Pioglitazone Is Associated with Lower Major Adverse Cardiovascular and Cerebrovascular Events than DPP4-Inhibitors in Diabetic Patients with End-Stage Renal Disease: A Taiwan Nationwide Cohort Study, 2006–2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design
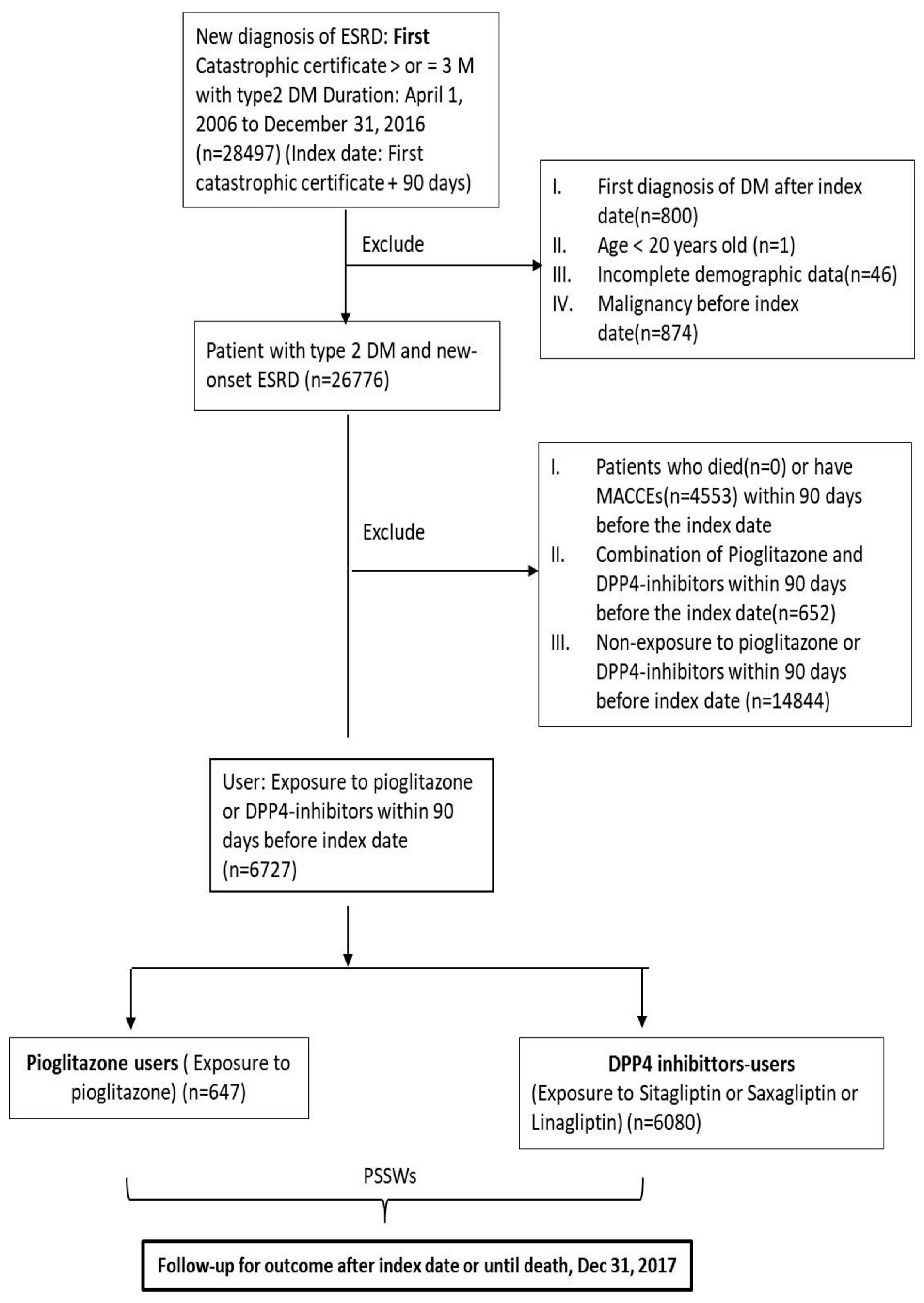
2.3. Patient Selection
2.4. Exposure
2.5. Covariates and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
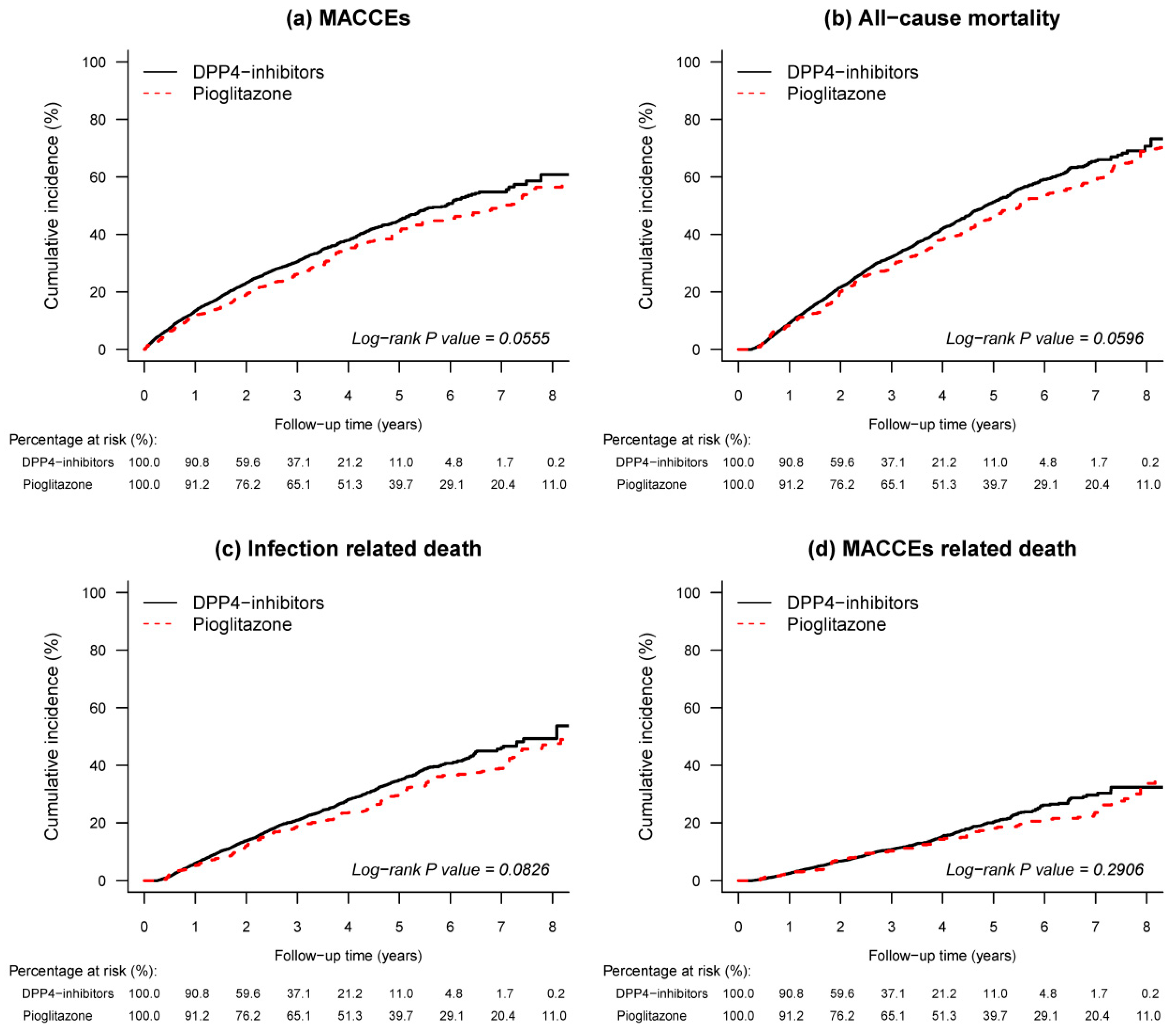
3.2. Outcomes
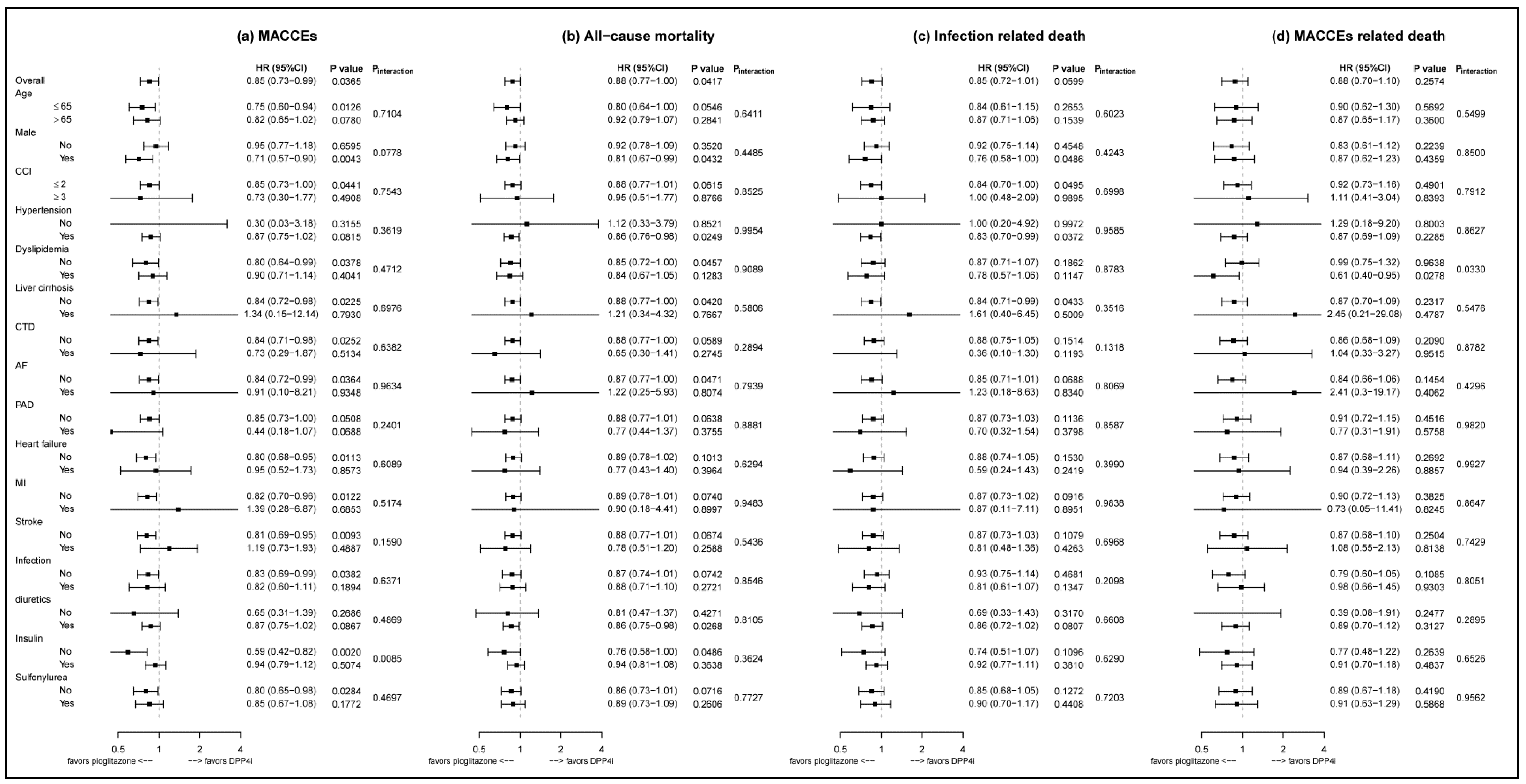
3.3. Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maeda, S.; Sawayama, Y.; Furusyo, N.; Shigematsu, M.; Hayashi, J. The association between fatal vascular events and risk factors for carotid atherosclerosis in patients on maintenance hemodialysis: Plaque number of dialytic atherosclerosis study. Atherosclerosis 2009, 204, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Zen, K.; Tamaki, N.; Nishimura, M.; Nakatani, E.; Moroi, M.; Nishimura, T.; Hasebe, N.; Kikuchi, K. Cardiac event risk stratification in patients with end-stage renal disease: Sub-analysis of the B-SAFE study. Int. J. Cardiol. 2016, 202, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.U.; Gillespie, I.A.; Kronenberg, F.; Richards, S.; Stenvinkel, P.; Anker, S.D.; Wheeler, D.C.; de Francisco, A.L.; Marcelli, D.; Froissart, M.; et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 2015, 88, 1117–1125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Y.; Stamm, C.; Nobre, D.; Pruijm, M.; Teta, D.; Cherpillod, A.; Halabi, G.; Phan, O.; Fumeaux, Z.; Bullani, R.; et al. Changing trends in end-stage renal disease patients with diabetes. Swiss. Med. Wkly 2017, 147, w14458. [Google Scholar]
- Hwang, S.J.; Tsai, J.C.; Chen, H.C. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology (Carlton) 2010, 15 (Suppl. 2), 3–9. [Google Scholar] [CrossRef]
- Abe, M.; Okada, K. DPP-4 Inhibitors in Diabetic Patients with Chronic Kidney Disease and End-Stage Kidney Disease on Dialysis in Clinical Practice. Contrib. Nephrol. 2015, 185, 98–115. [Google Scholar] [PubMed]
- Gianchandani, R.Y.; Neupane, S.; Iyengar, J.J.; Heung, M. Pathophysiology and management of hypoglycemiain end-stage renal disease patients: A review. Endocr. Pract. 2017, 23, 353–362. [Google Scholar] [CrossRef]
- American Diabetes Association Addendum. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), s98–s110. [Google Scholar] [CrossRef]
- Park, S.H.; Nam, J.Y.; Han, E.; Lee, Y.H.; Lee, B.W.; Kim, B.S.; Cha, B.S.; Kim, C.S.; Kang, E.S. Efficacy of different dipeptidyl peptidase-4 (DPP-4) inhibitors on metabolic parameters in patients with type 2 diabetes undergoing dialysis. Medicine (Baltimore) 2016, 95, e4543. [Google Scholar] [CrossRef]
- Trevisan, R. The Role of Vildagliptin in the Therapy of Type 2 Diabetic Patients with Renal Dysfunction. Diabetes Ther. 2017, 8, 1215–1226. [Google Scholar] [CrossRef]
- Walker, S.R.; Komenda, P.; Khojah, S.; Al-Tuwaijri, W.; MacDonald, K.; Hiebert, B.; Tangri, N.; Nadurak, S.W.D.; Ferguson, T.W.; Rigatto, C.; et al. Dipeptidyl Peptidase-4 Inhibitors in Chronic Kidney Disease: A Systematic Review of Randomized Clinical Trials. Nephron 2017, 136, 85–94. [Google Scholar] [CrossRef]
- Gallwitz, B. Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factors. Ther. Adv. Endocrinol. Metab. 2013, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Ou, S.M.; Chen, Y.T.; Shih, C.J. Effects of DPP-4 inhibitors on cardiovascular outcomes in patients with type 2 diabetes and end-stage renal disease. Int. J. Cardiol. 2016, 218, 170–175. [Google Scholar] [CrossRef]
- Wang, M.T.; Lin, S.C.; Tang, P.L.; Hung, W.T.; Cheng, C.C.; Yang, J.S.; Chang, H.T.; Liu, C.P.; Mar, G.Y.; Huang, W.C. The impact of DPP-4 inhibitors on long-term survival among diabetic patients after first acute myocardial infarction. Cardiovasc. Diabetol. 2017, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ushiyama, C.; Osada, S.; Shimada, N.; Ebihara, I.; Koide, H. Effect of pioglitazone on dyslipidemia in hemodialysis patients with type 2 diabetes. Ren. Fail. 2001, 23, 863–864. [Google Scholar] [PubMed]
- Manley, H.J.; Allcock, N.M. Thiazolidinedione safety and efficacy in ambulatory patients receiving hemodialysis. Pharmacotherapy 2003, 23, 861–865. [Google Scholar] [CrossRef]
- Abe, M.; Okada, K.; Kikuchi, F.; Matsumoto, K. Clinical investigation of the effects of pioglitazone on the improvement of insulin resistance and blood pressure in type 2-diabetic patients undergoing hemodialysis. Clin. Nephrol. 2008, 70, 220–228. [Google Scholar] [CrossRef]
- Khanderia, U.; Pop-Busui, R.; Eagle, K.A. Thiazolidinediones in type 2 diabetes: A cardiology perspective. Ann. Pharm. 2008, 42, 1466–1474. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Mohamed, M.; Ahmad, I.; Kamal, M.A.; Donnelly, R.; Idris, I.; Gan, S.H. Efficacy and Safety of Pioglitazone Monotherapy in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Sci. Rep. 2019, 9, 5389. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Kwong, J.S.W.; Li, L.; Li, Y.; Sun, X. Efficacy and safety of thiazolidinediones in diabetes patients with renal impairment: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 1717. [Google Scholar] [CrossRef]
- Han, L.; Shen, W.J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-beta/delta and PPAR-gamma. Future Cardiol. 2017, 13, 279–296. [Google Scholar] [CrossRef]
- Legchenko, E.; Chouvarine, P.; Borchert, P.; Fernandez-Gonzalez, A.; Snay, E.; Meier, M.; Maegel, L.; Mitsialis, S.A.; Rog-Zielinska, E.A.; Kourembanas, S.; et al. PPARgamma agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci. Transl. Med. 2018, 10, eaao0303. [Google Scholar] [CrossRef]
- Culman, J.; Zhao, Y.; Gohlke, P.; Herdegen, T. PPAR-gamma: Therapeutic target for ischemic stroke. Trends Pharmacol. Sci. 2007, 28, 244–249. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefebvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Schneider, C.A.; Ferrannini, E.; Defronzo, R.; Schernthaner, G.; Yates, J.; Erdmann, E. Effect of pioglitazone on cardiovascular outcome in diabetes and chronic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 182–187. [Google Scholar] [CrossRef]
- Krentz, A. Thiazolidinediones: Effects on the development and progression of type 2 diabetes and associated vascular complications. Diabetes Metab. Res. Rev. 2009, 25, 112–126. [Google Scholar] [CrossRef]
- Yoshii, H.; Onuma, T.; Yamazaki, T.; Watada, H.; Matsuhisa, M.; Matsumoto, M.; Kitagawa, K.; Kitakaze, M.; Yamasaki, Y.; Kawamori, R. Effects of pioglitazone on macrovascular events in patients with type 2 diabetes mellitus at high risk of stroke: The PROFIT-J study. J. Atheroscler Thromb. 2014, 21, 563–573. [Google Scholar] [CrossRef]
- Zhao, S.J.; Zhong, Z.S.; Qi, G.X.; Shi, L.Y.; Chen, L.; Tian, W. Effect of Pioglitazone in Preventing In-Stent Restenosis after Percutaneous Coronary Intervention in Patients with Type 2 Diabetes: A Meta-Analysis. PLoS ONE 2016, 11, e0155273. [Google Scholar] [CrossRef]
- Strongman, H.; Korhonen, P.; Williams, R.; Bahmanyar, S.; Hoti, F.; Christopher, S.; Majak, M.; Kool-Houweling, L.; Linder, M.; Dolin, P.; et al. Pioglitazone and risk of mortality in patients with type 2 diabetes: Results from a European multidatabase cohort study. BMJ Open Diabetes Res. Care 2017, 5, e000364. [Google Scholar] [CrossRef]
- De Jong, M.; van der Worp, H.B.; van der Graaf, Y.; Visseren, F.L.J.; Westerink, J. Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc. Diabetol. 2017, 16, 134. [Google Scholar] [CrossRef]
- Liao, H.W.; Saver, J.L.; Wu, Y.L.; Chen, T.H.; Lee, M.; Ovbiagele, B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: A systematic review and meta-analysis. BMJ Open 2017, 7, e013927. [Google Scholar] [CrossRef]
- National Health Insurance Administration. Ministry of Health and Welfare, Taiwan. National Health Insurance Annual Statistical Report; National Health Insurance Administration: Taipei City, Taiwan, 2018.
- Sharma, M.; Nazareth, I.; Petersen, I. Observational studies of treatment effectiveness: Worthwhile or worthless? Clin. Epidemiol. 2019, 11, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.C.; Paget, S.A.; Lachs, M.S.; Reid, M.C.; Charlson, M.E. The risk of comorbidity. Ann. Rheum. Dis. 2012, 71, 635–637. [Google Scholar] [CrossRef]
- Xu, S.; Ross, C.; Raebel, M.A.; Shetterly, S.; Blanchette, C.; Smith, D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 2010, 13, 273–277. [Google Scholar] [CrossRef]
- McCaffrey, D.F.; Griffin, B.A.; Almirall, D.; Slaughter, M.E.; Ramchand, R.; Burgette, L.F. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat. Med. 2013, 32, 3388–3414. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Fisman, E.Z.; Tenenbaum, A. Antidiabetic treatment with gliptins: Focus on cardiovascular effects and outcomes. Cardiovasc. Diabetol. 2015, 14, 129. [Google Scholar] [CrossRef]
- Wilcox, R.; Kupfer, S.; Erdmann, E. Effects of pioglitazone on major adverse cardiovascular events in high-risk patients with type 2 diabetes: Results from PROspective pioglitAzone Clinical Trial In macro Vascular Events (PROactive 10). Am. Heart. J. 2008, 155, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, S.M.; Thadhani, R.; Ikizler, T.A.; Feldman, H.I. Thiazolidinedione use is associated with better survival in hemodialysis patients with non-insulin dependent diabetes. Kidney Int. 2009, 75, 961–968. [Google Scholar] [CrossRef]
- Gianchandani, R.Y.; Neupane, S.; Heung, M. Hypoglycemia in Hospitalized Hemodialysis Patients with Diabetes: An Observational Study. J. Diabetes Sci. Technol. 2018, 12, 33–38. [Google Scholar] [CrossRef]
- Rhee, C.M.; Leung, A.M.; Kovesdy, C.P.; Lynch, K.E.; Brent, G.A.; Kalantar-Zadeh, K. Updates on the management of diabetes in dialysis patients. Semin. Dial. 2014, 27, 135–145. [Google Scholar] [CrossRef]
- Herman, M.E.; O’Keefe, J.H.; Bell, D.S.H.; Schwartz, S.S. Insulin Therapy Increases Cardiovascular Risk in Type 2 Diabetes. Prog. Cardiovasc. Dis. 2017, 60, 422–434. [Google Scholar] [CrossRef]
- Mazzone, T.; Meyer, P.M.; Feinstein, S.B.; Davidson, M.H.; Kondos, G.T.; D’Agostino, R.B.; Perez, A.; Provost, J.-C.; Haffner, S.M. Effect of Pioglitazone Compared with Glimepiride on Carotid Intima-Media Thickness in Type 2 DiabetesA Randomized Trial. JAMA 2006, 296, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Nicholls, S.J.; Wolski, K.; Nesto, R.; Kupfer, S.; Perez, A.; Jure, H.; De Larochelliere, R.; Staniloae, C.S.; Mavromatis, K.; et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: The PERISCOPE randomized controlled trial. (1538–3598 (Electronic)). JAMA 2008, 299, 1561–1573. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Jandeleit-Dahm, K.A. Dipeptidyl peptidase-4 inhibitors and cardiovascular and renal disease in type 2 diabetes: What have we learned from the CARMELINA trial? Diab. Vasc. Dis. Res. 2019, 16, 303–309. [Google Scholar] [CrossRef]
- Chen, D.Y.; Wang, S.H.; Mao, C.T.; Tsai, M.L.; Lin, Y.S.; Chou, C.C.; Wen, M.S.; Wang, C.C.; Hsieh, I.C.; Hung, K.C.; et al. Sitagliptin and cardiovascular outcomes in diabetic patients with chronic kidney disease and acute myocardial infarction: A nationwide cohort study. Int. J. Cardiol. 2015, 181, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Huang, Y.Y.; Wu, Y.L.; Lin, C.W.; Chen, P.C.; Chang, C.J.; Hsieh, S.H.; Sun, J.H.; Chen, S.T.; Lin, C.H. Coadministration of DPP-4 inhibitor and insulin therapy does not further reduce the risk of cardiovascular events compared with DPP-4 inhibitor therapy in diabetic foot patients: A nationwide population-based study. Diabetol. Metab. Syndr. 2018, 10, 75. [Google Scholar] [CrossRef]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef] [PubMed]
| Before PSSW | After PSSW | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone (n = 647) | DPP4i (n = 6080) | ASMD | Pioglitazone (n = 647) | DPP4i (n = 6080) | ASMD | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||
| Age (year) | ||||||||||
| Mean ± SD | 64.64 | ±11.75 | 65.07 | ±12.50 | 0.0352 | 64.98 | ±11.00 | 65.05 | ±12.43 | 0.0054 |
| <65 | 308 | (47.60) | 2916 | (47.96) | 0.1119 | 264.1 | (48.19) | 2906.5 | (47.96) | 0.0278 |
| ≥65 | 339 | (52.39) | 3164 | (52.04) | 283.9 | (51.81) | 3153.7 | (52.04) | ||
| Gender | ||||||||||
| Male | 299 | (46.21) | 3171 | (52.15) | 0.1191 | 282.3 | (51.51) | 3130.7 | (51.66) | 0.0029 |
| Female | 348 | (53.79) | 2909 | (47.85) | 265.7 | (48.49) | 2929.5 | (48.34) | ||
| Income level | ||||||||||
| ≥25,000 | 58 | (8.96) | 558 | (9.18) | 0.0776 | 45.4 | (8.28) | 553.9 | (9.14) | 0.0886 |
| 15,000–25,000 | 167 | (25.81) | 1464 | (24.08) | 137.9 | (25.16) | 1466.8 | (24.20) | ||
| <15,000 or dependent | 422 | (65.22) | 4058 | (66.75) | 364.8 | (66.56) | 4039.5 | (66.66) | ||
| Place of residence | ||||||||||
| Urban | 141 | (21.79) | 1626 | (26.74) | 0.1497 | 149.4 | (27.27) | 1596.7 | (26.35) | 0.0498 |
| Suburban | 163 | (25.19) | 1590 | (26.15) | 142.0 | (25.91) | 1579.8 | (26.07) | ||
| Rural | 343 | (53.02) | 2864 | (47.10) | 205.4 | (37.48) | 2363.2 | (39.00) | ||
| Missing | 74 | (11.44) | 506 | (8.32) | 51.2 | (9.34) | 520.2 | (8.59) | ||
| Charlson comorbidity index | ||||||||||
| 0 | 357 | (55.18) | 3015 | (49.59) | 0.0945 | 305.5 | (55.74) | 3019.2 | (49.82) | 0.1106 |
| 1 | 191 | (29.52) | 2018 | (33.19) | 163. 1 | (29.76) | 2006.6 | (33.11) | ||
| 2 | 69 | (10.66) | 741 | (12.19) | 54.2 | (9.90) | 734.0 | (12.11) | ||
| 3 | 25 | (3.86) | 243 | (4.00) | 22.0 | (4.01) | 238.3 | (3.93) | ||
| 4+ | 5 | (0.78) | 63 | (1.03) | 3.3 | (0.59) | 62.2 | (1.03) | ||
| Comorbidities (within 1 year before index date) | ||||||||||
| Hypertension | 617 | (95.40) | 5835 | (96.00) | 0.0298 | 525.9 | (96.00) | 5815.5 | (96.00) | 0.0000 |
| Dyslipidemia | 284 | (43.90) | 2691 | (44.30) | 0.0073 | 245.7 | (44.80) | 2678.7 | (44.20) | 0.0134 |
| Liver cirrhosis | 20 | (3.09) | 233 | (3.83) | 0.0405 | 15.7 | (2.87) | 228.5 | (3.77) | 0.0519 |
| Connective tissue disease | 28 | (4.33) | 276 | (4.54) | 0.0103 | 21.0 | (3.82) | 273.8 | (4.52) | 0.0361 |
| Atrial fibrillation | 16 | (2.47) | 245 | (4.03) | 0.0878 | 16.5 | (3.01) | 235.5 | (3.89) | 0.0496 |
| Peripheral artery disease | 33 | (5.10) | 345 | (5.67) | 0.0254 | 20.7 | (3.77) | 338.8 | (5.59) | 0.0890 |
| Hospitalization history (within 3 years before index date) | ||||||||||
| Heart failure | 43 | (6.65) | 653 | (10.70) | 0.1457 | 43.6 | (7.95) | 628.0 | (10.40) | 0.0867 |
| Myocardial infarction | 18 | (2.78) | 205 | (3.37) | 0.0341 | 18.4 | (3.36) | 202.0 | (3.33) | 0.0016 |
| Stroke | 51 | (7.88) | 443 | (7.29) | 0.0225 | 32.3 | (5.89) | 443.1 | (7.31) | 0.0594 |
| Infection | 195 | (30.10) | 2002 | (32.90) | 0.0600 | 184.31 | (33.60) | 1982.13 | (32.70) | 0.0205 |
| Medication (within 90 days before index date) | ||||||||||
| ACEi or ARB | 452 | (69.90) | 3712 | (61.10) | 0.1860 | 343.5 | (62.70) | 3746.0 | (61.80) | 0.0185 |
| Other anti-HTN | 598 | (92.40) | 5701 | (93.80) | 0.0528 | 518.6 | (94.60) | 5678.2 | (93.70) | 0.0414 |
| Diuretics | 590 | (91.20) | 5514 | (90.70) | 0.0174 | 499.9 | (91.20) | 5502.0 | (90.80) | 0.0154 |
| Aspirin or Plavix | 252 | (38.90) | 2453 | (40.30) | 0.0285 | 209.8 | (38.30) | 2439.3 | (40.30) | 0.0421 |
| NSAIDs | 166 | (25.70) | 1229 | (20.20) | 0.1297 | 114.3 | (20.80) | 1252.0 | (20.70) | 0.0048 |
| Insulin | 442 | (68.30) | 3299 | (54.30) | 0.2915 | 331.3 | (60.50) | 3366.3 | (55.50) | 0.1036 |
| Sulfonylurea | 264 | (40.80) | 1014 | (16.70) | 0.5528 | 109.5 | (20.00) | 1141.3 | (18.80) | 0.0305 |
| Acarbose | 10 | (1.55) | 52 | (0.86) | 0.0634 | 5.7 | (1.04) | 55.1 | (0.91) | 0.0140 |
| Meglitinides | 274 | (42.30) | 1445 | (23.80) | 0.4028 | 146.3 | (26.70) | 1543.9 | (25.50) | 0.0291 |
| GLP-1 | 0 | (0.00) | 1 | (0.01) | 0.0181 | 0.0 | (0.00) | 0.9 | (0.02) | 0.0176 |
| Anti-cholesterol | 281 | (43.40) | 2721 | (44.80) | 0.0266 | 248.4 | (45.30) | 2707.7 | (44.70) | 0.0133 |
| Pioglitazone (n = 647) | DPP4i (n = 6080) | Cox Results | ||||
|---|---|---|---|---|---|---|
| Events | Rate (95% CI) | Events | Rate (95% CI) | HR (95% CI) | p Value | |
| Before PSSW | ||||||
| MACCEs | 234 | 10.18 (8.87–11.48) | 1754 | 12.64 (12.05–13.24) | 0.82 (0.71–0.95) | 0.0063 |
| All-cause mortality | 377 | 13.07 (11.75–14.39) | 2224 | 13.23 (12.68–13.78) | 0.88 (0.78–0.98) | 0.0262 |
| Infection related death | 226 | 7.84 (6.81–8.86) | 1343 | 7.99 (7.56–8.42) | 0.89 (0.77–1.03) | 0.1214 |
| MACCEs related death | 117 | 4.06 (3.32–4.79) | 680 | 4.05 (3.74–4.35) | 0.83 (0.67–1.02) | 0.0785 |
| After PSSW | ||||||
| MACCEs | 190.3 | 10.48 (8.99–11.97) | 1766.9 | 12.62 (12.03–13.21) | 0.85 (0.73–0.99) | 0.0365 |
| All-cause mortality | 291.7 | 12.86 (11.39–14.34) | 2247.4 | 13.22 (12.67–13.77) | 0.88 (0.77–0.99) | 0.0417 |
| Infection related death | 168.3 | 7.42 (6.30–8.54) | 1357.3 | 7.98 (7.56–8.41) | 0.85 (0.72–1.01) | 0.0599 |
| MACCEs related death | 95.2 | 4.20 (3.35–5.04) | 687.6 | 4.04 (3.74–4.35) | 0.88 (0.70–1.1) | 0.2574 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-H.; Yang, H.-Y.; Yen, C.-L.; Wu, C.-Y.; Jenq, C.-C.; Kuo, G.; Peng, W.-S.; Liu, J.-R.; Tian, Y.-C.; Yang, C.-W.; et al. Pioglitazone Is Associated with Lower Major Adverse Cardiovascular and Cerebrovascular Events than DPP4-Inhibitors in Diabetic Patients with End-Stage Renal Disease: A Taiwan Nationwide Cohort Study, 2006–2016. J. Clin. Med. 2020, 9, 3578. https://doi.org/10.3390/jcm9113578
Lin M-H, Yang H-Y, Yen C-L, Wu C-Y, Jenq C-C, Kuo G, Peng W-S, Liu J-R, Tian Y-C, Yang C-W, et al. Pioglitazone Is Associated with Lower Major Adverse Cardiovascular and Cerebrovascular Events than DPP4-Inhibitors in Diabetic Patients with End-Stage Renal Disease: A Taiwan Nationwide Cohort Study, 2006–2016. Journal of Clinical Medicine. 2020; 9(11):3578. https://doi.org/10.3390/jcm9113578
Chicago/Turabian StyleLin, Min-Hao, Huang-Yu Yang, Chieh-Li Yen, Chao-Yi Wu, Chang-Chyi Jenq, George Kuo, Wei-Sheng Peng, Jia-Rou Liu, Ya-Chung Tian, Chih-Wei Yang, and et al. 2020. "Pioglitazone Is Associated with Lower Major Adverse Cardiovascular and Cerebrovascular Events than DPP4-Inhibitors in Diabetic Patients with End-Stage Renal Disease: A Taiwan Nationwide Cohort Study, 2006–2016" Journal of Clinical Medicine 9, no. 11: 3578. https://doi.org/10.3390/jcm9113578
APA StyleLin, M.-H., Yang, H.-Y., Yen, C.-L., Wu, C.-Y., Jenq, C.-C., Kuo, G., Peng, W.-S., Liu, J.-R., Tian, Y.-C., Yang, C.-W., Anderson, G. F., & See, L.-C. (2020). Pioglitazone Is Associated with Lower Major Adverse Cardiovascular and Cerebrovascular Events than DPP4-Inhibitors in Diabetic Patients with End-Stage Renal Disease: A Taiwan Nationwide Cohort Study, 2006–2016. Journal of Clinical Medicine, 9(11), 3578. https://doi.org/10.3390/jcm9113578





