Can Polyphenols in Eye Drops Be Useful for Trabecular Protection from Oxidative Damage?
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Experimental Conditions
2.3. DCF Assay
2.4. Alamar Blue Assay
2.5. Western Blotting
2.6. qPCR
2.7. Apoptosis Array
2.8. Statistical Analysis
3. Results
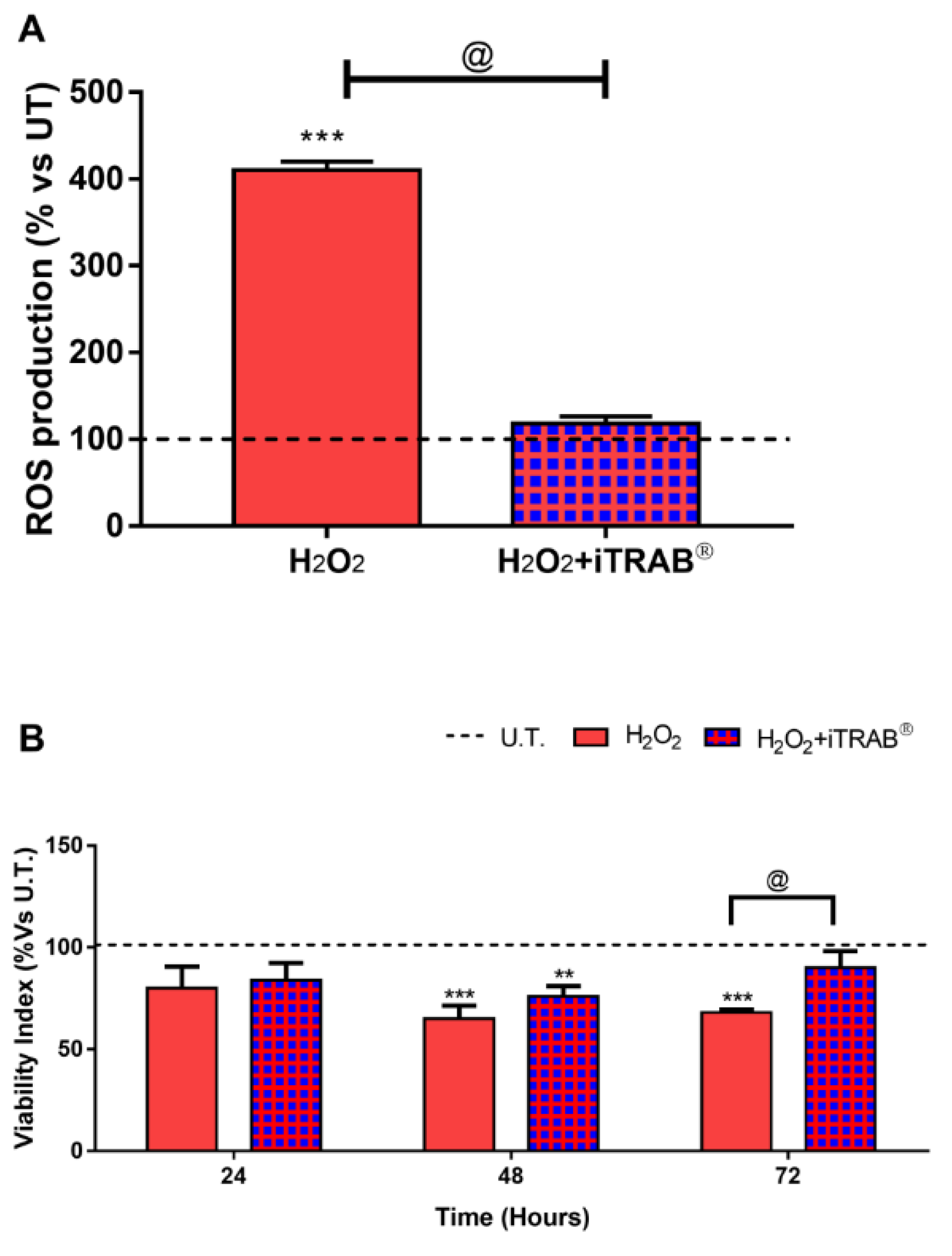
3.1. ROS Production (Fluorimetric DCF Assay)
3.2. Viability Index (Alamar Blue Assay)
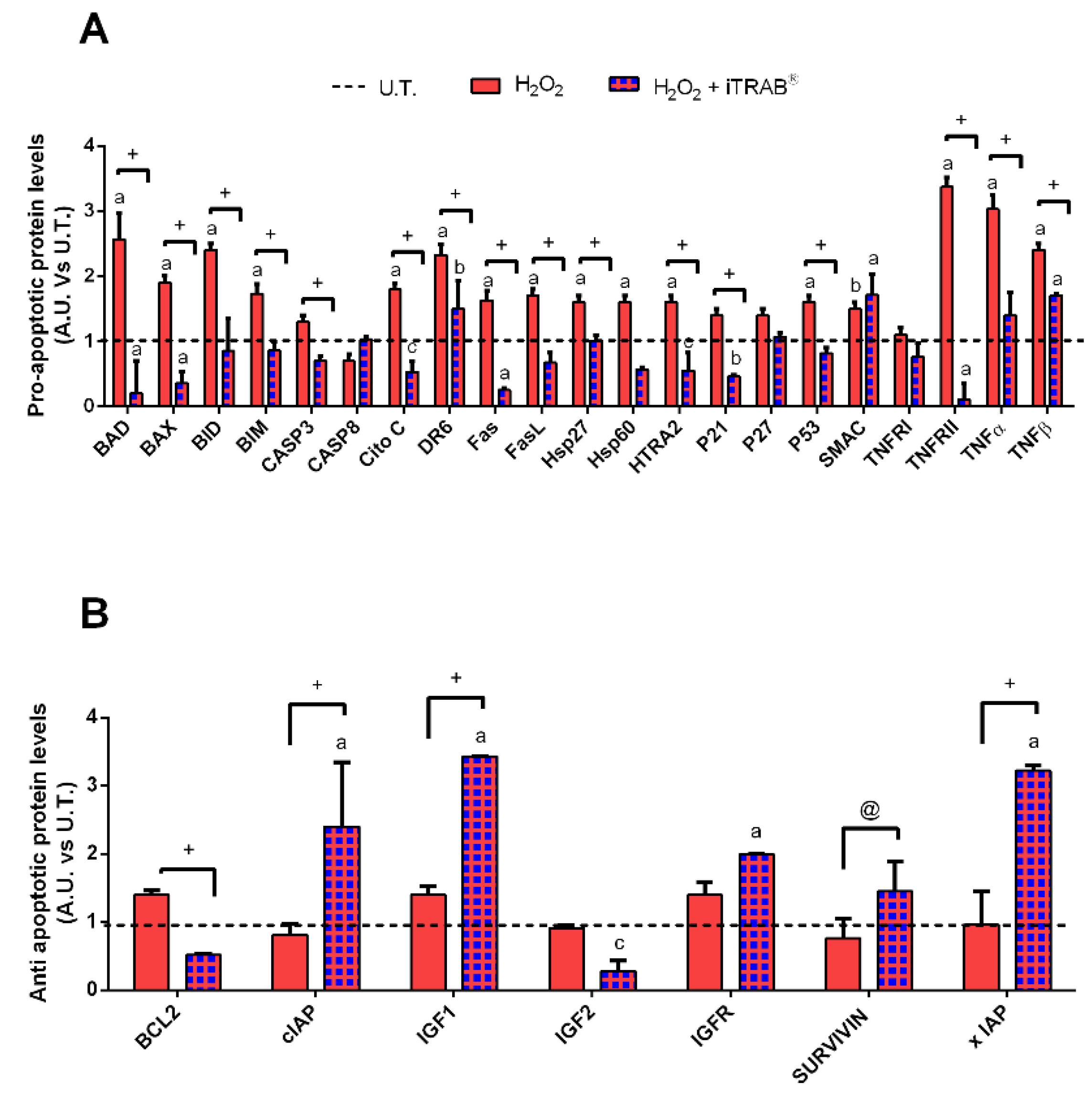
3.3. Apoptosis Pathway (Human Apoptosis Antibody Array C1)
3.4. Western Blot
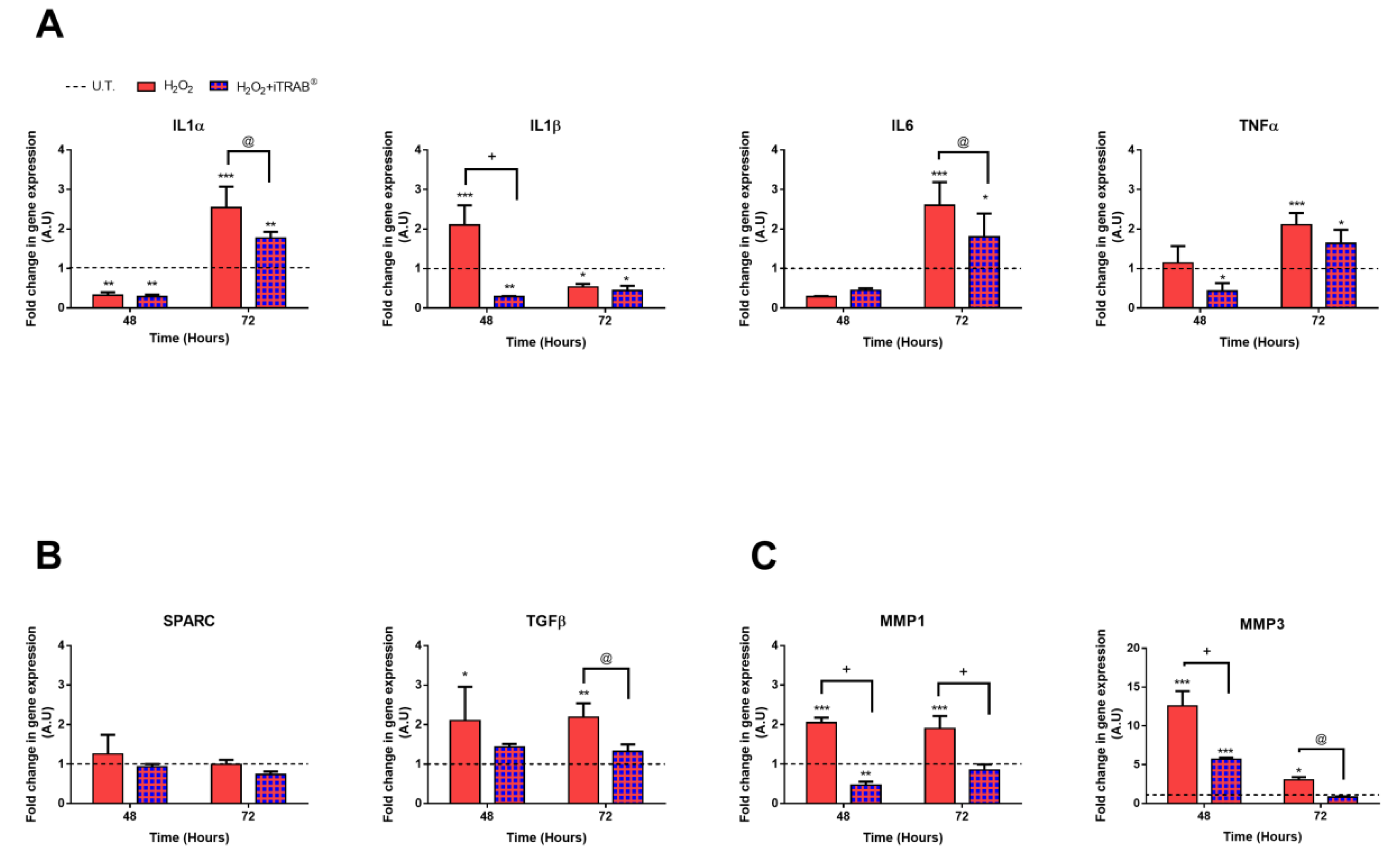
3.5. Gene Expression Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sacca, S.C.; Corazza, P.; Gandolfi, S.A.; Ferrari, D.; Sukkar, S.G.; Iorio, E.L.; Traverso, C.E. Substances of Interest That Support Glaucoma Therapy. Nutrients 2019, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, V.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Vernazza, S.; Iorio, E.L.; Tirendi, S.; Bassi, A.M.; Gandolfi, S.; Izzotti, A. Molecular changes in glaucomatous trabecular meshwork. Correlations with retinal ganglion cell death and novel strategies for neuroprotection. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 256, pp. 151–188. [Google Scholar]
- Tabrez, S.; Priyadarshini, M.; Urooj, M.; Shakil, S.; Ashraf, G.M.; Khan, M.S.; Kamal, M.A.; Alam, Q.; Jabir, N.R.; Abuzenadah, A.M.; et al. Cancer Chemoprevention by Polyphenols and Their Potential Application as Nanomedicine. J. Environ. Sci. Health Part C 2013, 31, 67–98. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric Oxide Production and Signaling in Inflammation. Curr. Drug Target Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Španinger, E.; Hrnčič, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molcules 2016, 21, 901. [Google Scholar] [CrossRef]
- De Kok, T.M.; Van Breda, S.G.; Manson, M.M. Mechanisms of combined action of different chemopreventive dietary compounds. Eur. J. Nutr. 2008, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In Vitro and in Vivo Antitumoral Effects of Combinations of Polyphenols, or Polyphenols and Anticancer Drugs: Perspectives on Cancer Treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, B.R.; Hansen, J.M. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem. J. 2009, 424, 491–500. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, J.; Qi, J. Role of nuclear factor (erythroid-derived 2)-like 2 in the age-resistant properties of the glaucoma trabecular meshwork. Exp. Ther. Med. 2017, 14, 791–796. [Google Scholar] [CrossRef]
- Izzotti, A.; Saccà, S.C.; Longobardi, M.; Cartiglia, C. Sensitivity of Ocular Anterior Chamber Tissues to Oxidative Damage and Its Relevance to the Pathogenesis of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5251–5258. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Izzotti, A. Focus on molecular events in the anterior chamber leading to glaucoma. Cell. Mol. Life Sci. 2013, 71, 2197–2218. [Google Scholar] [CrossRef]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Bagnis, A.; Saccà, S.C. The role of oxidative stress in glaucoma. Mutat. Res. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef]
- Vernazza, S.; Tirendi, S.; Bassi, A.M.; Traverso, C.E.; Saccà, S.C. Neuroinflammation in Primary Open-Angle Glaucoma. J. Clin. Med. 2020, 9, 3172. [Google Scholar] [CrossRef]
- Alvarado, J.; Murphy, C.; Juster, R. Trabecular Meshwork Cellularity in Primary Open-angle Glaucoma and Nonglaucomatous Normals. Ophthalmology 1984, 91, 564–579. [Google Scholar] [CrossRef]
- Avotri, S.; Eatman, D.; Russell-Randall, K. Effects of Resveratrol on Inflammatory Biomarkers in Glaucomatous Human Trabecular Meshwork Cells. Nutrients 2019, 11, 984. [Google Scholar] [CrossRef]
- Acott, T.S.; Kelley, M.J. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008, 86, 543–561. [Google Scholar] [CrossRef]
- Saccà, S.C.; Izzotti, A. Oxidative stress and glaucoma: Injury in the anterior segment of the eye. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 173, pp. 385–407. ISBN 978-0-444-53256-5. [Google Scholar]
- Pinazo-Durán, M.D.; Shoaie-Nia, K.; Zanon-Moreno, V.; Sanz-González, S.M.; Del Castillo, F.B.; García-Medina, J.J. Strategies to Reduce Oxidative Stress in Glaucoma Patients. Curr. Neuropharmacol. 2018, 16, 903–918. [Google Scholar] [CrossRef]
- D’Azy, C.B.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166915. [Google Scholar] [CrossRef]
- Overby, D.R.; Stamer, W.D.; Johnson, M. The changing paradigm of outflow resistance generation: Towards synergistic models of the JCT and inner wall endothelium. Exp. Eye Res. 2009, 88, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Y.; Luo, L. Effect of Myricetin on Primary Open-angle Glaucoma. Transl. Neurosci. 2018, 9, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Gandolfi, S.; Bagnis, A.; Manni, G.; Damonte, G.; Traverso, C.E.; Izzotti, A. From DNA damage to functional changes of the trabecular meshwork in aging and glaucoma. Ageing Res. Rev. 2016, 29, 26–41. [Google Scholar] [CrossRef]
- Almeida, S.; Alves, M.G.; Sousa, M.; Oliveira, P.F.; Silva, B.M. Are Polyphenols Strong Dietary Agents Against Neurotoxicity and Neurodegeneration? Neurotox. Res. 2016, 30, 345–366. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free. Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- D’Angelo, S. Current Evidence on the Effect of Dietary Polyphenols Intake on Brain Health. Curr. Nutr. Food Sci. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P.V. Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef]
- Vernazza, S.; Tirendi, S.; Scarfì, S.; Passalacqua, M.; Oddone, F.; Traverso, C.E.; Rizzato, I.; Bassi, A.M.; Saccà, S.C. 2D- and 3D-cultures of human trabecular meshwork cells: A preliminary assessment of an in vitro model for glaucoma study. PLoS ONE 2019, 14, e0221942. [Google Scholar] [CrossRef]
- Saccà, S.C.; Tirendi, S.; Scarfì, S.; Passalacqua, M.; Oddone, F.; Traverso, C.E.; Vernazza, S.; Bassi, A.M. An advanced in vitro model to assess glaucoma onset. ALTEX 2020. [CrossRef]
- Available online: https://www.cellapplications.com/human-trabecular-meshwork-cells-htmc (accessed on 4 November 2020).
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Eucciferri, N.; Esbrana, T.; Ahluwalia, A. Allometric Scaling and Cell Ratios in Multi-Organ in vitro Models of Human Metabolism. Front. Bioeng. Biotechnol. 2014, 2, 74. [Google Scholar] [CrossRef]
- Sacca, S.C.; Pulliero, A.; La Maestra, S.; Geretto, M.; Profumo, A.; Ilderbayev, O.; Rosano, C.; Izzotti, A. Protection of trabecular meshwork cells by eyedrops containing high concentration of polyphenols. New Front. Ophthalmol. 2019, 5, 5. [Google Scholar] [CrossRef]
- Poehlmann, A.; Reissig, K.; Schönfeld, P.; Walluscheck, D.; Schinlauer, A.; Hartig, R.; Lessel, W.; Guenther, T.; Silver, A.; Roessner, A. Repeated H2O2exposure drives cell cycle progression in aninvitromodel of ulcerative colitis. J. Cell. Mol. Med. 2013, 17, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Kaczara, P.; Sarna, T.; Burke, J.M. Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress. Free. Radic. Biol. Med. 2010, 48, 1064–1070. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader11Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may be suitable. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Aarskog, N.K.; Vedeler, C. Real-time quantitative polymerase chain reaction. Qual. Life Res. 2000, 107, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2009, 3, research0034.1. [Google Scholar] [CrossRef]
- Tektas, O.-Y.; Lütjen-Drecoll, E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 2009, 88, 769–775. [Google Scholar] [CrossRef]
- Foster, A.; Resnikoff, S. The impact of Vision 2020 on global blindness. Eye 2005, 19, 1133–1135. [Google Scholar] [CrossRef]
- Saccà, S.C.; La Maestra, S.; Micale, R.T.; Larghero, P.; Travaini, G.; Baluce, B.; Izzotti, A. Ability of Dorzolamide Hydrochloride and Timolol Maleate to Target Mitochondria in Glaucoma Therapy. Arch. Ophthalmol. 2011, 129, 48. [Google Scholar] [CrossRef][Green Version]
- Fahmy, H.M.; Saad, E.A.E.-M.S.; Sabra, N.M.; El-Gohary, A.A.; Mohamed, F.F.; Gaber, M.H. Treatment merits of Latanoprost/Thymoquinone – Encapsulated liposome for glaucomatus rabbits. Int. J. Pharm. 2018, 548, 597–608. [Google Scholar] [CrossRef]
- Izzotti, A.; Longobardi, M.; Cartiglia, C.; Saccà, S.C. Mitochondrial Damage in the Trabecular Meshwork Occurs Only in Primary Open-Angle Glaucoma and in Pseudoexfoliative Glaucoma. PLoS ONE 2011, 6, e14567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, T.; Jiang, Y.; Wu, L.; Cai, X.; Sun, X.; Sun, X. Photooxidative damage in retinal pigment epithelial cells via GRP78 and the protective role of grape skin polyphenols. Food Chem. Toxicol. 2014, 74, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Vargas, J.L.C.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Liton, P.B.; Qiu, J.; Epstein, D.L.; Challa, P.; Gonzalez, P. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem. Toxicol. 2009, 47, 198–204. [Google Scholar] [CrossRef]
- Inoue-Mochita, M.; Inoue, T.; Kojima, S.; Futakuchi, A.; Fujimoto, T.; Sato-Ohira, S.; Tsutsumi, U.; Tanihara, H. Interleukin-6–mediated trans-signaling inhibits transforming growth factor-β signaling in trabecular meshwork cells. J. Biol. Chem. 2018, 293, 10975–10984. [Google Scholar] [CrossRef]
- De Groef, L.; Van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the Trabecular Meshwork: Promising Targets for Future Glaucoma Therapies? Investig. Ophthalmol. Vis. Sci. 2013, 54, 7756–7763. [Google Scholar] [CrossRef]
- Kelley, M.J.; Rose, A.Y.; Song, K.; Chen, Y.; Bradley, J.M.; Rookhuizen, D.; Acott, T.S. Synergism of TNF and IL-1 in the Induction of Matrix Metalloproteinase-3 in Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2634–2643. [Google Scholar] [CrossRef]
- Li, G.; Luna, C.; Liton, P.B.; Navarro, I.; Epstein, D.L.; Gonzalez, P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol. Vis. 2007, 13, 2282–2288. [Google Scholar]
- Tezel, G.; Wax, M.B. Increased Production of Tumor Necrosis Factor-α by Glial Cells Exposed to Simulated Ischemia or Elevated Hydrostatic Pressure Induces Apoptosis in Cocultured Retinal Ganglion Cells. J. Neurosci. 2000, 20, 8693–8700. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Cannon, J.G. Cytokines in Aging and Muscle Homeostasis. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1995, 50A, 120–123. [Google Scholar] [CrossRef]
- Fleenor, D.L.; Shepard, A.R.; Hellberg, P.E.; Jacobson, N.; Pang, I.-H.; Clark, A.F. TGFβ2-Induced Changes in Human Trabecular Meshwork: Implications for Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2006, 47, 226–234. [Google Scholar] [CrossRef]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef]
- Wang, J.; Harris, A.; Prendes, M.A.; Alshawa, L.; Gross, J.C.; Wentz, S.M.; Rao, A.B.; Kim, N.J.; Synder, A.; Siesky, B. Targeting Transforming Growth Factor-β Signaling in Primary Open-Angle Glaucoma. J. Glaucoma 2017, 26, 390–395. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Matricellular Proteins in Cardiac Adaptation and Disease. Physiol. Rev. 2012, 92, 635–688. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H. Matrix metalloproteinases. In Zinc Metalloproteases in Health and Disease; CRC Press: Boca Raton, FL, USA, 1996; pp. 173–224. [Google Scholar]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G. The Role of Matrix Metalloproteinases in Tumor Invasion, Metastasis, and Angiogenesis. Surg. Oncol. Clin. N. Am. 2001, 10, 383–392. [Google Scholar] [CrossRef]

| Gene | GenBank | F | R |
|---|---|---|---|
| IL1α | NM_000575.4 | CAATCTgTgTCTCTgAgTATC | TCAACCgTCTCTTCTTCA |
| IL1β | NM_000576.2 | TgATggCTTATTACAgTggCAATg | gTAgTggTggTCggAgATTCg |
| IL6 | NM_001318095.1 | CAgATTTgAgAgTAgTgAggAAC | CgCAgAATgAgATgAgTTgTC |
| TNFα | NM_000594.4 | GTGAGGAGGACGAACATC | GAGCCAGAAGAGGTTGAG |
| TGFβ2 | NM_001135599.3 | AACCTCTAACCATTCTCTACTACA | CgTCgTCATCATCATTATCATCA |
| SPARC | NM_003118.4 | ATggTTCCTgTAAgCACTAA | TgAATgAATgAATgAATgAATgAC |
| MMP1 | NM_001145938.1 | ggTgATgAAgCAgCCCAgATg | CAgAggTgTgACATTACTCCAgAg |
| MMP3 | NM_002422.5 | TAATAATTCTTCACCTAAgTCTCT | AgATTCACgCTCAAgTTC |
| HPRT-1 | NM_000194.3 | GGTCAGGCAGTATAATCCAAAG | TTCATTATAGTCAAGGGCATATCC |
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | POS | POS | NEG | NEG | Blank | Blank | bad | bax | Bcl2 | Bcl2-w | BID | BIM | Caspase3 | Caspase8 |
| 2 | ||||||||||||||
| 3 | CD40 | CD40L | cIAP2 | CytoC | DR6 | Fas | FasL | Blank | Hsp27 | Hsp60 | Hsp70 | HTRA2 | IGF1 | IGF2 |
| 4 | ||||||||||||||
| 5 | IGFBP1 | IGFBP2 | IGFBP3 | IGFBP4 | IGFBP5 | IGFBP6 | IGF-1R | Livin | P21 | P27 | P53 | SMAC | Survivn | TNF RI |
| 6 | ||||||||||||||
| 7 | TNF RII | TNFα | TNFβ | TRAIL R1 | TRAIL R2 | TRAIL R3 | TRAIL R4 | XIAP | Blank | Blank | NEG | NEG | POS | POS |
| 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saccà, S.C.; Izzotti, A.; Vernazza, S.; Tirendi, S.; Scarfì, S.; Gandolfi, S.; Bassi, A.M. Can Polyphenols in Eye Drops Be Useful for Trabecular Protection from Oxidative Damage? J. Clin. Med. 2020, 9, 3584. https://doi.org/10.3390/jcm9113584
Saccà SC, Izzotti A, Vernazza S, Tirendi S, Scarfì S, Gandolfi S, Bassi AM. Can Polyphenols in Eye Drops Be Useful for Trabecular Protection from Oxidative Damage? Journal of Clinical Medicine. 2020; 9(11):3584. https://doi.org/10.3390/jcm9113584
Chicago/Turabian StyleSaccà, Sergio Claudio, Alberto Izzotti, Stefania Vernazza, Sara Tirendi, Sonia Scarfì, Stefano Gandolfi, and Anna Maria Bassi. 2020. "Can Polyphenols in Eye Drops Be Useful for Trabecular Protection from Oxidative Damage?" Journal of Clinical Medicine 9, no. 11: 3584. https://doi.org/10.3390/jcm9113584
APA StyleSaccà, S. C., Izzotti, A., Vernazza, S., Tirendi, S., Scarfì, S., Gandolfi, S., & Bassi, A. M. (2020). Can Polyphenols in Eye Drops Be Useful for Trabecular Protection from Oxidative Damage? Journal of Clinical Medicine, 9(11), 3584. https://doi.org/10.3390/jcm9113584







