Information Recall in Pre-Operative Consultation for Glioma Surgery Using Actual Size Three-Dimensional Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Procedure
2.3. Visualization Tools
2.4. Analysis
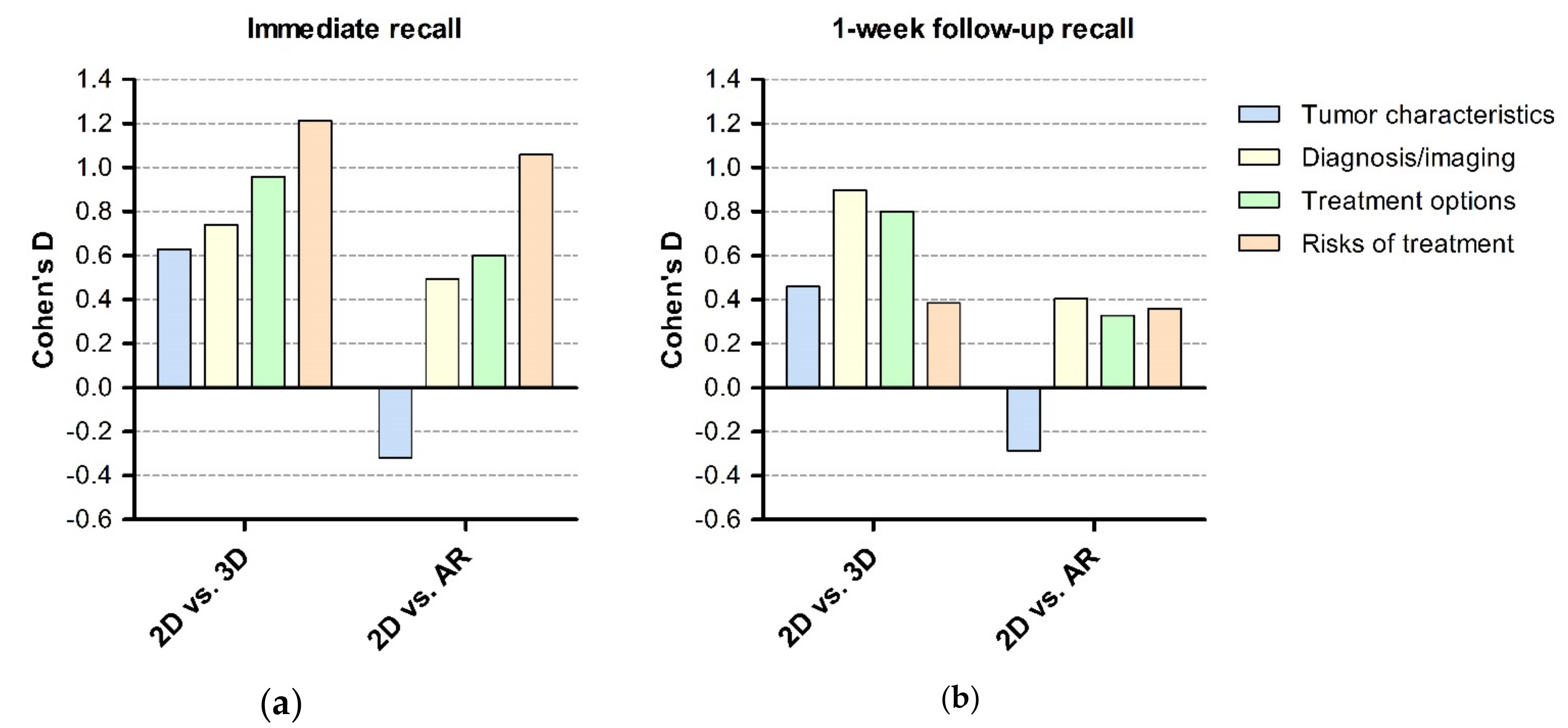
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Pre-Developed Script
General Information (All Groups)
- Lesion found on imaging, suspected of low-grade glioma.
- There are four different grades and the lesion is suspected to be a grade 2 glioma but inevitably grows into a higher grade.
- Surgery provides better prognosis than a wait and see approach. This is why we will discuss the options for surgery and its risks.
- The tumor pathology will give more insight into whether post-operative treatment (chemotherapy, radiotherapy) are indicated.
- To assess the possible risks, we made a functional MRI (fMRI) to map the functional areas and tracts related to the tumor. During the fMRI, they had to tap their fingers, move their toes, think of different words and, finally, say different verbs out loud.
Group 1
- Left and right are reversed.
- T2 FLAIR: The tumor size (mention the maximum diameter, further specified in the script for each case) and location (brain lobe).
- fMRI: activation of functional areas (e.g., hand-, leg-, lip-, tongue function and speech), tractography (e.g., corticospinal and superior longitudinal fasciculus) and their function. Their relation to the tumor. The enhancing areas that display the artifacts.
Group 2 and 3
- •
- Left and right are reversed.
- •
- T2 FLAIR: The tumor size (mention the maximum diameter, further specified in the script for each case) and location (brain lobe).
- •
- fMRI: activation of functional areas (e.g., hand, leg, lip, tongue function and speech), tractography (e.g., corticospinal and superior longitudinal fasciculus) and their function. Their relation to the tumor. The enhancing areas that display the artifacts.
- •
- The tumor, relevant brain areas and tractography are made into a 3D model:
- ◦
- Group 2: The 3D model is printed. Different colors represent different areas (red = tumor, orange = motor function (hand, foot, lips), blue = speech, yellow = motor tract, purple = speech tract). Orient the model so that it projects how it is displayed in the participants head.
- ◦
- Group 3: the 3D model is made into an AR model, shown on the iPad. Different colors represent different areas (red = tumor, orange = motor function (hand, foot, lips), blue = speech, yellow = motor tract, purple = speech tract). Show the model first with and then without the surrounding cerebrum. Hand the iPad to the participant, who is free to move around to see the tumor from different angles.
Treatment Options and Risks (All Groups)
- •
- There are four treatment options: watchful waiting, biopsy, partial resection (debulking) and complete resection.
- •
- Watchful waiting means following the tumor through scanning after a set number of months and checking for tumor growth. This eliminates all risks related to surgery, but is not the preferred option, since the tumor will grow and develop into a higher grade.
- •
- Biopsy means taking multiple small pieces of the tumor through a small burr hole in the skull. This gives us more information about the diagnosis, but with less risks than extensive surgery. Risks (chances are approximately 1% of happening) include bleeding followed by neurological deficit (paralysis or speech deficit), epilepsy and infection. Also, the amount of tumor that is removed during surgery is minimal, thus the advantages for prognosis seen in resection do not apply for biopsy. The tumor can be heterogenous, so there can be a risk of sampling error (meaning not getting the exact diagnosis). Finally, there is a small risk that the biopsy is insufficient after which a second biopsy is needed.
- ◦
- Show the angle in which the biopsy will be taken (depending on the group either on MRI, 3D model of AR model).
- •
- Partial resection (debulking) means taking a piece of tumor that is safely possible. The opening is larger than in a biopsy. This way, we keep away from the relevant brain areas and tracts. While the chances are lower, there is still a risk for neurological deficit (paralysis or speech deficit) if bleeding occurs. This lower risk is an advantage (compared to complete resection), but the disadvantage is the limited resection and thus it does not carry the advantages for progression in complete resection.
- ◦
- Show the part of the tumor that will be removed in partial resection (debulking) surgery and its relation to the eloquent brain areas and tracts (depending on the group either on (f)MRI, 3D model of AR model).
- •
- Complete resection means that the aim is to remove as much tumor as safely possible. To this end, the functions will be monitored during surgery. For speech, the patient needs to be awake for some time during the surgery. Complete resection means that a large part of the tumor cannot evolve into a higher grade. However, it is impossible to resect one hundred percent, since the infiltrative growth. With the large amount of tumor that is resected, the right diagnosis will be made. It is known that this surgery provides the best prognosis. The disadvantage is the highest risk chance: bleeding (2%), permanent neurological deficit (10%), temporary neurological deficit (50%), epilepsy and infection.
- ◦
- Show the functional areas and mention that these functions will be monitored during surgery (depending on the group either on (f)MRI, 3D model of AR model).
- ◦
- Explain the details of an awake procedure: pre-operative preparing through psychological tests and exercises (similar to the exercises during surgery), peri-operative procedure (sleeping during surgery preparation i.e., catheter and head clamp placement, opening of skin and skull, awake during testing and sleeping during closing).
Appendix B. Questionnaire
Subdomain 1: Tumor Characteristics
- What type of tumor is shown?
- What is the tumor grade?
- On which side is the tumor located?
- In which lobe is the tumor located?
- What is the approximate diameter of the tumor?
Subdomain 2: Imaging and Diagnosis
- Select one or more from the following options:
- Which of the following functions is/are tested during the functional MRI?
- Which of the following functions is/are located in close relation to tumor?
- Which of the following brain tracts is/are located in close relation to tumor?
Subdomain 3: Treatment Options
- Describe the different treatment options that the doctor discussed with you.
- What does ‘watchfull waiting’ mean?
- What is the advantage of watchfull waiting?
- What is the disadvantage of watchfull waiting?
- 3What is a ‘biopsy’?
- What is the goal of a biopsy?
- Select one or more from the following options: What is/are the advantage(s) of biopsy compared to tumor removal?
- Select one or more from the following options: What is/are the disadvantage(s) of biopsy compared to tumor removal?
- What does ‘tumor resection’ mean?
- What is the difference between partial and complete resection?
- Select one or more from the following options: What is/are the advantage(s) of complete resection?
- Select one or more from the following options: What is/are the disadvantage(s) of complete resection?
- At what point will surgery be stopped during tumor resection?
- How is damage to relevant brain areas prevented during surgery?
- Select one or more from the following options: Which function(s) is/are monitored during surgery?
Subdomain 4: Risks of Treatment
- Select one or more from the following options: Which complication(s) can occur after a biopsy?
- Select one or more from the following options: Which complication(s) can occur after tumor resection?
- Select one or more from the following options: Which function(s) is/are at risk during complete resection surgery?
References
- World Health Organization. Health Promotion Glossary; Education and Communications (HPR); Health Education and Health Promotion Unit (HEP); World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Yeh, M.Y.; Wu, S.C.; Tung, T.H. The relation between patient education, patient empowerment and patient satisfaction: A cross-sectional-comparison study. Appl. Nurs. Res. 2018, 39, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.; Hetrick, S.; Jorm, A. Shared decision-making: Benefits, barriers and current opportunities for application. Australas. Psychiatry 2010, 18, 394–397. [Google Scholar] [CrossRef]
- Rutten, L.J.; Arora, N.K.; Bakos, A.D.; Aziz, N.; Rowland, J. Information needs and sources of information among cancer patients: A systematic review of research (1980–2003). Patient Educ. Couns. 2005, 57, 250–261. [Google Scholar] [CrossRef]
- Ha, J.F.; Longnecker, N. Doctor-patient communication: A review. Ochsner J. 2010, 10, 38–43. [Google Scholar] [PubMed]
- Kessels, R.P.C. Patients’ memory for medical information. J. R. Soc. Med. 2003, 96, 219–222. [Google Scholar] [PubMed]
- McGuire, L.C. Remembering what the doctor said: Organization and adults’ memory for medical information. Exp. Aging Res. 1996, 22, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.W.B.; McKinstry, B. A systematic review of interventions to improve recall of medical advice in healthcare consultations. J. R. Soc. Med. 2009, 102, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.E.; Wellings, R.; Griffiths, F.; Hutchinson, C.; Kunar, M. Do medical images aid understanding and recall of medical information? An experimental study comparing the experience of viewing no image, a 2D medical image and a 3D medical image alongside a diagnosis. Patient Educ. Couns. 2017, 100, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.D.; Zhou, M.C.; Liu, S.C.; Wu, J.F.; Wang, R.; Chen, C.M. Effectiveness of personalized 3D printed models for patient education in degenerative lumbar disease. Patient Educ. Couns. 2019, 102, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Wake, N.; Rosenkrantz, A.B.; Huang, R.; Park, K.U.; Wysock, J.S.; Taneja, S.S.; Huang, W.C.; Sodickson, D.K.; Chandarana, H. Patient-specific 3D printed and augmented reality kidney and prostate cancer models: Impact on patient education. 3D Print Med. 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.S.; Choi, C.H.; Han, I.H.; Lee, J.H.; Choi, H.J.; Lee, J.I. Obtaining Informed Consent Using Patient Specific 3D Printing Cerebral Aneurysm Model. J. Korean Neurosurg Soc. 2019, 62, 398–404. [Google Scholar] [CrossRef] [PubMed]
- van de Belt, T.H.; Nijmeijer, H.; Grim, D.; Engelen, L.; Vreeken, R.; van Gelder, M.; Ter Laan, M. Patient-Specific Actual-Size Three-Dimensional Printed Models for Patient Education in Glioma Treatment: First Experiences. World Neurosurg. 2018, 117, e99–e105. [Google Scholar] [CrossRef] [PubMed]
- Biglino, G.; Koniordou, D.; Gasparini, M.; Capelli, C.; Leaver, L.K.; Khambadkone, S.; Schievano, S.; Taylor, A.M.; Wray, J. Piloting the Use of Patient-Specific Cardiac Models as a Novel Tool to Facilitate Communication During Cinical Consultations. Pediatr. Cardiol. 2017, 38, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, J.L.; Maddox, M.M.; Dorsey, P.; Feibus, A.; Thomas, R.; Lee, B.R. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: A pilot study. Urology 2014, 84, 268–272. [Google Scholar] [CrossRef]
- Sander, I.M.; Liepert, T.T.; Doney, E.L.; Leevy, W.M.; Liepert, D.R. Patient Education for Endoscopic Sinus Surgery: Preliminary Experience Using 3D-Printed Clinical Imaging Data. J. Funct. Biomater. 2017, 8. [Google Scholar] [CrossRef]
- Wessel, I.; van der Kooy, P.; Merckelbach, H. Differential recall of central and peripheral details of emotional slides is not a stable phenomenon. Memory 2000, 8, 95–109. [Google Scholar] [CrossRef]
- Ley, P. Memory for medical information. Br. J. Soc. Clin. Psychol 1979, 18, 245–255. [Google Scholar] [CrossRef]

| 2D (n = 21) | 3D (n = 20) | AR (n = 20) | Total (n = 61) | |
|---|---|---|---|---|
| Mean age (SD) | 41.1 (14.6) | 36.7 (15.7) | 41.1 (15.1) | 39.6 (15.1) |
| Gender | ||||
| • Men (%) | 7 (33) | 5 (25) | 9 (45) | 21 (34) |
| • Women (%) | 14 (67) | 15 (75) | 11 (55) | 40 (66) |
| Level of education | ||||
| • Lower vocational training (%) | 1 (5) | 1 (2) | ||
| • General secondary education (%) | 2 (10) | 2 (10) | 4 (7) | |
| • Higher professional training (%) | 13 (62) | 10 (50) | 10 (50) | 33 (54) |
| • Academic degree (%) | 7 (33) | 8 (40) | 8 (40) | 23 (37) |
| Pre-existing anatomical knowledge | ||||
| • 0 (none) | 4 (19) | 6 (30) | 7 (35) | 17 (28) |
| • 1 (television) | 11 (52) | 6 (30) | 5 (25) | 22 (36) |
| • 2 (books, own line of work) | 5 (24) | 7 (35) | 7 (35) | 19 (31) |
| • 3 (doctor)* | 1 (5) | 1 (5) | 1 (5) | 3 (5) |
| Professional background | ||||
| • Healthcare | 6 | 5 | 7 | 18 |
| • Education | 6 | 8 | 3 | 17 |
| • Sales, hospitality, recreation | 2 | 3 | 2 | 7 |
| • Construction | 2 | 0 | 1 | 3 |
| • Information technology (IT) | 0 | 1 | 2 | 3 |
| • Other/student | 5 | 3 | 5 | 13 |
| Mean amount of days between first and second assessment (SD) | 8.8 (1.7) | 8.2 (1.5) | 9.1 (2.6) | 8.7 (2.0) |
| 2D (n = 21) | 3D (n = 20) | AR (n = 20) | Total (n = 61) | |
|---|---|---|---|---|
| Immediate recall (95% CI) | 83.60 (81.74–85.47) | 89.91 (88.52–91.30) | 87.97 (86.14–89.81) | 87.10 (85.95–88.26) |
| 1-Week Follow-up (95% CI) | 83.05 (80.02–86.08) | 88.06 (86.41–89.72) | 84.89 (82.02–87.79) | 85.30 (83.78–86.81) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sezer, S.; Piai, V.; Kessels, R.P.C.; ter Laan, M. Information Recall in Pre-Operative Consultation for Glioma Surgery Using Actual Size Three-Dimensional Models. J. Clin. Med. 2020, 9, 3660. https://doi.org/10.3390/jcm9113660
Sezer S, Piai V, Kessels RPC, ter Laan M. Information Recall in Pre-Operative Consultation for Glioma Surgery Using Actual Size Three-Dimensional Models. Journal of Clinical Medicine. 2020; 9(11):3660. https://doi.org/10.3390/jcm9113660
Chicago/Turabian StyleSezer, Sümeyye, Vitoria Piai, Roy P.C. Kessels, and Mark ter Laan. 2020. "Information Recall in Pre-Operative Consultation for Glioma Surgery Using Actual Size Three-Dimensional Models" Journal of Clinical Medicine 9, no. 11: 3660. https://doi.org/10.3390/jcm9113660
APA StyleSezer, S., Piai, V., Kessels, R. P. C., & ter Laan, M. (2020). Information Recall in Pre-Operative Consultation for Glioma Surgery Using Actual Size Three-Dimensional Models. Journal of Clinical Medicine, 9(11), 3660. https://doi.org/10.3390/jcm9113660





