Which Are the Most Suitable Stents for Interventional Endoscopic Ultrasound?
Abstract
1. Introduction
2. Selection of Appropriate Stents for Endoscopic Ultrasound (EUS)-Guided Drainage Procedures
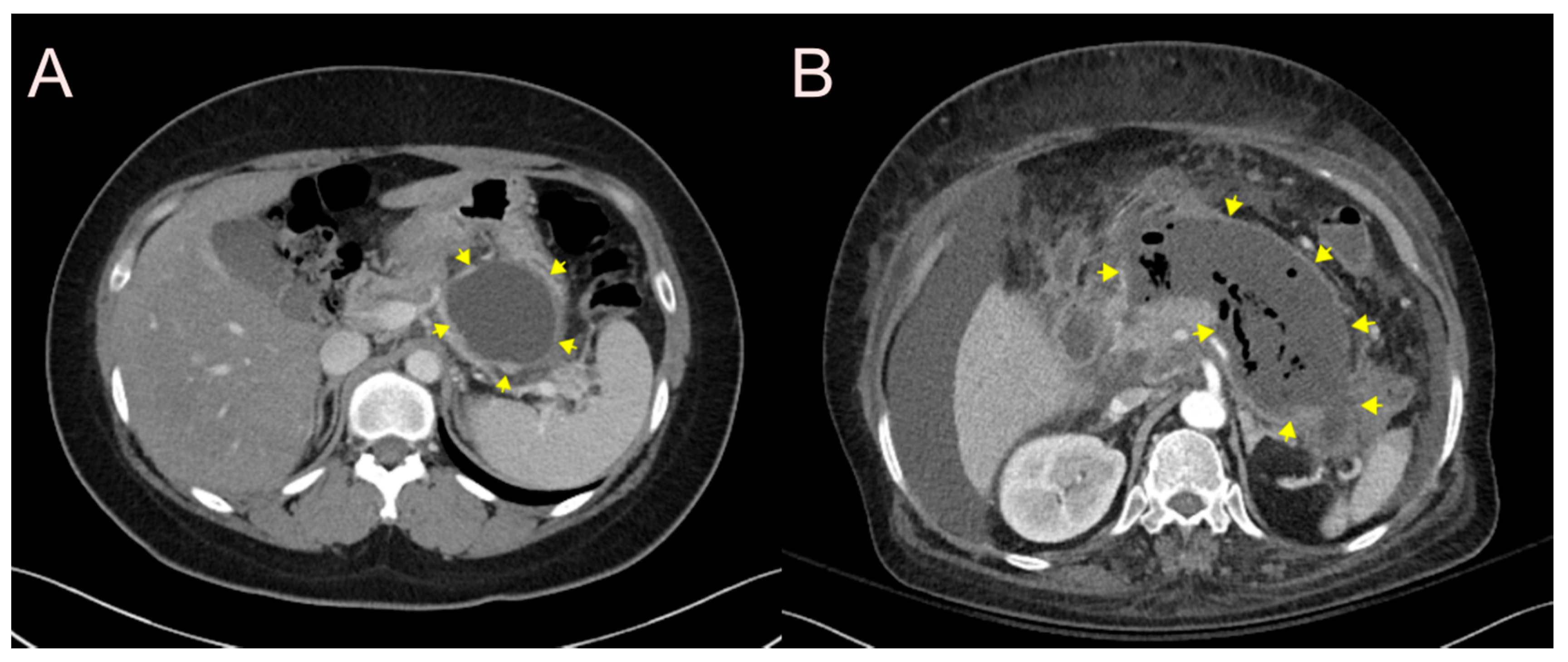
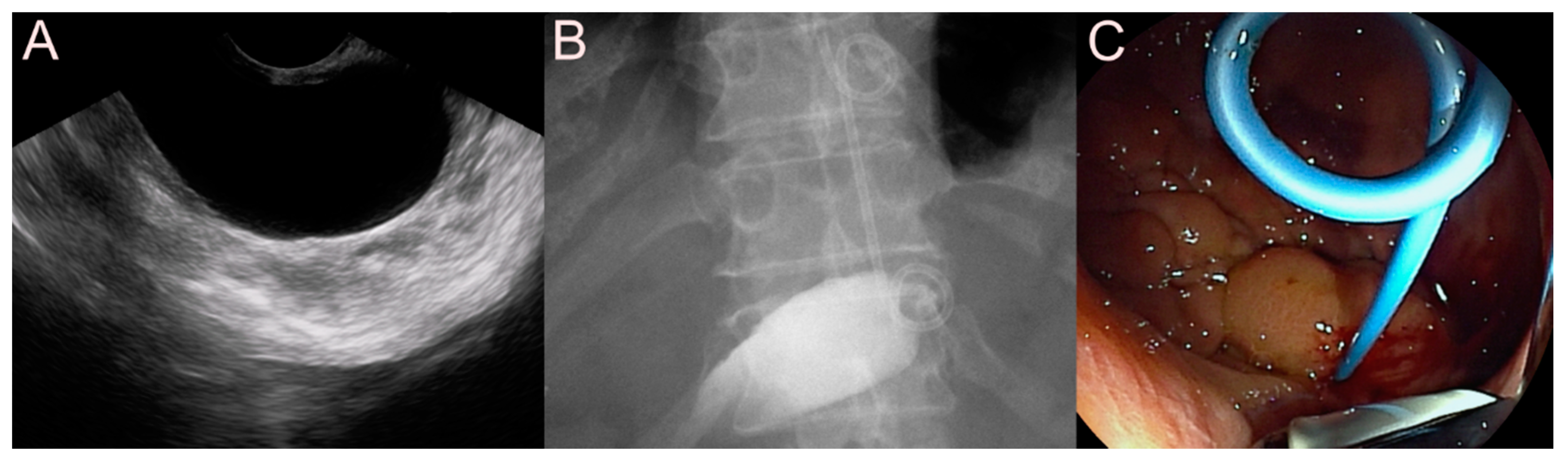
2.1. Stent for EUS-Guided Peripancreatic Fluid Collection (PFC) Drainage
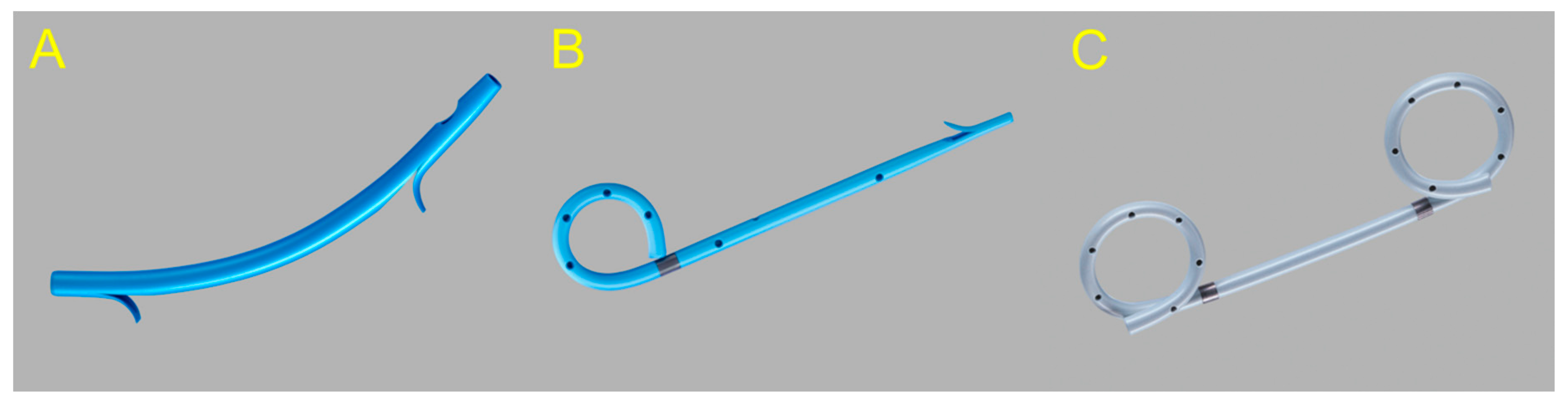
2.1.1. Use of the Plastic Stent
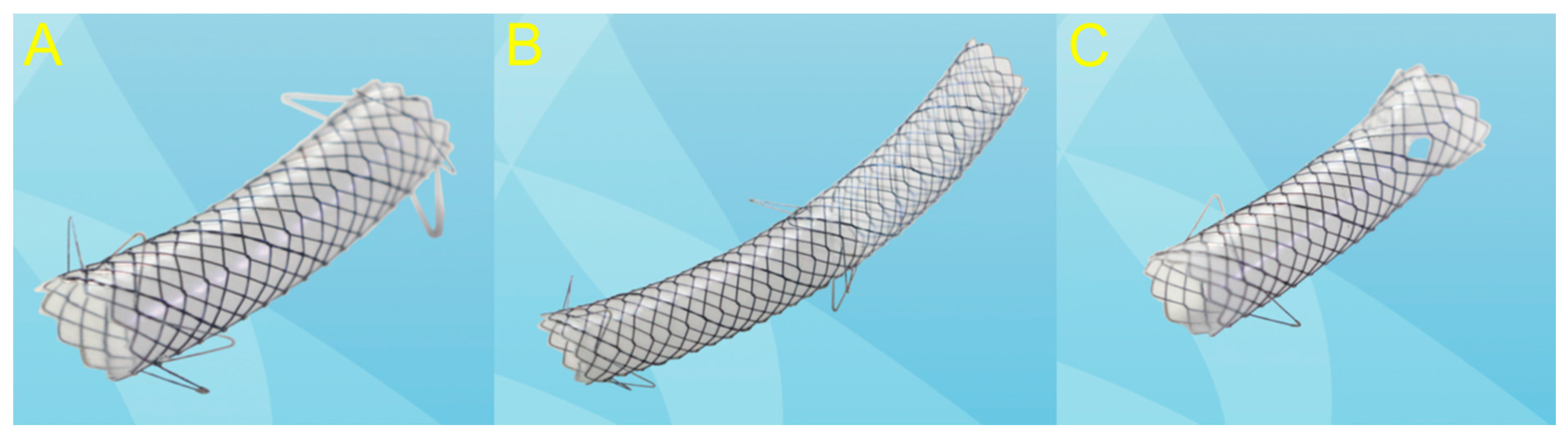
2.1.2. Use of the Self-Expandable Metal Stent (SEMS)
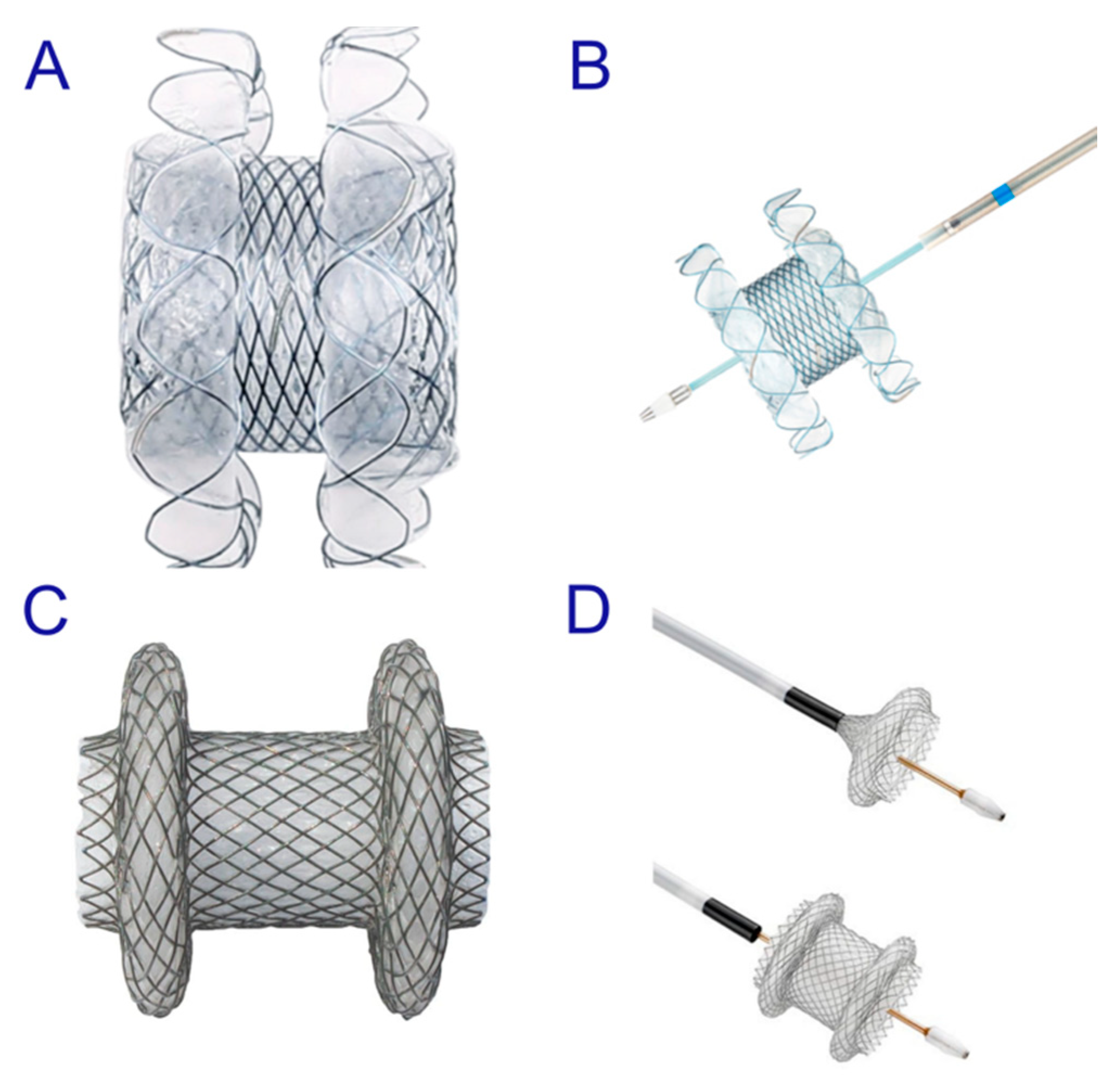
2.1.3. Use of the Lumen-Apposing Metal Stent (LAMS)
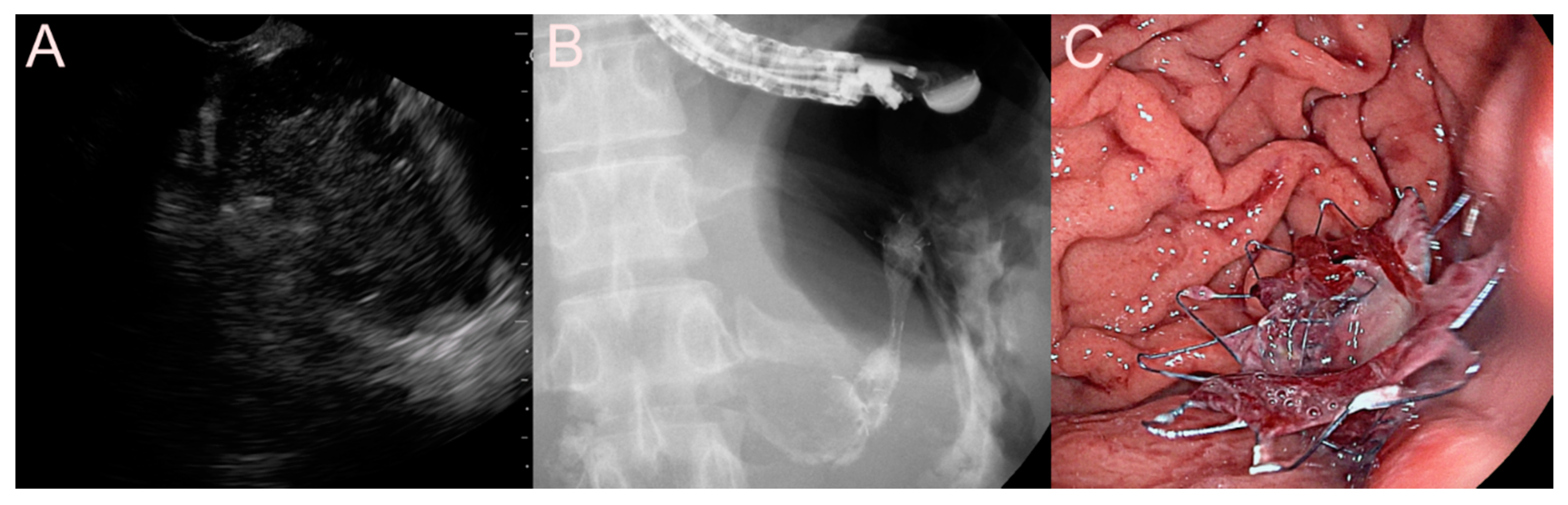
2.2. Stent for EUS-Guided Bile Duct (BD) Drainage
2.2.1. Use of the Plastic Stent
2.2.2. Use of the SEMS
2.2.3. Use of the LAMS
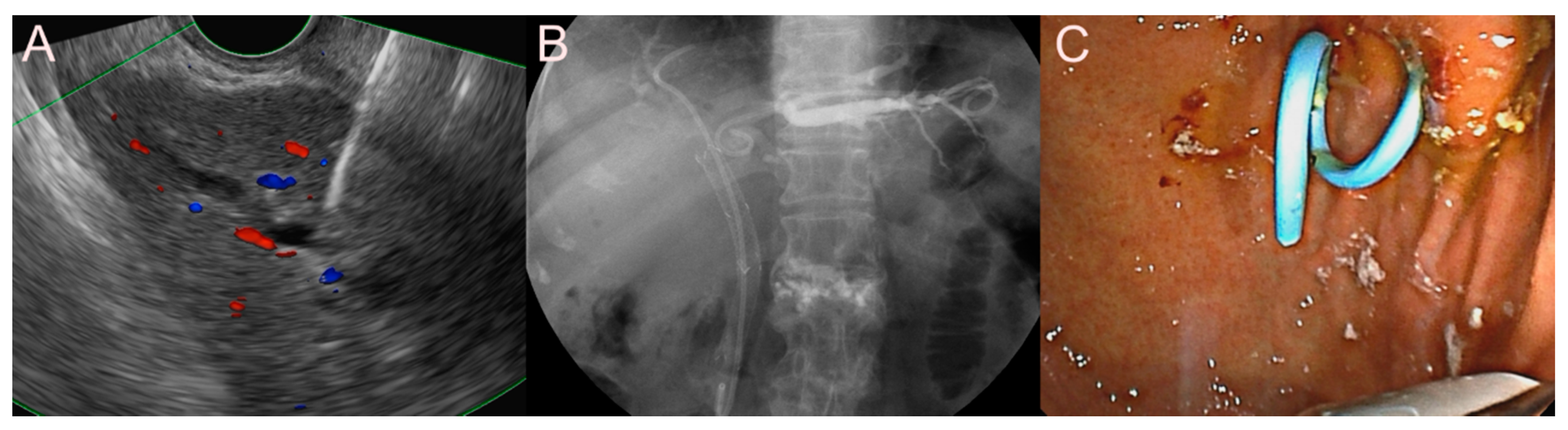
2.3. Stent for EUS-Guided Gallbladder (GB) Drainage
2.3.1. Use of the Plastic Stent
2.3.2. Use of the SEMS
2.3.3. Use of the LAMS
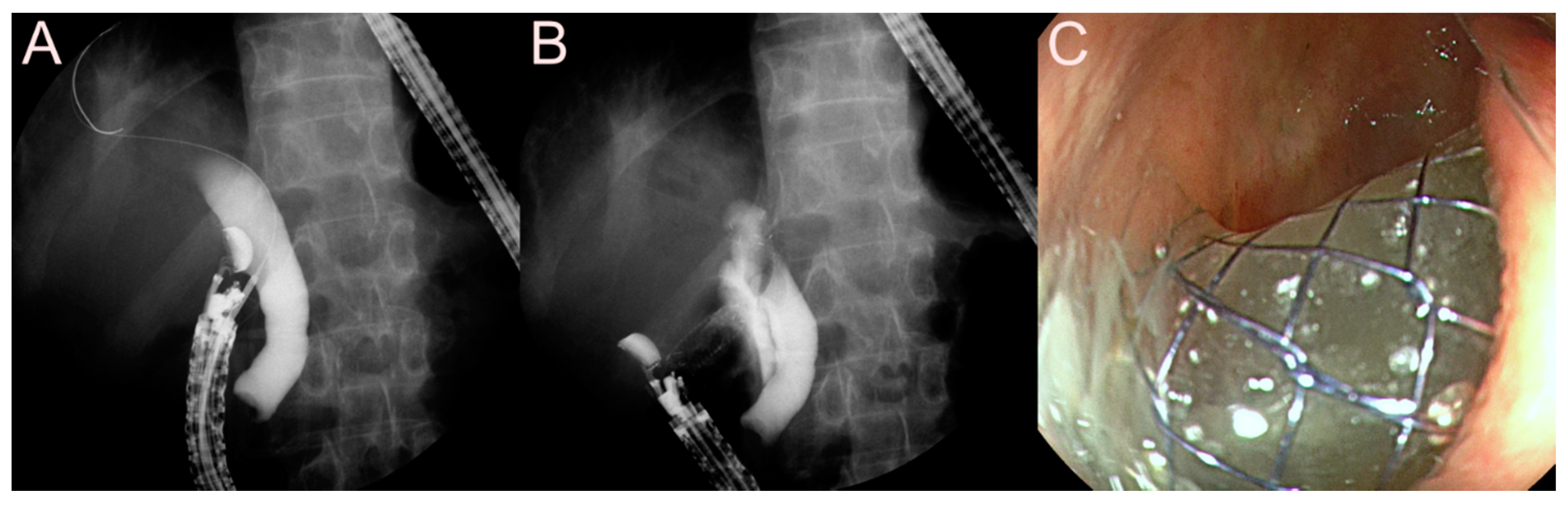
2.4. Stent for EUS-Guided Pancreatic Duct (PD) Drainage
2.4.1. Use of the Plastic Stent
2.4.2. Use of the SEMS
2.5. Stent for EUS-Guided Creation of Entero-Enteric Anastomosis
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, B.U.; Song, T.J.; Lee, S.S.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Newly designed, fully covered metal stents for endoscopic ultrasound (EUS)-guided transmural drainage of peripancreatic fluid collections: A prospective randomized study. Endoscopy 2014, 46, 1078–1084. [Google Scholar] [CrossRef]
- Shah, R.J.; Shah, J.N.; Waxman, I.; Kowalski, T.E.; Sanchez-Yague, A.; Nieto, J.; Brauer, B.C.; Gaidhane, M.; Kahaleh, M. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin. Gastroenterol. Hepatol. 2015, 13, 747–752. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Adler, D.G.; Nieto, J.; Shah, J.N.; Binmoeller, K.F.; Kane, S.; Yan, L.; Laique, S.N.; Kowalski, T.; Loren, D.E.; et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: A large retrospective, multicenter U.S. experience (with videos). Gastrointest. Endosc. 2016, 83, 699–707. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Tyberg, A.; Khashab, M.A.; Kumta, N.A.; Karia, K.; Nieto, J.; Siddiqui, U.D.; Waxman, I.; Joshi, V.; Benias, P.C.; et al. Endoscopic Therapy With Lumen-apposing Metal Stents Is Safe and Effective for Patients With Pancreatic Walled-off Necrosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1797–1803. [Google Scholar] [CrossRef]
- Song, T.J.; Lee, S.S.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Preliminary report on a new hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest. Endosc. 2014, 80, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, S.S.; Choi, J.H.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy 2014, 46, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Park, S.W.; Hyun, B.; Lee, J.; Koh, D.H.; Chung, D. Identification of risk factors for obstructive cholecystitis following placement of biliary stent in unresectable malignant biliary obstruction: A 5-year retrospective analysis in single center. Surg. Endosc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.W.; Lee, S.S.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest. Endosc. 2011, 74, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Seewald, S.; Thonke, F.; Ang, T.L.; Omar, S.; Seitz, U.; Groth, S.; Zhong, Y.; Yekebas, E.; Izbicki, J.; Soehendra, N. One-step, simultaneous double-wire technique facilitates pancreatic pseudocyst and abscess drainage (with videos). Gastrointest. Endosc. 2006, 64, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; DeFilippis, E.M.; Kedia, P.; Gaidhane, M.; Boumitri, C.; Lim, H.W.; Han, E.; Singh, H.; Ghumman, S.S.; Kowalski, T.; et al. Metal versus plastic for pancreatic pseudocyst drainage: Clinical outcomes and success. Gastrointest. Endosc. 2015, 82, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.L.; Kongkam, P.; Kwek, A.B.; Orkoonsawat, P.; Rerknimitr, R.; Fock, K.M. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc. Ultrasound 2016, 5, 320–327. [Google Scholar] [CrossRef]
- Lang, G.D.; Fritz, C.; Bhat, T.; Das, K.K.; Murad, F.M.; Early, D.S.; Edmundowicz, S.A.; Kushnir, V.M.; Mullady, D.K. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: Comparison of efficacy and adverse event rates. Gastrointest. Endosc. 2018, 87, 150–157. [Google Scholar] [CrossRef]
- Bapaye, A.; Dubale, N.A.; Sheth, K.A.; Bapaye, J.; Ramesh, J.; Gadhikar, H.; Mahajani, S.; Date, S.; Pujari, R.; Gaadhe, R. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: Comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig. Endosc. 2017, 29, 104–110. [Google Scholar] [CrossRef]
- Ang, T.L.; Teo, E.K.; Fock, K.M. EUS-guided drainage of infected pancreatic pseudocyst: Use of a 10F Soehendra dilator to facilitate a double-wire technique for initial transgastric access (with videos). Gastrointest. Endosc. 2008, 68, 192–194. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hasan, M.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: May not be business as usual. Gut 2017, 66, 2054–2056. [Google Scholar] [CrossRef] [PubMed]
- Pelaez-Luna, M.; Vege, S.S.; Petersen, B.T.; Chari, S.T.; Clain, J.E.; Levy, M.J.; Pearson, R.K.; Topazian, M.D.; Farnell, M.B.; Kendrick, M.L.; et al. Disconnected pancreatic duct syndrome in severe acute pancreatitis: Clinical and imaging characteristics and outcomes in a cohort of 31 cases. Gastrointest. Endosc. 2008, 68, 91–97. [Google Scholar] [CrossRef]
- Tann, M.; Maglinte, D.; Howard, T.J.; Sherman, S.; Fogel, E.; Madura, J.A.; Lehman, G.A. Disconnected pancreatic duct syndrome: Imaging findings and therapeutic implications in 26 surgically corrected patients. J. Comput. Assist. Tomogr. 2003, 27, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Park, S.W.; Nam, E.; Jung, J.H.; Jo, J.H. Comparative efficacy of stents in endoscopic ultrasonography-guided peripancreatic fluid collection drainage: A systematic review and network meta-analysis. J. Gastroenterol. Hepatol. 2020, 35, 941–952. [Google Scholar] [CrossRef]
- Ang, T.L.; Seewald, S. Fully covered self-expandable metal stents: The "be all and end all" for pancreatic fluid collections? Gastrointest. Endosc. 2015, 82, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.K.; Sutton, B.; Hawes, R.; Varadarajulu, S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut 2019, 68, 1200–1209. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Kowalski, T.E.; Loren, D.E.; Khalid, A.; Soomro, A.; Mazhar, S.M.; Isby, L.; Kahaleh, M.; Karia, K.; Yoo, J.; et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest. Endosc. 2017, 85, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.L.; Teoh, A.Y.B. Endoscopic ultrasonography-guided drainage of pancreatic fluid collections. Dig. Endosc. 2017, 29, 463–471. [Google Scholar] [CrossRef]
- Park, D.H.; Jeong, S.U.; Lee, B.U.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest. Endosc. 2013, 78, 91–101. [Google Scholar] [CrossRef]
- Park, D.H.; Jang, J.W.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011, 74, 1276–1284. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, S.S.; Oh, D.; Song, T.J.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest. Endosc. 2017, 85, 1067–1075. [Google Scholar] [CrossRef]
- Paik, W.H.; Park, D.H. Outcomes and limitations: EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2019, 8, S44–S49. [Google Scholar]
- Umeda, J.; Itoi, T.; Tsuchiya, T.; Sofuni, A.; Itokawa, F.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Kamada, K.; Tanaka, R.; et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos). Gastrointest. Endosc. 2015, 82, 390–396. [Google Scholar] [CrossRef]
- Ogura, T.; Masuda, D.; Imoto, A.; Takeushi, T.; Kamiyama, R.; Mohamed, M.; Umegaki, E.; Higuchi, K. EUS-guided hepaticogastrostomy combined with fine-gauge antegrade stenting: A pilot study. Endoscopy 2014, 46, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Itoi, T.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; Asai, Y.; Matsunami, Y.; et al. EUS-guided antegrade metal stenting with hepaticoenterostomy using a dedicated plastic stent with a review of the literature (with video). Endosc. Ultrasound 2018, 7, 404–412. [Google Scholar]
- Bories, E.; Pesenti, C.; Caillol, F.; Lopes, C.; Giovannini, M. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy 2007, 39, 287–291. [Google Scholar] [CrossRef]
- Khashab, M.A.; Valeshabad, A.K.; Modayil, R.; Widmer, J.; Saxena, P.; Idrees, M.; Iqbal, S.; Kalloo, A.N.; Stavropoulos, S.N. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: Rendezvous versus direct transluminal techniques (with videos). Gastrointest. Endosc. 2013, 78, 734–741. [Google Scholar] [CrossRef]
- Park, D.H. Endoscopic ultrasound-guided biliary drainage of hilar biliary obstruction. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 664–668. [Google Scholar] [CrossRef]
- Nakai, Y.; Isayama, H.; Yamamoto, N.; Matsubara, S.; Ito, Y.; Sasahira, N.; Hakuta, R.; Umefune, G.; Takahara, N.; Hamada, T.; et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016, 48, 1125–1128. [Google Scholar] [CrossRef]
- Kawakubo, K.; Isayama, H.; Kato, H.; Itoi, T.; Kawakami, H.; Hanada, K.; Ishiwatari, H.; Yasuda, I.; Kawamoto, H.; Itokawa, F.; et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 328–334. [Google Scholar] [CrossRef]
- Martins, F.P.; Rossini, L.G.; Ferrari, A.P. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: Fatal complication. Endoscopy 2010, 42 (Suppl. 2), E126–E127. [Google Scholar] [CrossRef] [PubMed]
- Kahaleh, M.; Hernandez, A.J.; Tokar, J.; Adams, R.B.; Shami, V.M.; Yeaton, P. Interventional EUS-guided cholangiography: Evaluation of a technique in evolution. Gastrointest. Endosc. 2006, 64, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Isayama, H.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Tsuji, S.; Ishii, K.; Ikeuchi, N.; Tanaka, R.; et al. Stent selection and tips on placement technique of EUS-guided biliary drainage: Transduodenal and transgastric stenting. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 664–672. [Google Scholar] [CrossRef]
- Kunda, R.; Perez-Miranda, M.; Will, U.; Ullrich, S.; Brenk, E.D.; Dollhopf, M.; Meier, M.; Larghi, A. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg. Endosc. 2016, 30, 5002–5008. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Teoh, A.Y.B.; Itoi, T.; Yamao, K.; Hara, K.; Nakai, Y.; Isayama, H.; Kitano, M. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction: A prospective multicenter study. Gastrointest. Endosc. 2018, 87, 1138–1146. [Google Scholar] [CrossRef]
- Jacques, J.; Privat, J.; Pinard, F.; Fumex, F.; Valats, J.C.; Chaoui, A.; Cholet, F.; Godard, B.; Grandval, P.; Legros, R.; et al. Endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents: A retrospective analysis. Endoscopy 2019, 51, 540–547. [Google Scholar] [CrossRef]
- Jain, D.; Bhandari, B.S.; Agrawal, N.; Singhal, S. Endoscopic Ultrasound-Guided Gallbladder Drainage Using a Lumen-Apposing Metal Stent for Acute Cholecystitis: A Systematic Review. Clin. Endosc. 2018, 51, 450–462. [Google Scholar] [CrossRef]
- Dollhopf, M.; Larghi, A.; Will, U.; Rimbas, M.; Anderloni, A.; Sanchez-Yague, A.; Teoh, A.Y.B.; Kunda, R. EUS-guided gallbladder drainage in patients with acute cholecystitis and high surgical risk using an electrocautery-enhanced lumen-apposing metal stent device. Gastrointest. Endosc. 2017, 86, 636–643. [Google Scholar] [CrossRef]
- Walter, D.; Teoh, A.Y.; Itoi, T.; Perez-Miranda, M.; Larghi, A.; Sanchez-Yague, A.; Siersema, P.D.; Vleggaar, F.P. EUS-guided gall bladder drainage with a lumen-apposing metal stent: A prospective long-term evaluation. Gut 2016, 65, 6–8. [Google Scholar] [CrossRef]
- Cho, S.H.; Oh, D.; Song, T.J.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Lee, Y.N.; Moon, J.H.; Lee, S.S. Comparison of the effectiveness and safety of lumen-apposing metal stents and anti-migrating tubular self-expandable metal stents for EUS-guided gallbladder drainage in high surgical risk patients with acute cholecystitis. Gastrointest. Endosc. 2020, 91, 543–550. [Google Scholar] [CrossRef]
- Irani, S.; Ngamruengphong, S.; Teoh, A.; Will, U.; Nieto, J.; Abu Dayyeh, B.K.; Gan, S.I.; Larsen, M.; Yip, H.C.; Topazian, M.D.; et al. Similar Efficacies of Endoscopic Ultrasound Gallbladder Drainage With a Lumen-Apposing Metal Stent Versus Percutaneous Transhepatic Gallbladder Drainage for Acute Cholecystitis. Clin. Gastroenterol. Hepatol. 2017, 15, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Sobani, Z.A.; Ling, C.; Rustagi, T. Endoscopic Ultrasound-Guided Gallbladder Drainage. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef]
- Jang, J.W.; Lee, S.S.; Song, T.J.; Hyun, Y.S.; Park, D.Y.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Yun, S.C. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology 2012, 142, 805–811. [Google Scholar] [CrossRef]
- Khan, M.A.; Atiq, O.; Kubiliun, N.; Ali, B.; Kamal, F.; Nollan, R.; Ismail, M.K.; Tombazzi, C.; Kahaleh, M.; Baron, T.H. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest. Endosc. 2017, 85, 76–87.e3. [Google Scholar] [CrossRef]
- Baron, T.H.; Topazian, M.D. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest. Endosc. 2007, 65, 735–737. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, M.H.; Park, D.H.; Song, T.J.; Eum, J.; Lee, S.S.; Seo, D.W.; Lee, S.K. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest. Endosc. 2010, 72, 86–91. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, M.H.; Moon, S.H.; Lee, S.S.; Seo, D.W.; Lee, S.K. Feasibility and safety of placement of a newly designed, fully covered self-expandable metal stent for refractory benign pancreatic ductal strictures: A pilot study (with video). Gastrointest. Endosc. 2008, 68, 1182–1189. [Google Scholar] [CrossRef]
- Takagi, W.; Ogura, T.; Sano, T.; Onda, S.; Okuda, A.; Masuda, D.; Imoto, A.; Takeuchi, T.; Fukunishi, S.; Higuchi, K. EUS-guided cholecystoduodenostomy for acute cholecystitis with an anti-stent migration and anti-food impaction system; a pilot study. Therap. Adv. Gastroenterol. 2016, 9, 19–25. [Google Scholar] [CrossRef]
- Kahaleh, M.; Perez-Miranda, M.; Artifon, E.L.; Sharaiha, R.Z.; Kedia, P.; Penas, I.; De la Serna, C.; Kumta, N.A.; Marson, F.; Gaidhane, M.; et al. International collaborative study on EUS-guided gallbladder drainage: Are we ready for prime time? Dig. Liver Dis. 2016, 48, 1054–1057. [Google Scholar] [CrossRef]
- Chen, Y.I.; Barkun, A.N.; Adam, V.; Bai, G.; Singh, V.K.; Bukhari, M.; Gutierrez, O.B.; Elmunzer, B.J.; Moran, R.; Fayad, L.; et al. Cost-effectiveness analysis comparing lumen-apposing metal stents with plastic stents in the management of pancreatic walled-off necrosis. Gastrointest. Endosc. 2018, 88, 267–276. [Google Scholar] [CrossRef]
- Itoi, T.; Baron, T.H.; Khashab, M.A.; Tsuchiya, T.; Irani, S.; Dhir, V.; Bun Teoh, A.Y. Technical review of endoscopic ultrasonography-guided gastroenterostomy in 2017. Dig. Endosc. 2017, 29, 495–502. [Google Scholar] [CrossRef]
- Irani, S.; Baron, T.H.; Grimm, I.S.; Khashab, M.A. EUS-guided gallbladder drainage with a lumen-apposing metal stent (with video). Gastrointest. Endosc. 2015, 82, 1110–1115. [Google Scholar] [CrossRef]
- Chan, S.M.; Teoh, A.Y.B.; Yip, H.C.; Wong, V.W.Y.; Chiu, P.W.Y.; Ng, E.K.W. Feasibility of per-oral cholecystoscopy and advanced gallbladder interventions after EUS-guided gallbladder stenting (with video). Gastrointest. Endosc. 2017, 85, 1225–1232. [Google Scholar] [CrossRef]
- de la Serna-Higuera, C.; Perez-Miranda, M.; Gil-Simon, P.; Ruiz-Zorrilla, R.; Diez-Redondo, P.; Alcaide, N.; Sancho-del Val, L.; Nunez-Rodriguez, H. EUS-guided transenteric gallbladder drainage with a new fistula-forming, lumen-apposing metal stent. Gastrointest. Endosc. 2013, 77, 303–308. [Google Scholar] [CrossRef]
- Law, R.; Grimm, I.S.; Stavas, J.M.; Baron, T.H. Conversion of Percutaneous Cholecystostomy to Internal Transmural Gallbladder Drainage Using an Endoscopic Ultrasound-Guided, Lumen-Apposing Metal Stent. Clin. Gastroenterol. Hepatol. 2016, 14, 476–480. [Google Scholar] [CrossRef]
- Chapman, C.G.; Waxman, I.; Siddiqui, U.D. Endoscopic Ultrasound (EUS)-Guided Pancreatic Duct Drainage: The Basics of When and How to Perform EUS-Guided Pancreatic Duct Interventions. Clin. Endosc. 2016, 49, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Fujii, L.L.; Topazian, M.D.; Abu Dayyeh, B.K.; Baron, T.H.; Chari, S.T.; Farnell, M.B.; Gleeson, F.C.; Gostout, C.J.; Kendrick, M.L.; Pearson, R.K.; et al. EUS-guided pancreatic duct intervention: Outcomes of a single tertiary-care referral center experience. Gastrointest. Endosc. 2013, 78, 854–864.e1. [Google Scholar] [CrossRef]
- Itoi, T.; Kasuya, K.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Yasuda, I.; Nakai, Y.; Isayama, H.; Moriyasu, F. Endoscopic ultrasonography-guided pancreatic duct access: Techniques and literature review of pancreatography, transmural drainage and rendezvous techniques. Dig. Endosc. 2013, 25, 241–252. [Google Scholar] [CrossRef]
- Fujii-Lau, L.L.; Levy, M.J. Endoscopic ultrasound-guided pancreatic duct drainage. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 51–57. [Google Scholar] [CrossRef]
- Kurihara, T.; Itoi, T.; Sofuni, A.; Itokawa, F.; Mariyasu, F. Endoscopic ultrasonography-guided pancreatic duct drainage after failed endoscopic retrograde cholangiopancreatography in patients with malignant and benign pancreatic duct obstructions. Dig. Endosc. 2013, 25 (Suppl. 2), 109–116. [Google Scholar] [CrossRef]
- Francois, E.; Kahaleh, M.; Giovannini, M.; Matos, C.; Deviere, J. EUS-guided pancreaticogastrostomy. Gastrointest. Endosc. 2002, 56, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kahaleh, M.; Hernandez, A.J.; Tokar, J.; Adams, R.B.; Shami, V.M.; Yeaton, P. EUS-guided pancreaticogastrostomy: Analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest. Endosc. 2007, 65, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kikuyama, M.; Itoi, T.; Ota, Y.; Matsumura, K.; Tsuchiya, T.; Itokawa, F.; Sofuni, A.; Yamao, K. Therapeutic endoscopy for stenotic pancreatodigestive tract anastomosis after pancreatoduodenectomy (with videos). Gastrointest. Endosc. 2011, 73, 376–382. [Google Scholar] [CrossRef]
- Tyberg, A.; Sharaiha, R.Z.; Kedia, P.; Kumta, N.; Gaidhane, M.; Artifon, E.; Giovannini, M.; Kahaleh, M. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: A multicenter international collaborative study. Gastrointest. Endosc. 2017, 85, 164–169. [Google Scholar] [CrossRef]
- Matsunami, Y.; Itoi, T.; Sofuni, A.; Tsuchiya, T.; Kamada, K.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; et al. Evaluation of a new stent for EUS-guided pancreatic duct drainage: Long-term follow-up outcome. Endosc. Int. Open 2018, 6, E505–E512. [Google Scholar]
- Itoi, T.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Ikeuchi, N.; Tanaka, R.; Umeda, J.; Tonozuka, R.; Honjo, M.; Mukai, S.; et al. Initial evaluation of a new plastic pancreatic duct stent for endoscopic ultrasonography-guided placement. Endoscopy 2015, 47, 462–465. [Google Scholar] [CrossRef]
- Shimamura, Y.; Mosko, J.; Teshima, C.; May, G.R. Endoscopic Ultrasound-Guided Pancreatic Duct Intervention. Clin. Endosc. 2017, 50, 112–116. [Google Scholar] [CrossRef]
- Krafft, M.R.; Nasr, J.Y. Anterograde Endoscopic Ultrasound-Guided Pancreatic Duct Drainage: A Technical Review. Dig. Dis. Sci. 2019, 64, 1770–1781. [Google Scholar] [CrossRef]
- Widmer, J.; Sharaiha, R.Z.; Kahaleh, M. Endoscopic ultrasonography-guided drainage of the pancreatic duct. Gastrointest. Endosc. Clin. N. Am. 2013, 23, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Park, D.H.; Cho, M.K.; Nam, K.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Feasibility and safety of a fully covered self-expandable metal stent with antimigration properties for EUS-guided pancreatic duct drainage: Early and midterm outcomes (with video). Gastrointest. Endosc. 2016, 83, 366–373. [Google Scholar] [CrossRef]
- Tyberg, A.; Kumta, N.; Karia, K.; Zerbo, S.; Sharaiha, R.Z.; Kahaleh, M. EUS-guided gastrojejunostomy after failed enteral stenting. Gastrointest. Endosc. 2015, 81, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, N.; Itoi, T.; Tsuchiya, T.; Nagakawa, Y.; Tsuchida, A. One-step EUS-guided gastrojejunostomy with use of lumen-apposing metal stent for afferent loop syndrome treatment. Gastrointest. Endosc. 2015, 82, 166. [Google Scholar] [CrossRef]
- Khashab, M.A.; Tieu, A.H.; Azola, A.; Ngamruengphong, S.; El Zein, M.H.; Kumbhari, V. EUS-guided gastrojejunostomy for management of complete gastric outlet obstruction. Gastrointest. Endosc. 2015, 82, 745. [Google Scholar] [CrossRef]
- Tyberg, A.; Perez-Miranda, M.; Sanchez-Ocana, R.; Penas, I.; de la Serna, C.; Shah, J.; Binmoeller, K.; Gaidhane, M.; Grimm, I.; Baron, T.; et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: A multicenter, international experience. Endosc. Int. Open 2016, 4, E276–E281. [Google Scholar] [CrossRef]
- Itoi, T.; Ishii, K.; Ikeuchi, N.; Sofuni, A.; Gotoda, T.; Moriyasu, F.; Dhir, V.; Teoh, A.Y.; Binmoeller, K.F. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut 2016, 65, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Binmoeller, K.F.; Shah, J.N. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: A porcine study. Endoscopy 2012, 44, 499–503. [Google Scholar] [CrossRef]
- Amin, S.; Sethi, A. Endoscopic Ultrasound-Guided Gastrojejunostomy. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 707–713. [Google Scholar] [CrossRef]
- Kedia, P.; Tyberg, A.; Kumta, N.A.; Gaidhane, M.; Karia, K.; Sharaiha, R.Z.; Kahaleh, M. EUS-directed transgastric ERCP for Roux-en-Y gastric bypass anatomy: A minimally invasive approach. Gastrointest. Endosc. 2015, 82, 560–565. [Google Scholar] [CrossRef]
- Kedia, P.; Kumta, N.A.; Widmer, J.; Sundararajan, S.; Cerefice, M.; Gaidhane, M.; Sharaiha, R.; Kahaleh, M. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: A novel technique. Endoscopy 2015, 47, 159–163. [Google Scholar] [CrossRef]
- Tyberg, A.; Kedia, P.; Tawadros, A.; Tarnasky, P.R.; Gaidhane, M.; Nieto, J.; Kahaleh, M. EUS-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography (EDGE): The First Learning Curve. J. Clin. Gastroenterol. 2020, 54, 569–572. [Google Scholar] [CrossRef]
- Krafft, M.R.; Hsueh, W.; James, T.W.; Runge, T.M.; Baron, T.H.; Khashab, M.A.; Irani, S.S.; Nasr, J.Y. The EDGI new take on EDGE: EUS-directed transgastric intervention (EDGI), other than ERCP, for Roux-en-Y gastric bypass anatomy: A multicenter study. Endosc. Int. Open 2019, 7, E1231–E1240. [Google Scholar] [CrossRef]

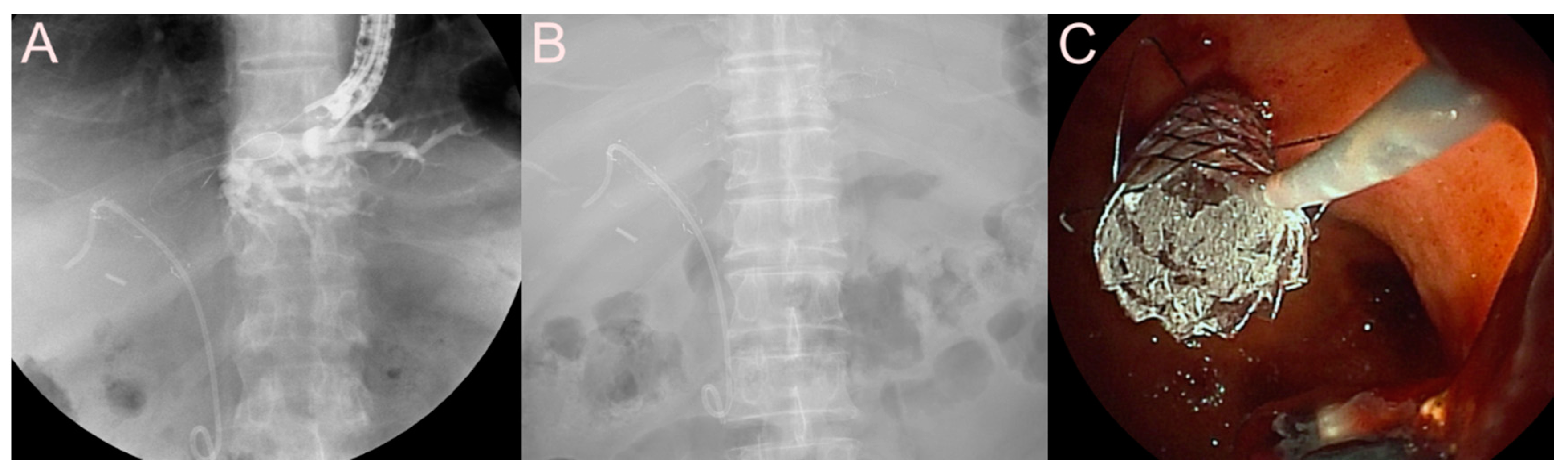
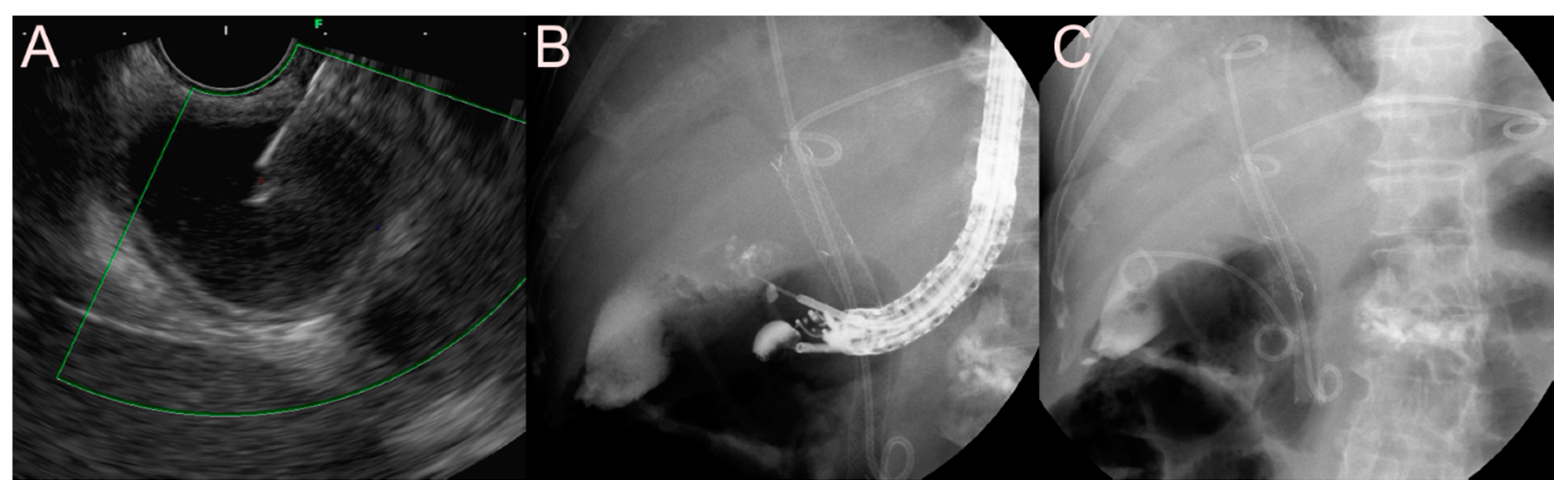
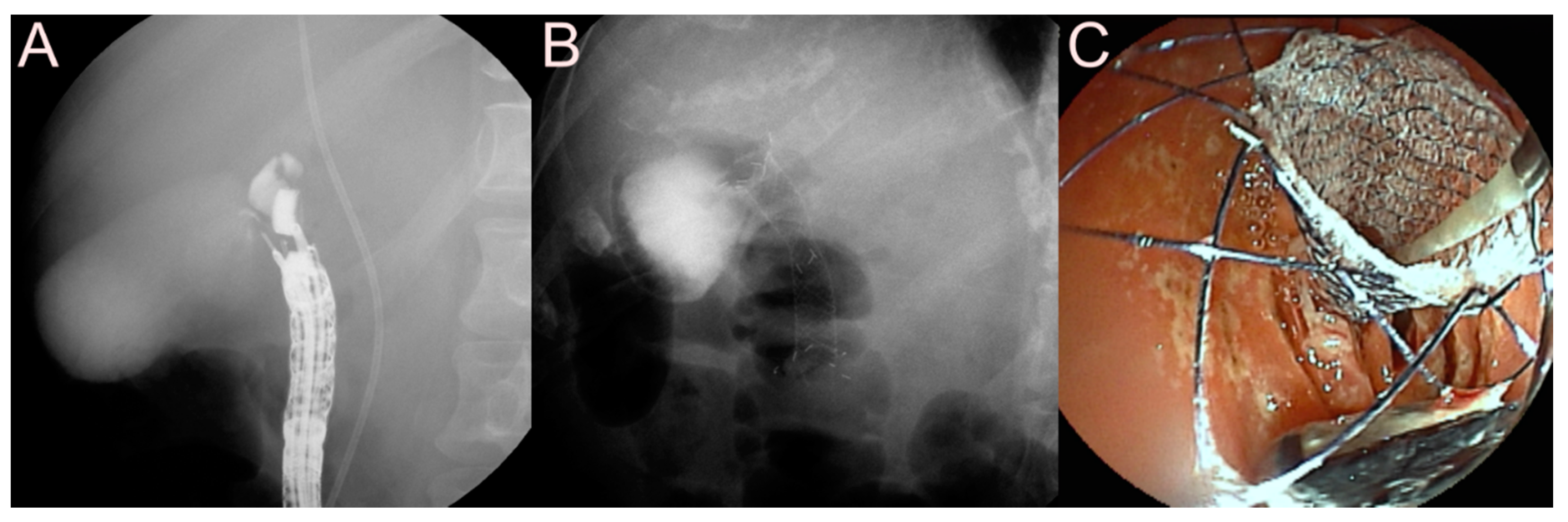
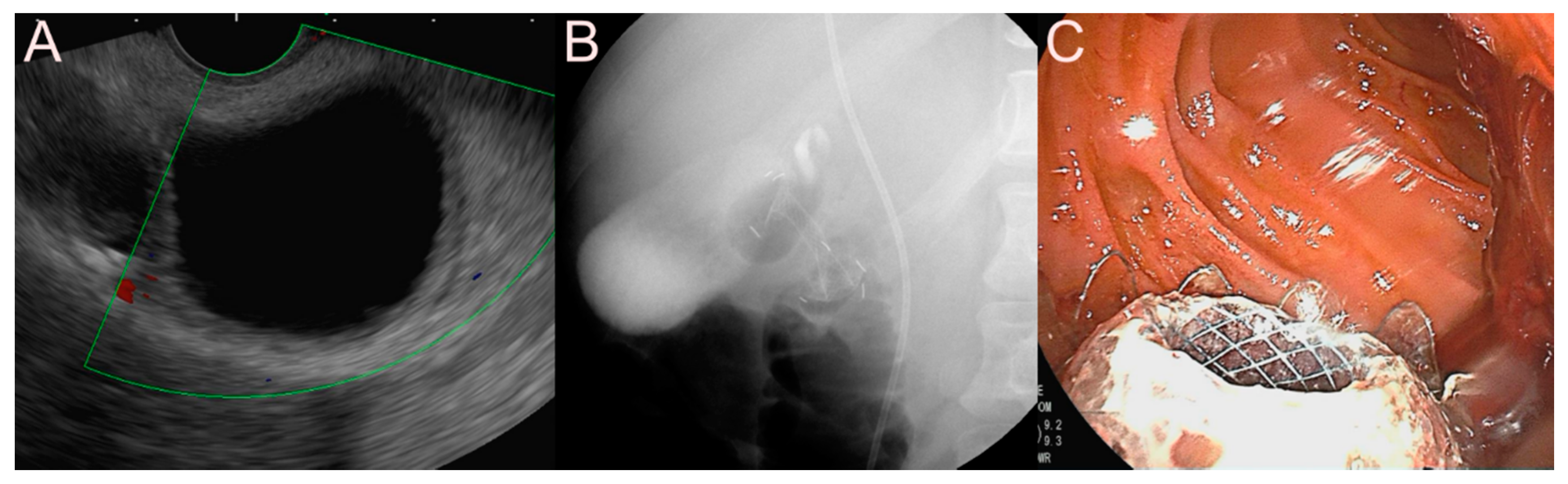

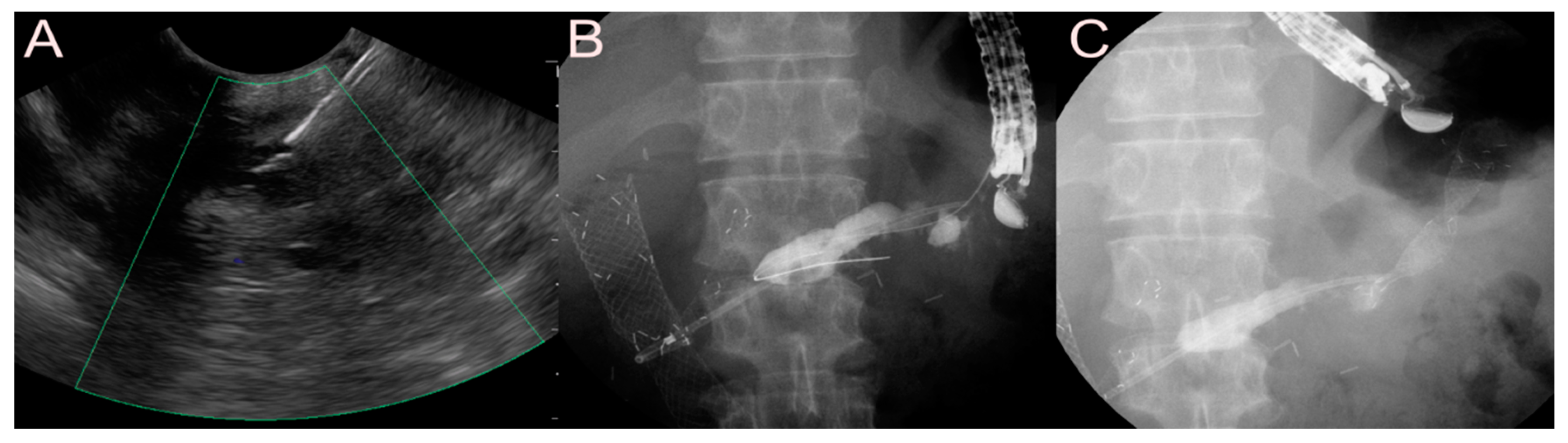
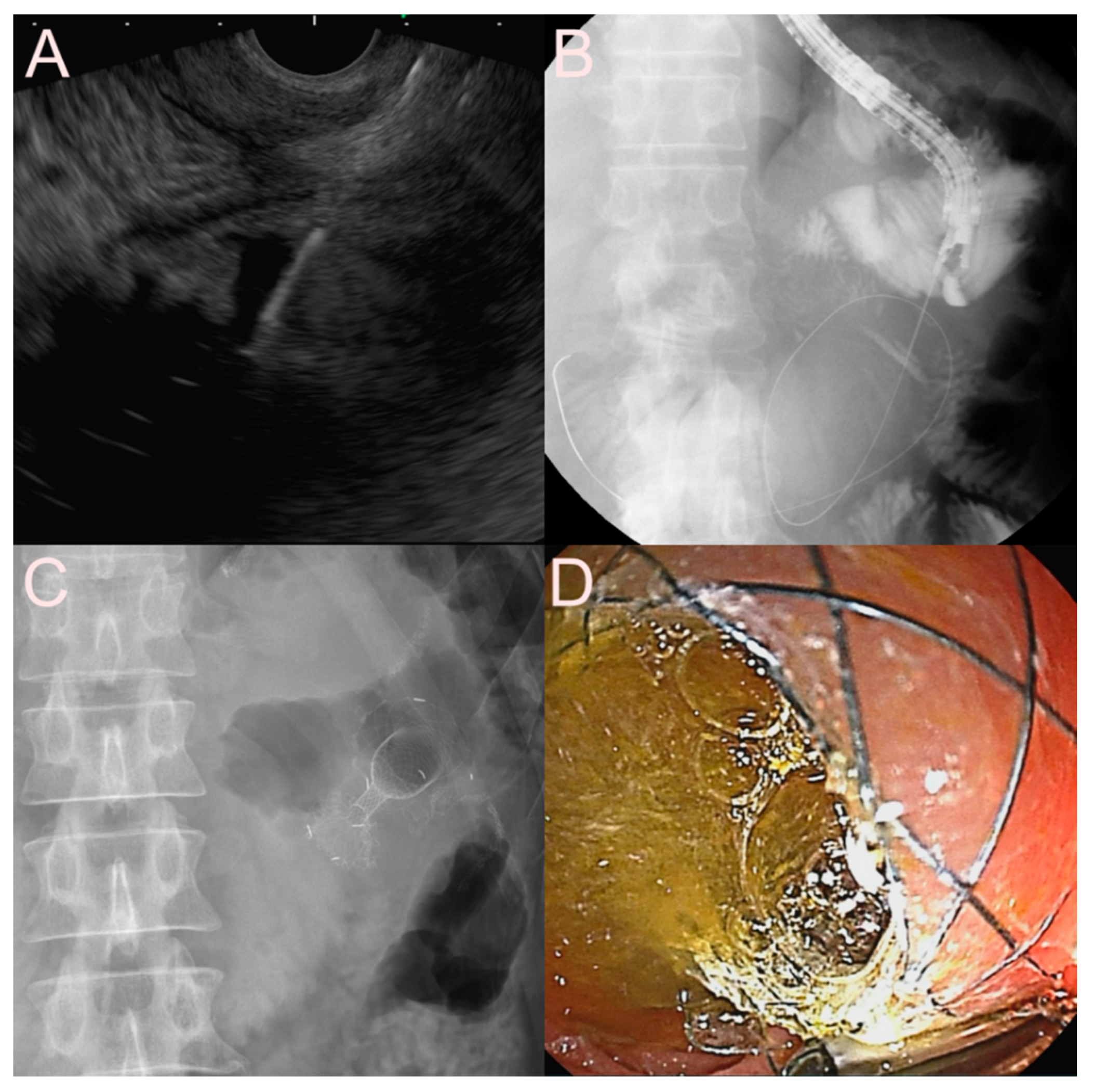
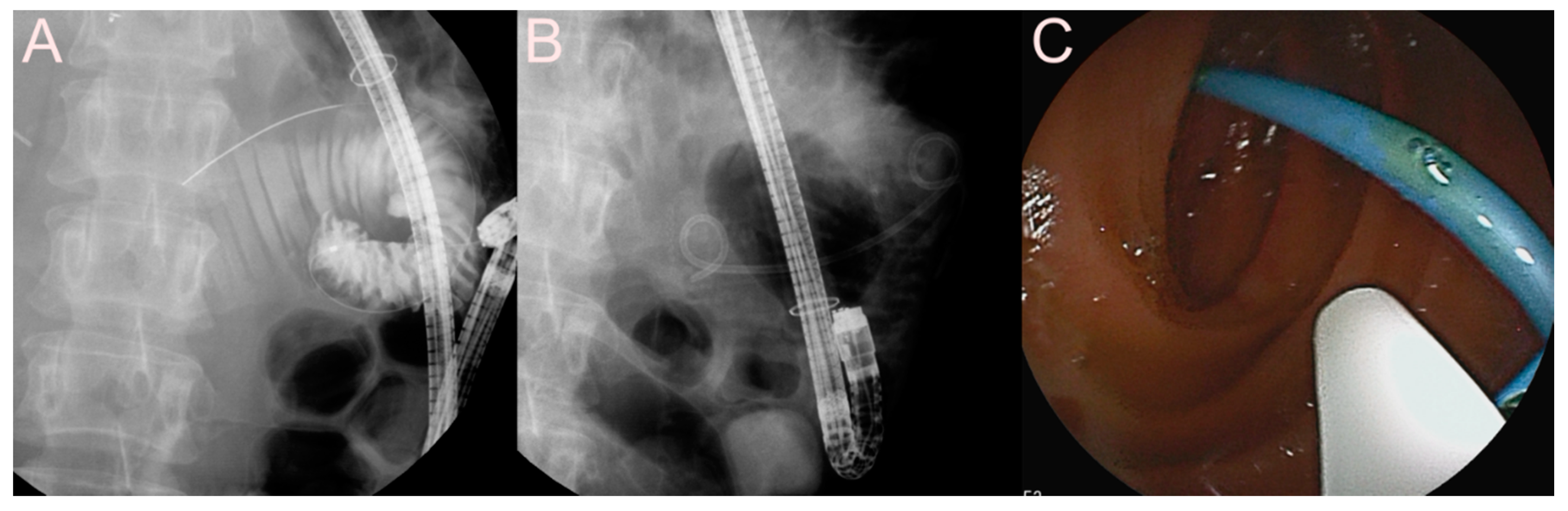
| Possible Subtype | Advantages | Disadvantages | |
|---|---|---|---|
| Plastic stent |
|
|
|
| Fully covered self-expandable metal stent (FCSEMS) |
|
|
|
| (Lumen-apposing metal stent) LAMS |
|
|
|
| Possible Subtype | Advantages | Disadvantages | |
|---|---|---|---|
| Plastic stent |
|
|
|
| Fully covered self-expandable metal stent (FCSEMS) |
|
|
|
| Lumen-apposing metal stent (LAMS) |
|
|
|
| Possible Subtype | Advantages | Disadvantages | |
|---|---|---|---|
| Plastic stent |
|
|
|
| Fully covered self-expandable metal stent (FCSEMS) |
|
|
|
| Lumen-apposing metal stent (LAMS) |
|
|
|
| Possible Subtype | Advantages | Disadvantages | |
|---|---|---|---|
| Plastic stent |
|
|
|
| Fully covered self-expandable metal stent (FCSEMS) |
|
|
|
| Lumen-apposing metal stent (LAMS) |
|
| Possible Subtype | Advantages | Disadvantages | |
|---|---|---|---|
| Plastic stent |
|
|
|
| Fully covered self-expandable metal stent (FCSEMS) |
|
|
|
| Lumen-apposing metal stent (LAMS) |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.W.; Lee, S.S. Which Are the Most Suitable Stents for Interventional Endoscopic Ultrasound? J. Clin. Med. 2020, 9, 3595. https://doi.org/10.3390/jcm9113595
Park SW, Lee SS. Which Are the Most Suitable Stents for Interventional Endoscopic Ultrasound? Journal of Clinical Medicine. 2020; 9(11):3595. https://doi.org/10.3390/jcm9113595
Chicago/Turabian StylePark, Se Woo, and Sang Soo Lee. 2020. "Which Are the Most Suitable Stents for Interventional Endoscopic Ultrasound?" Journal of Clinical Medicine 9, no. 11: 3595. https://doi.org/10.3390/jcm9113595
APA StylePark, S. W., & Lee, S. S. (2020). Which Are the Most Suitable Stents for Interventional Endoscopic Ultrasound? Journal of Clinical Medicine, 9(11), 3595. https://doi.org/10.3390/jcm9113595





