305. [Google Scholar] [CrossRef]
- Gardiner, A.S.; McBee, W.C.; Edwards, R.P.; Austin, M.; Lesnock, J.L.; Bhargava, R.; Guido, R.; Khan, S.A. MicroRNA analysis in human papillomavirus (HPV)-associated cervical neoplasia and cancer. Infect. Agents Cancer 2010, 5, A55. [Google Scholar] [CrossRef]
- Cheung, T.-H.; Man, K.-N.M.; Yu, M.-Y.; Yim, S.-F.; Siu, N.S.; Lo, K.W.; Doran, G.; Wong, R.R.; Wang, V.W.; Smith, D.I.; et al. Dysregulated microRNAs in the pathogenesis and progression of cervical neoplasm. Cell Cycle 2012, 11, 2876–2884. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Zhou, H.; Peng, Y.; Li, J.; Lin, Z. Aberrant microRNA expression in human cervical carcinomas. Med Oncol. 2011, 29, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Eom, K.; Kim, J.; Bang, H.; Wang, H.-Y.; Ahn, S.; Kim, G.; Jang, H.; Kim, S.; Lee, D.; et al. MiR-9, miR-21, and miR-155 as potential biomarkers for HPV positive and negative cervical cancer. BMC Cancer 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Villegas-Ruiz, V.; Juárez-Méndez, S.; Pérez-González, O.A.; Arreola, H.; Paniagua-García, L.; Parra-Melquiadez, M.; Peralta-Rodríguez, R.; López-Romero, R.; Monroy-García, A.; Mantilla-Morales, A.; et al. Heterogeneity of microRNAs expression in cervical cancer cells: Over-expression of miR-196a. Int. J. Clin. Exp. Pathol. 2014, 7, 1389–1401. [Google Scholar] [PubMed]
- Gao, C.; Zhou, C.; Zhuang, J.; Liu, L.; Liu, C.; Li, H.; Liu, G.; Wei, J.; Sun, C. MicroRNA expression in cervical cancer: Novel diagnostic and prognostic biomarkers. J. Cell. Biochem. 2018, 119, 7080–7090. [Google Scholar] [CrossRef]
- Shishodia, G.; Shukla, S.; Srivastava, Y.; Masaldan, S.; Mehta, S.; Bhambhani, S.; Sharma, S.; Mehrotra, R.; Das, B.C.; Bharti, A.C. Alterations in microRNAs miR-21 and let-7a correlate with aberrant STAT3 signaling and downstream effects during cervical carcinogenesis. Mol. Cancer 2015, 14, 1–13. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Z.; Zhang, W.; Hu, X.-X. miR-31 functions as an oncogene in cervical cancer. Arch. Gynecol. Obstet. 2015, 292, 1083–1089. [Google Scholar] [CrossRef]
- Gocze, K.; Gombos, K.; Juhász, K.; Kovacs, K.; Kajtar, B.; Benczik, M.; Gocze, P.; Patczai, B.; Arany, I.; Ember, I. Unique microRNA expression profiles in cervical cancer. Anticancer. Res. 2013, 33, 2561–2567. [Google Scholar]
- Bierkens, M.; Krijgsman, O.; Wilting, S.M.; Bosch, L.; Jaspers, A.; Meijer, G.A.; Meijer, C.J.; Snijders, P.J.F.; Ylstra, B.; Steenbergen, R.D. Focal aberrations indicateEYA2andhsa-miR-375as oncogene and tumor suppressor in cervical carcinogenesis. Genes Chromosom. Cancer 2012, 52, 56–68. [Google Scholar] [CrossRef]
- Babion, I.; Snoek, B.C.; Novianti, P.W.; Jaspers, A.; Van Trommel, N.; Heideman, D.A.M.; Meijer, C.J.; Snijders, P.J.; Steenbergen, R.D.; Wilting, S.M. Triage of high-risk HPV-positive women in population-based screening by miRNA expression analysis in cervical scrapes; a feasibility study. Clin. Epigenetics 2018, 10, 76. [Google Scholar] [CrossRef]
- Tian, Q.; Li, Y.; Wang, F.; Li, Y.; Xu, J.; Shen, Y.; Ye, F.; Wang, X.; Cheng, X.; Chen, Y.; et al. MicroRNA Detection in cervical exfoliated cells as a triage for human Papillomavirus-positive women. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Fujii, T.; Kukimoto, I.; Yamada, H.; Yamamoto, N.; Kuroda, M.; Otani, S.; Ichikawa, R.; Nishio, E.; Torii, Y.; et al. Identification of miRNAs in cervical mucus as a novel diagnostic marker for cervical neoplasia. Sci. Rep. 2018, 8, 7070. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, G.; Corrie, S.R.; Feng, Q.; Morihara, J.; Stern, J.; Hawes, S.E.; Kiviat, N.B. Expression of Mir-21 and Mir-143 in cervical specimens ranging from histologically normal through to invasive cervical cancer. PLoS ONE 2011, 6, e28423. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, J.S.; Ye, F.; Cheng, X.D.; Zhou, C.Y.; Lü, W.G.; Xie, X. Reduced miR-100 expression in cervical cancer and precursors and its carcinogenic effect through targeting PLK1 protein. Eur. J. Cancer 2011, 47, 2166–2174. [Google Scholar] [CrossRef]
- Wang, P.; Zhai, G.; Bai, Y. Values of miR-34a and miR-218 expression in the diagnosis of cervical cancer and the prediction of prognosis. Oncol. Lett. 2018, 15, 3580–3585. [Google Scholar] [CrossRef]
- Ribeiro, J.; Marinho-Dias, J.; Monteiro, P.; Loureiro, J.; Baldaque, I.I.; Medeiros, R.; Sousa, H. miR-34a and miR-125b expression in HPV infection and cervical cancer development. BioMed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Yuan, C.; Cui, B.; Zou, X.; Qiao, Y. High-risk human papillomavirus reduces the expression of MicroRNA-218 in women with cervical intraepithelial neoplasia. J. Int. Med Res. 2010, 38, 1730–1736. [Google Scholar] [CrossRef]
- Ofir, M.; Hacohen, D.; Ginsberg, D. miR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E. Mol. Cancer Res. 2011, 9, 440–447. [Google Scholar] [CrossRef]
- Han, M.-S.; Lee, J.M.; Kim, S.-N.; Kim, J.-H.; Kim, H.-S. Human Papillomavirus 16 oncoproteins downregulate the expression of miR-148a-3p, miR-190a-5p, and miR-199b-5p in cervical cancer. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Jayamohan, S.; Kannan, M.; Moorthy, R.K.; Rajasekaran, N.; Jung, H.S.; Shin, Y.K.; Arockiam, A.J.V. Dysregulation of miR-375/AEG-1 axis by human Papillomavirus 16/18-E6/E7 promotes cellular proliferation, migration, and invasion in cervical cancer. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Graham, S.V.; Faizo, A.A.A. Control of human papillomavirus gene expression by alternative splicing. Virus Res. 2017, 231, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, Q.; Lin, L.; Xu, C. MiR-875 and miR-3144 switch the human papillomavirus 16 E6/E6 * mRNA ratio through the EGFR pathway and a direct targeting effect. Gene 2018, 679, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Pietilä, T.; Rönty, M.; Michon, F.; Frilander, M.J.; Ritari, J.; Tarkkanen, J.; Paulín, L.; Auvinen, P.; Auvinen, E. Identification and validation of human Papillomavirus encoded microRNAs. PLoS ONE 2013, 8, e70202. [Google Scholar] [CrossRef] [PubMed]
- Chirayil, R.; Kincaid, R.P.; Dahlke, C.; Kuny, C.; Dälken, N.; Spohn, M.; Lawson, B.; Grundhoff, A.; Sullivan, C.S. Identification of virus-encoded microRNAs in divergent Papillomaviruses. PLoS Pathog. 2018, 14, e1007156. [Google Scholar] [CrossRef]
- Virtanen, E.; Pietilä, T.; Nieminen, P.; Qian, K.; Auvinen, E. Low expression levels of putative HPV encoded microRNAs in cervical samples. SpringerPlus 2016, 5, 1856. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Han, Y.; Song, C.; Wei, H.; Chen, Y.; Huang, K.; Li, S.; Ma, D.; Wang, S.; Wang, J.; et al. Systematic review and meta-analysis of the prognostic significance of microRNAs in cervical cancer. Oncotarget 2017, 9, 17141–17148. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Y.; Huang, Y.; Yu, H.; Huang, Y.; Wu, L.; Huang, L. Up-regulated miR155 reverses the epithelial-mesenchymal transition induced by EGF and increases chemo-sensitivity to cisplatin in human caski cervical cancer cells. PLoS ONE 2012, 7, e52310. [Google Scholar] [CrossRef]
- Qin, W.; Dong, P.; Ma, C.; Mitchelson, K.; Deng, T.; Zhang, L.; Sun, Y.; Feng, X.; Ding, Y.; Lu, X.; et al. MicroRNA-133b is a key promoter of cervical carcinoma development through the activation of the ERK and AKT1 pathways. Oncogene 2012, 31, 4067–4075. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Y.; Caramuta, S.; Larsson, C.; Lui, W.-O. miR-205 expression promotes cell proliferation and migration of human cervical cancer cells. PLoS ONE 2012, 7, e46990. [Google Scholar] [CrossRef]
- Zhang, L.; Zhan, X.; Yan, D.; Wang, Z. Circulating microRNA-21 is involved in lymph node metastasis in cervical cancer by targeting RASA1. Int. J. Gynecol. Cancer 2016, 26, 810–816. [Google Scholar] [CrossRef]
- Cheng, Y.; Geng, L.; Zhao, L.; Zuo, P.; Wang, J. Human papillomavirus E6-regulated microRNA-20b promotes invasion in cervical cancer by targeting tissue inhibitor of metalloproteinase 2. Mol. Med. Rep. 2017, 16, 5464–5470. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Mi, Y.; Jin, H.; Cao, J.; Li, H.; Han, L.; Huang, T.; Zhang, X.; Ren, S.; et al. microRNA-499a promotes the progression and chemoresistance of cervical cancer cells by targeting SOX6. Apoptosis 2020, 25, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lee, L.; Scicluna, P.; Kavak, E.; Larsson, C.; Sandberg, R.; Lui, W.-O. Novel functions and targets of miR-944 in human cervical cancer cells. Int. J. Cancer 2014, 136, E230–E241. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.L.A.; Tsang, T.Y.; Yau, P.L.; Kwok, T.T. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene 2011, 30, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.T.; Leung, C.O.; Ye, T.-M.; Liu, W.; Chiu, P.C.; Lam, K.K.; Lee, K.-F.; Yeung, W.S. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinog. 2010, 31, 1037–1044. [Google Scholar] [CrossRef]
- Geng, D.; Song, X.; Ning, F.; Song, Q.; Yin, H. MiR-34a Inhibits viability and invasion of human Papillomavirus-positive cervical cancer cells by targeting E2F3 and regulating surviving. Int. J. Gynecol. Cancer 2015, 25, 707–713. [Google Scholar] [CrossRef]
- Fan, Z.; Cui, H.; Xu, X.; Lin, Z.; Zhang, X.; Kang, L.; Han, B.; Meng, J.; Yan, Z.; Yan, X.; et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget 2015, 6, 25266–25280. [Google Scholar] [CrossRef]
- Natalia, M.-A.; Alejandro, G.-T.; Virginia, T.-V.J.; Alvarez-Salas, L.M.; Marat, A.-S.L. MARK1 is a novel target for miR-125a-5p: Implications for cell migration in cervical tumor cells. MicroRNA 2018, 7, 54–61. [Google Scholar] [CrossRef]
- Xue, M.; Qin, X.; Wan, Y.; Wang, S. MicroRNA-125a-5p modulates human cervical carcinoma proliferation and migration by targeting ABL2. Drug Des. Dev. Ther. 2015, 10, 71–79. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Wang, Z.; Cai, H. MiR-195 inhibits the proliferation of human cervical cancer cells by directly targeting cyclin D1. Tumor Biol. 2016, 37, 6457–6463. [Google Scholar] [CrossRef]
- Zhou, Q.; Han, L.R.; Zhou, Y.X.; Li, Y. MiR-195 suppresses cervical cancer migration and invasion through targeting Smad3. Int. J. Gynecol. Cancer 2016, 26, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kinoshita, T.; Nohata, N.; Itesako, T.; Yoshino, H.; Enokida, H.; Nakagawa, M.; Shozu, M.; Seki, N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int. J. Oncol. 2013, 42, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Kogo, R.; How, C.; Chaudary, N.; Bruce, J.; Shi, W.; Hill, R.P.; Zahedi, P.; Yip, K.W.; Liu, F.-F. The microRNA-218~Survivin axis regulates migration, invasion, and lymph node metastasis in cervical cancer. Oncotarget 2014, 6, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Song, Q.; Zeng, R.; Li, J.; Li, J.; Lin, X.; Chen, X.; Zhang, J.; Zheng, Y. MicroRNA-218 inhibits EMT, migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical cancer. Oncotarget 2016, 7, 45622–45636. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, H.; Jia, C.Y.; Cheng, W.; Yu, M.; Peng, M.; Zhu, Y.; Zhao, Q.; Dong, Y.W.; Shao, K.; et al. MicroRNA-223 regulates FOXO1 expression and cell proliferation. FEBS Lett. 2012, 586, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Zhou, J.; Xu, J.; Peng, C.; Ye, F.; Shen, Y.; Lu, W.; Wan, X.; Xie, X. miR-375 Is Down-regulated in squamous cervical cancer and inhibits cell migration and invasion via targeting transcription factor SP1. Am. J. Pathol. 2011, 179, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Chen, G.; Wang, Y.; Zhang, L.; Long, Y.; Yuan, M.; Yang, D.; Liu, S.; Cheng, Y.; Zhang, L. Identification of candidate biomarkers correlated with the diagnosis and prognosis of cervical cancer via integrated bioinformatics analysis. OncoTargets Ther. 2019, 12, 4517–4532. [Google Scholar] [CrossRef]
- Chen, S.; Gao, C.; Wu, Y.; Huang, Z. Identification of prognostic miRNA signature and lymph node metastasis-related key genes in cervical cancer. Front. Pharmacol. 2020, 11, 544. [Google Scholar] [CrossRef]
- Wang, F.; Liu, M.; Li, X.; Tang, H. MiR-214 reduces cell survival and enhances cisplatin-induced cytotoxicity via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett. 2013, 587, 488–495. [Google Scholar] [CrossRef]
- Chen, Y.; Ke, G.; Han, D.; Liang, S.; Yang, G.; Wu, X. MicroRNA-181a enhances the chemoresistance of human cervical squamous cell carcinoma to cisplatin by targeting PRKCD. Exp. Cell Res. 2014, 320, 12–20. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Wang, X.; Chen, L. Up-regulation of microRNA-664 inhibits cell growth and increases cisplatin sensitivity in cervical cancer. Int. J. Clin. Exp. Med. 2015, 8, 18123–18129. [Google Scholar] [PubMed]
- Yang, H.; Wu, X.-L.; Wu, K.-H.; Zhang, R.; Ju, L.-L.; Ji, Y.; Zhang, Y.-W.; Xue, S.-L.; Zhang, Y.-X.; Yang, Y.-F.; et al. MicroRNA-497 regulates cisplatin chemosensitivity of cervical cancer by targeting transketolase. Am. J. Cancer Res. 2016, 6, 2690–2699. [Google Scholar] [PubMed]
- Shen, Y.; Wang, P.; Li, Y.; Ye, F.; Wang, F.; Wan, X.; Cheng, X.; Lu, W.; Xie, X. miR-375 is upregulated in acquired paclitaxel resistance in cervical cancer. Br. J. Cancer 2013, 109, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Cui, H.; Yu, H.; Ji, Q.; Kang, L.; Han, B.; Wang, J.; Dong, Q.; Li, Y.; Yan, Z.; et al. MiR-125a promotes paclitaxel sensitivity in cervical cancer through altering STAT3 expression. Oncogenesis 2016, 5, e197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, L.; Liu, S.; Zeng, S.; Zhang, L. miR-375 modulates radiosensitivity of HR-HPV-positive cervical cancer cells by targeting UBE3A through the p53 pathway. Med Sci. Monit. 2015, 21, 2210–2217. [Google Scholar] [CrossRef]
- Song, L.; Song, L.; Zhang, L.; Yao, H.; Gao, F.; Xu, D.; Lili, S. MiR-21 modulates radiosensitivity of cervical cancer through inhibiting autophagy via the PTEN/Akt/HIF-1α feedback loop and the Akt-mTOR signaling pathway. Tumor Biol. 2016, 37, 12161–12168. [Google Scholar] [CrossRef]
- Pedroza-Torres, A.; Campos-Parra, A.D.; Millan-Catalan, O.; Loissell-Baltazar, Y.; Zamudio-Meza, H.; De Leï, N.D.C.; Montalvo-Esquivel, G.; Isla-Ortiz, D.; Herrera, L.; Ngeles-Zaragoza, I.; et al. MicroRNA-125 modulates radioresistance through targeting p21 in cervical cancer. Oncol. Rep. 2018. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, X.; Huo, D.; Zhao, Y.; Zhang, H. MiR-449b-5p regulates cell proliferation, migration and radioresistance in cervical cancer by interacting with the transcription suppressor FOXP1. Eur. J. Pharmacol. 2019, 856, 172399. [Google Scholar] [CrossRef]
- Ding, F.; Gao, B.; Wu, X.; Gong, C.; Wang, W.; Zhang, S. miR-122-5p modulates the radiosensitivity of cervical cancer cells by regulating cell division cycle 25A (CDC25A). FEBS Open Bio 2019, 9, 1869–1879. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Wright, T.C.; Stoler, M.H.; Behrens, C.M.; Sharma, A.; Zhang, G.; Wright, T.L. Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test. Gynecol. Oncol. 2015, 136, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Cobucci, R.; Maisonnette, M.; Macêdo, E.; Filho, F.S.; Rodovalho, P.; Nóbrega, M.; Gonçalves, A. Pap test accuracy and severity of squamous intraepithelial lesion. Indian J. Cancer 2016, 53, 74. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Bergeron, C.; Klinkhamer, P.; Martin-Hirsch, P.; Siebers, A.G.; Bulten, J. Liquid compared with conventional cervical cytology. Obstet. Gynecol. 2008, 111, 167–177. [Google Scholar] [CrossRef]
- Siebers, A.G.; Klinkhamer, P.J.J.M.; Grefte, J.M.M.; Massuger, L.F.; Vedder, J.E.M.; Beijers-Broos, A.; Bulten, J.; Arbyn, M. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors. JAMA 2009, 302, 1757–1764. [Google Scholar] [CrossRef]
- Katki, H.A.; Schiffman, M.; Castle, P.E.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Cheung, L.C.; Raine-Bennett, T.; Gage, J.C.; Kinney, W.K. Five-year risks of CIN 3+ and cervical cancer among women who test pap-negative but are HPV-positive. J. Low. Genit. Tract Dis. 2013, 17, S56–S63. [Google Scholar] [CrossRef]
- Conrad, R.D.; Liu, A.H.; Wentzensen, N.; Zhang, R.R.; Dunn, S.T.; Wang, S.S.; Schiffman, M.; Gold, M.A.; Walker, J.L.; Zuna, R.E. Cytologic patterns of cervical adenocarcinomas with emphasis on factors associated with underdiagnosis. Cancer Cytopathol. 2018, 126, 950–958. [Google Scholar] [CrossRef]
- Wentzensen, N.; Fetterman, B.; Castle, P.E.; Schiffman, M.; Wood, S.N.; Stiemerling, E.; Tokugawa, D.; Bodelon, C.; Poitras, N.; Lorey, T.; et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J. Natl. Cancer Inst. 2015, 107, djv257. [Google Scholar] [CrossRef]
- Floore, A.; Hesselink, A.; Oštrbenk, A.; Alcaniz, E.; Rothe, B.; Pedersen, H.; Hortal, M.T.; Doorn, S.; Quint, W.; Petry, K.U.; et al. Intra- and inter-laboratory agreement of the FAM19A4/mir124-2 methylation test: Results from an international study. J. Clin. Lab. Anal. 2019, 33, e22854. [Google Scholar] [CrossRef]
- Dick, S.; Kremer, W.W.; De Strooper, L.M.; Lissenberg-Witte, B.I.; Steenbergen, R.D.; Meijer, C.J.; Berkhof, J.; Heideman, D.A.M. Long-term CIN3+ risk of HPV positive women after triage with FAM19A4/miR124-2 methylation analysis. Gynecol. Oncol. 2019, 154, 368–373. [Google Scholar] [CrossRef]
- Liu, S.S.; Chan, K.K.L.; Chu, D.K.H.; Wei, T.N.; Lau, L.S.K.; Ngu, S.F.; Chu, M.M.Y.; Tse, K.Y.; Ip, P.P.C.; Ng, E.K.O.; et al. Oncogenic micro RNA signature for early diagnosis of cervical intraepithelial neoplasia and cancer. Mol. Oncol. 2018, 12, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wu, Y.; Zhang, Q.; Gao, G.; Zhang, C.; Xiang, Y. Expression profile of circulating microRNAs as a promising fingerprint for cervical cancer diagnosis and monitoring. Mol. Clin. Oncol. 2015, 3, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Nagamitsu, Y.; Nishi, H.; Sasaki, T.; Takaesu, Y.; Terauchi, F.; Isaka, K. Profiling analysis of circulating microRNA expression in cervical cancer. Mol. Clin. Oncol. 2016, 5, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Liu, P.; Ma, C.-F. A circulating serum miRNA panel as early detection biomarkers of cervical intraepithelial neoplasia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4846–4851. [Google Scholar] [PubMed]
- Zheng, M.; Hou, L.; Ma, Y.; Zhou, L.; Wang, F.; Cheng, B.; Wang, W.; Lu, B.; Liu, P.; Lu, W.; et al. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol. Cancer 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Trakunram, K.; Champoochana, N.; Chaniad, P.; Thongsuksai, P.; Raungrut, P. MicroRNA isolation by trizol-based method and its stability in stored serum and cDNA derivatives. Asian Pac. J. Cancer Prev. 2019, 20, 1641–1647. [Google Scholar] [CrossRef]
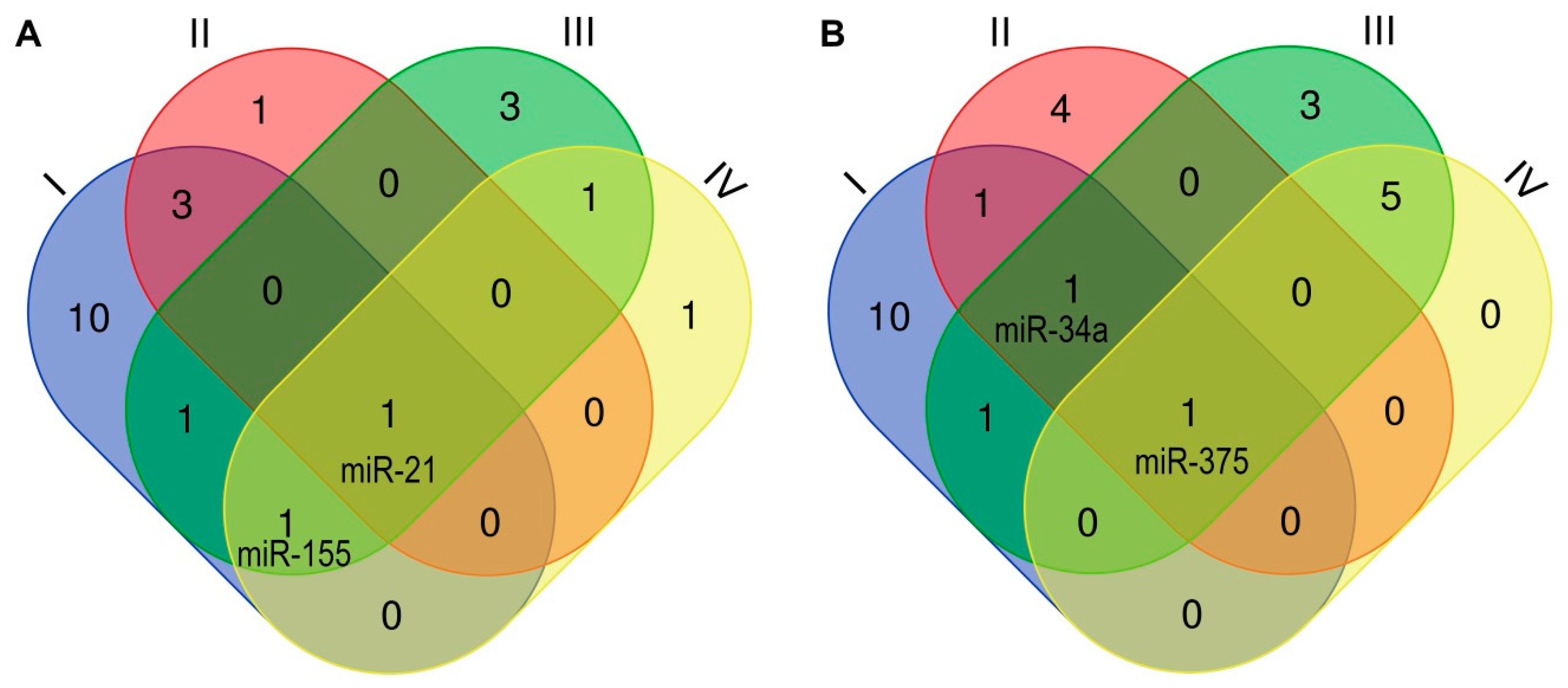
| Early Transient [References] | Early Continuous [References] | Late [References] | |
|---|---|---|---|
| Overexpressed | miR-10a [86,93] | miR-10a [88] | miR-9 [86,90,93,95,102] |
| miR-34b [93] | miR-34b [86] | miR-15a [86,90] | |
| miR-28 [86,102] | miR-15b [85,86,89,90,102] | ||
| miR-92a [86,87,89,103] | miR-17 [86,89,90] | ||
| miR-93 [89,103] | miR-20b [86,89,90,93,94,104] | ||
| miR-16 [92] | miR-16 [85,86,87,88,89,90,97] | ||
| miR-21 [86,92,98,105] | miR-21 [90,95,97] | ||
| miR-25 [86] | miR-25 [87,89] | ||
| miR-141 [92] | miR-141 [86,90,94] | ||
| miR-155 [89] | miR-155 [85,86,95] | ||
| miR-196a [88,96] | miR-27a 1 [87,88] | ||
| miR-200a [86,94,97] | miR-31 [90,94,99] | ||
| miR-106a [86,88,89] | |||
| miR-106b [90,92,97] | |||
| miR-124 [91,92] | |||
| miR-142-5p [88,94] | |||
| miR-223 [85,92,97] | |||
| miR-224 [89,94] | |||
| Underexpressed | miR-100 [87] | miR-100 [89,94,106] | miR-100 [86,102] |
| miR-29a 1 [87,88,89] | miR-10b [89,94] | ||
| miR-34a 1 [89,103] | miR-34a 1 [100,107,108] | ||
| miR-99a [88,89] | miR-99a [86,94,97] | ||
| miR-125b [108] | miR-125b [86,89,97,102] | ||
| miR-195 [89,90,94] | miR-195 [86] | ||
| miR-199a [88] | miR-199a [86] | ||
| miR-218 [89,90,92,103,109] | miR-218 [86,91,94,107] | ||
| miR-375 [89,101,103] | miR-375 [86,102] | ||
| miR-497 [90,94] | miR-497 [86] | ||
| miR-513 [88] | miR-513 [86] | ||
| miR-143 1 [88,94] | miR-143 1 [85] | ||
| miR-145 [88] | miR-145 [85] | ||
| miR-424 [89,93,103] | miR-424 [85] | ||
| miR-145 1 [88,89,94] | miR-376 [86,90,94] | ||
| miR-149 [86,102] | |||
| miR-193b 2 [86,97] | |||
| miR-203 [86,88,97,100,102] |
| miRNA | Target | Processes Related to Metastization | Reference | |
|---|---|---|---|---|
| Overexpressed | miR-20b | TIMP metallopeptidase inhibitor 2 (TIMP2) | Migration, invasion, EMT modulation | [123] |
| miR-21 | RAS p21 protein activator 1 (RASA1) | Migration, invasion | [122] | |
| miR-133b | Mammalian sterile 20-like kinase 2 (MST2), Cell division control protein 42 homolog (CDC42), Ras homolog gene family member A (RHOA) | Proliferation, colony formation | [120] | |
| miR-155 | Tumor protein p53 (TP53), mothers against decapentaplegic homolog 2 (SMAD2), cyclin D1 (CCND1) Epidermal growth factor (EGF) | Migration, invasion EMT modulation | [119] | |
| miR-205 | Connective tissue growth factor (CTGF), cysteine-rich angiogenic inducer 61 (CYR61) | Proliferation, migration | [121] | |
| miR-499a | Sex-determining region Y-box 6 (SOX6) | Proliferation, migration, invasion | [124] | |
| miR-944 | HECT domain ligase W2 (HECW2), S100P-binding protein (S100PBP) | Proliferation, migration, invasion | [125] | |
| Underexpressed | miR-23b | Urokinase plasminogen activator (uPA) | Migration, EMT modulation | [126] |
| miR-34a | Notch receptor 1 (NOTCH1), jagged canonical notch ligand 1 (JAGGED1), E2F transcription factor 3 (E2F3) | Invasion, EMT modulation | [127] [128] | |
| miR-125a | Signal transducer and activator of transcription 3 (STAT3) Microtubule affinity regulating kinase 1 (MARK1), ABL proto-oncogene 2, non-receptor tyrosine kinase (ABL2) | Migration, proliferation, EMT modulation | [129] [130] [131] | |
| miR-195 | Cyclin D1, mothers against decapentaplegic homolog 3 (SMAD3) | Proliferation, migration, invasion | [132] [133] | |
| miR-218 | Laminin subunit beta 3 (LAMB3), baculoviral IAP repeat-containing 5 (BIRC5), Scm-like with four Mbt domains 1 (SFMBT1), defective in cullin neddylation 1 domain-containing 1 (DCUN1D1) | Migration, invasion, EMT modulation | [134] [135] [136] | |
| miR-223 | Forkhead box O1 (FOXO1) | Proliferation, EMT modulation | [137] | |
| miR-375 | Sp1 transcription factor (SP1), Astrocyte elevated gene-1 (AEG-1) | Proliferation, migration, invasion, EMT modulation | [138] [112] |
| miRNA | Target | Response to Treatment | Reference | |
|---|---|---|---|---|
| Overexpressed | miR-21 | Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) | Radioresistance | [148] |
| miR-125 | p21 | Radioresistance | [149] | |
| miR-181a | Protein kinase C delta (PRKCD) | Resistance to cisplatin | [142] | |
| miR-375 | Not specified | Acquired resistance to paclitaxel | [145] | |
| miR-499a | SRY-box transcription factor 6 (SOX6) | Resistance to cisplatin | [124] | |
| Underexpressed | miR-122-5p | Cell division cycle 25A (CDC25A) | Radioresistance | [151] |
| miR-125a | Signal transducer and activator of transcription 3 (STAT3) | Resistance to cisplatin and paclitaxel | [146] | |
| miR-155 | Epidermal growth factor (EGF) SMAD family member 2 (SMAD2) Cyclin D1 (CCND1) | Resistance to cisplatin | [119] | |
| miR-214 | BCL2-like 2 (Bcl2L2) | Resistance to cisplatin | [141] | |
| miR-375 | Ubiquitin protein ligase E3A (UBE3A) | Radioresistance | [147] | |
| miR-449b-5p | Forkhead box P1 (FOXP1) | Radioresistance | [150] | |
| miR-497 | Transketolase (TKT) | Resistance to cisplatin | [144] | |
| miR-664 | E-cadherin | Resistance to cisplatin | [143] |
| Clinical Stage | Tested miRNA | AUC | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|
| LSIL | miR-451a miR-144-3p | 0.850 0.850 | 76.0 68.0 | 82.0 89.0 | [104] |
| miR-183 6 miR pattern | 0.990 0.998 | 95.0 97.9 | 97.0 98.6 | [163] | |
| HSIL | miR-451a miR-144-3p | 0.870 0.870 | 80.0 75.0 | 82.0 88.0 | [104] |
| miR-424 miR-424/375/218 | 0.840 0.874 | 76.0 74.4 | 78.1 85.3 | [103] | |
| miR-183 6 miR pattern | 0.980 0.996 | 92.0 97.2 | 92.0 96.6 | [163] | |
| CC | miR-451a miR-144-3p | 0.940 0.930 | 83.0 87.0 | 91.0 89.0 | [104] |
| miR-125b | 0.802 | 88.0 | 69.0 | [108] | |
| miR-141 6 miR pattern | 0.942 0.959 | 82.8 91.4 | 91.7 87.6 | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisarska, J.; Baldy-Chudzik, K. MicroRNA-Based Fingerprinting of Cervical Lesions and Cancer. J. Clin. Med. 2020, 9, 3668. https://doi.org/10.3390/jcm9113668
Pisarska J, Baldy-Chudzik K. MicroRNA-Based Fingerprinting of Cervical Lesions and Cancer. Journal of Clinical Medicine. 2020; 9(11):3668. https://doi.org/10.3390/jcm9113668
Chicago/Turabian StylePisarska, Justyna, and Katarzyna Baldy-Chudzik. 2020. "MicroRNA-Based Fingerprinting of Cervical Lesions and Cancer" Journal of Clinical Medicine 9, no. 11: 3668. https://doi.org/10.3390/jcm9113668
APA StylePisarska, J., & Baldy-Chudzik, K. (2020). MicroRNA-Based Fingerprinting of Cervical Lesions and Cancer. Journal of Clinical Medicine, 9(11), 3668. https://doi.org/10.3390/jcm9113668





