CAR T-Cells in Multiple Myeloma Are Ready for Prime Time
Abstract
1. Introduction
2. Engineered T-Cells in MM: CAR T-Cells
3. Target Selection
- (1)
- CD38 is expressed with high intensity in clonal plasma cells and has been shown to be a promising target for the treatment of MM. However, CD38 is also expressed, although with less intensity, on normal hematopoietic cells, such as red blood cells, natural killer cells, and other tissues, increasing the risk of on-target off-tumor toxicity [11]. There is already preclinical evidence showing an activity of CD38-targeting CAR T-cells, and several clinical trials are ongoing.
- (2)
- CD138 (Syndecan 1) is expressed in normal and clonal plasma cells and also on normal tissues such as epithelial cells potentially inducing “on target–off tumor” toxicity. However, in a preclinical study using CD138-directed CAR T-cells, no epithelial toxicity was observed [12]. The same experience was noted in a clinical report with five patients treated with CD138 directed CAR T-cell in China, and a phase 1 trial is ongoing [11].
- (3)
- The orphan G protein–coupled receptor, class C group 5 member D (GPRC5D), is expressed ubiquitously in malignant bone marrow plasma cells, hair follicles, and variably in the lung tissue [13]. Interestingly, expression in MM cells is 500 to 1000 times that found on normal cells [11]. CAR T-cells targeting GPRC5D have demonstrated promising preclinical activity [13], and clinical strategies targeting GPRC5D, particularly using bispecific antibodies, are under evaluation.
- (4)
- Signaling lymphocyte activation molecule F7 (SLAMF7 or CS1) is widely expressed on plasma cells, as well as subsets of normal B and T-cells, natural killer (NK) cells, monocytes, and dendritic cells, and it is already a target used in MM therapy with the antibody elotuzumab [11,14,15]. CAR T-cells targeting SLAMF7 have shown encouraging preclinical activity; however, SLAMF7 expression in lymphocyte subsets raised the problem of the specific fratricide of SLAMF7+/high target cells by SLAMF7-CAR T-cells, although SLAMF7−/low was preserved and able to remain viable [16]. Clinical trials evaluating SLAMF7-targeted CAR T-cells are ongoing.
- (5)
- CD19-directed CAR T-cell therapy is approved for the treatment of B acute lymphoblastic leukemia and diffuse large B cell lymphoma. CD19 is typically absent on the dominant multiple myeloma cell population, but it may be present on a minor subset with unique myeloma-propagating properties [17,18]. CD19-directed CAR T-cell therapy has been tested in MM patients after autologous stem cells therapy with interesting results [18] and also in combination with BCMA-directed CAR T-cells in small series of patients with promising data [19].
- (6)
- The activating receptor NKG2D (natural-killer group 2, member D) and its ligands play an important role in the NK, γδ+, and CD8+ T-cell-mediated immune response to tumors. Ligands for NKG2D are rarely detectable on the surface of healthy cells and tissues, but they are frequently expressed by tumor cell lines and in tumor tissues, which makes them attractive targets for CAR development [20]. NKG2D ligand-directed CAR T-cells have been evaluated both in preclinical and clinical settings (albeit a small number of patients) with promising results [21]. NKG2D ligand-directed CAR NK cells are also under development [22].
4. Clinical Data Using CAR T-Cells in MM
5. Limitations of Current Approaches and Potential Avenues
5.1. Peak Expansion and Response
5.2. Antigen Escape
5.3. CAR T-Cell Persistence and Duration of Response
5.4. Combination Therapies
6. Practical Considerations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Noonan, K.A.; Huff, C.A.; Davis, J.; Lemas, M.V.; Fiorino, S.; Bitzan, J.; Ferguson, A.; Emerling, A.; Luznik, L.; Matsui, W.; et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci. Transl. Med. 2015, 7, 288ra78. [Google Scholar] [CrossRef] [PubMed]
- Drent, E.; Themeli, M.; Poels, R.; De Jong-Korlaar, R.; Yuan, H.; De Bruijn, J.; Martens, A.C.; Zweegman, S.; Van De Donk, N.W.; Groen, R.W.; et al. A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization. Mol. Ther. 2017, 25, 1946–1958. [Google Scholar] [CrossRef] [PubMed]
- Madry, C.; Laabi, Y.; Callebaut, I.; Roussel, J.; Hatzoglou, A.; Le Coniat, M.; Mornon, J.P.; Berger, R.; Tsapis, A. The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. Int. Immunol. 1998, 10, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Rennert, P.; Schneider, P.; Cachero, T.G.; Thompson, J.; Trabach, L.; Hertig, S.; Holler, N.; Qian, F.; Mullen, C.; Strauch, K.; et al. A Soluble Form of B Cell Maturation Antigen, a Receptor for the Tumor Necrosis Factor Family Member April, Inhibits Tumor Cell Growth. J. Exp. Med. 2000, 192, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia 2020, 34, 985–1005. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-F.; Anderson, K.C.; Tai, Y.-T. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front. Immunol. 2018, 9, 1821. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Tanenbaum, E.J.; Patil, S.; Li, M.; Soof, C.M.; Vidisheva, A.; Waterman, G.N.; Hekmati, T.; Tang, G.; Wang, C.S.; et al. The clinical significance of B-cell maturation antigen as a therapeutic target and biomarker. Expert Rev. Mol. Diagn. 2018, 18, 319–329. [Google Scholar] [CrossRef]
- Laurent, S.A.; Hoffmann, F.S.; Kuhn, P.H.; Cheng, Q.; Chu, Y.; Schmidt-Supprian, M.; Wanngren, J. gamma-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat. Commun. 2015, 6, 7333. [Google Scholar] [CrossRef] [PubMed]
- Sidana, S.; Shah, N. CAR T-cell therapy: Is it prime time in myeloma? Blood Adv. 2019, 3, 3473–3480. [Google Scholar] [CrossRef]
- Sun, C.; Mahendravada, A.; Ballard, B.; Kale, B.; Ramos, C.; West, J.; Maguire, T.; McKay, K.; Lichtman, E.; Tuchman, S.; et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget 2019, 10, 2369–2383. [Google Scholar] [CrossRef]
- Smith, E.L.; Harrington, K.; Staehr, M.; Masakayan, R.; Jones, J.; Long, T.J.; Ng, K.Y.; Ghoddusi, M.; Purdon, T.J.; Wang, X.; et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci. Transl. Med. 2019, 11, eaau7746. [Google Scholar] [CrossRef] [PubMed]
- Hsi, E.D.; Steinle, R.; Balasa, B.; Szmania, S.; Draksharapu, A.; Shum, B.P.; Huseni, M.; Powers, D.; Nanisetti, A.; Zhang, Y.; et al. CS1, a Potential New Therapeutic Antibody Target for the Treatment of Multiple Myeloma. Clin. Cancer Res. 2008, 14, 2775–2784. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Palumbo, A.; San-Miguel, J.F.; Shpilberg, O.; Anderson, K.; Grosicki, S.; Spicka, I.; et al. Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth. Br. J. Haematol. 2017, 178, 896–905. [Google Scholar] [CrossRef]
- Gogishvili, T.; Danhof, S.; Prommersberger, S.; Rydzek, J.; Schreder, M.; Brede, C.; Einsele, H.; Hudecek, M. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood 2017, 130, 2838–2847. [Google Scholar] [CrossRef]
- Garfall, A.L.; Maus, M.V.; Hwang, W.-T.; Lacey, S.F.; Mahnke, Y.D.; Melenhorst, J.J.; Zheng, Z.; Vogl, D.T.; Cohen, A.D.; Weiss, B.M.; et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1040–1047. [Google Scholar] [CrossRef]
- Garfall, A.L.; Stadtmauer, E.A.; Hwang, W.T.; Lacey, S.F.; Melenhorst, J.J.; Krevvata, M.; Dhodapkar, K. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight 2019, 4, e127684. [Google Scholar] [CrossRef]
- Yan, L.; Yan, Z.; Shang, J.; Shi, X.; Jin, S.; Kang, L.; Qu, S.; Zhou, J.; Kang, H.; Wang, R.; et al. Sequential CD19- and Bcma-Specific Chimeric Antigen Receptor T Cell Treatment for RRMM: Report from a Single Center Study. Blood 2019, 134, 578. [Google Scholar] [CrossRef]
- Nausch, N.; Cerwenka, A. NKG2D ligands in tumor immunity. Oncogene 2008, 27, 5944–5958. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Murad, J.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Gicobi, J.K.; Schmucker, A.; Reder, J.; Sentman, C.L.; Gilham, D.E.; et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol. Res. 2018, 7, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Leivas, A.; Rio, P.; Mateos, R.; Paciello, M.L.; Garcia-Ortiz, A.; Fernandez, L.; Perez-Martinez, A.; Lee, D.A.; Powell, D.J.; Valeri, A.; et al. NKG2D-CAR Transduced Primary Natural Killer Cells Efficiently Target Multiple Myeloma Cells. Blood 2018, 132, 590. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, J.L.D.; Shah, N.; Jagannath, S.; Berdeja, J.G.; Lonial, S.; Raje, N.S.; Siegel, D.S.D.; Lin, Y.; Oriol, A.; et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): Initial KarMMa results. J. Clin. Oncol. 2020, 38, 8503. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Alsina, M.; Shah, N.D.; Siegel, D.S.; Jagannath, S.; Madduri, D.; Kaufman, J.L.; Munshi, N.C.; Rosenblatt, J.; Jasielec, J.K.; et al. Updated Results from an Ongoing Phase 1 Clinical Study of bb21217 Anti-Bcma CAR T Cell Therapy. Blood 2019, 134, 927. [Google Scholar] [CrossRef]
- Mailankody, S.; Jakubowiak, A.J.; Htut, M.; Costa, L.J.; Lee, K.; Ganguly, S.; Kaufman, J.L.; Siegel, D.S.D.; Bensinger, W.; Cota, M.; et al. Orvacabtagene autoleucel (orva-cel), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): Update of the phase 1/2 EVOLVE study (NCT03430011). J. Clin. Oncol. 2020, 38, 8504. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Deepu, M.; Berdeja, J.G.; Indrajeet, S.; Zudaire, E.; Yeh, T.-M.; Allred, A.J.; Olyslager, Y.; Banerjee, A.; Goldberg, J.D.; et al. Treatment of Relapsed/Refractory Multiple Myeloma with Jnj-4528, A B-Cell Maturation Antigen (Bcma)-Directed Chimeric Antigen Receptor (Car)-T Cell Therapy: Update of Phase 1b Results from Cartitude-1; EHA Library: The Hague, The Netherlands, 2020; p. EP926. [Google Scholar]
- Chen, L.; Xu, J.; Fu, S.W.; Jin, S.; Yang, S.; Yan, S.; Wu, W.; Liu, Y.; Zhang, W.; Weng, X.; et al. Updated Phase 1 Results of a First-in-Human Open-Label Study of Lcar-B38M, a Structurally Differentiated Chimeric Antigen Receptor T (CAR-T) Cell Therapy Targeting B-Cell Maturation Antigen (Bcma). Blood 2019, 134, 1858. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Wang, M.; Stroncek, D.; Maric, I.; Brudno, J.N.; Stetler-Stevenson, M.; Rose, B.J.J.; Feldman, S.; Hansen, B.; et al. Remissions of Multiple Myeloma during a First-in-Humans Clinical Trial of T Cells Expressing an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor. Blood 2015, 126, LBA-1. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Liu, J.; Wang, B.-Y.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. Updated Analysis of a Phase 1, Open-Label Study of LCAR-B38M, a Chimeric Antigen Receptor T Cell Therapy Directed Against B-Cell Maturation Antigen, in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2018, 132, 955. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Zhao, W.-H.; Liu, J.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. Long-Term Follow-up of a Phase 1, First-in-Human Open-Label Study of LCAR-B38M, a Structurally Differentiated Chimeric Antigen Receptor T (CAR-T) Cell Therapy Targeting B-Cell Maturation Antigen (BCMA), in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 579. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’Connor, R.S.; Hwang, W.-T.; et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018, 24, 563–571. [Google Scholar] [CrossRef]
- Jagannath, S.; Lin, Y.; Goldschmidt, H.; Reece, D.E.; Nooka, A.K.; Otero, P.R.; Matsue, K.; Shah, N.; Anderson, J.L.D.; Wilson, K.; et al. KarMMa-RW: A study of real-world treatment patterns in heavily pretreated patients with relapsed and refractory multiple myeloma (RRMM) and comparison of outcomes to KarMMa. J. Clin. Oncol. 2020, 38, 8525. [Google Scholar] [CrossRef]
- Zudaire, E.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Berdeja, J.G.; Geng, D.; Rizvi, S.; Nesheiwat, T.; Schecter, J.M.; Goldberg, J.D.; et al. Translational Analysis from CARTITUDE-1, an Ongoing Phase 1b/2 Study of JNJ-4528 BCMA-targeted CAR-T Cell Therapy in Relapsed and/or Refractory Multiple Myeloma (R/R MM), Indicates Preferential Expansion of CD8+ T Cell Central Memory Cell Subset. Blood 2019, 134, 928. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Cowan, A.J.; Pont, M.; Sather, B.D.; Turtle, M.C.J.; Till, B.G.; Nagengast, R.A.M.; Libby, I.E.N.; Becker, P.S.; Coffey, D.G.; Tuazon, S.A.; et al. Efficacy and Safety of Fully Human Bcma CAR T Cells in Combination with a Gamma Secretase Inhibitor to Increase Bcma Surface Expression in Patients with Relapsed or Refractory Multiple Myeloma. Blood 2019, 134, 204. [Google Scholar] [CrossRef]
- Da-Via, M.; Dietrich, O.; Truger, M.; Arampatzi, P.; Duell, J.; Zhou, X.; Tabares, P.; Danhof, S.; Kraus, S.; Meggendorfer, M.; et al. Biallelic Deletion of Chromosome 16p Encompassing the BCMA Locus as a Tumor-Intrinsic Resistance Mechanism to BCMA-Directed CAR T in Multiple Myeloma; EHA Library Rasche L: The Hague, The Netherlands, 2020; Volume 294800, p. EP883. [Google Scholar]
- Zhang, H.; Gao, L.; Liu, L.; Wang, J.; Wang, S.; Zhang, C.; Liu, Y.; Kong, P.; Liu, J.; He, J.; et al. A Bcma and CD19 Bispecific CAR-T for Relapsed and Refractory Multiple Myeloma. Blood 2019, 134, 3147. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.-R.; Yue, R.; Fu, Y.-L.; Liu, Z.-Y.; Feng, H.-Y.; Li, J.-G.; Han, S.-Y. PiggyBac transposon system with polymeric gene carrier transfected into human T cells. Am. J. Transl. Res. 2019, 11, 7126–7136. [Google Scholar]
- Shah, U.A.; Mailankody, S. CAR T and CAR NK cells in multiple myeloma: Expanding the targets. Best Pr. Res. Clin. Haematol. 2020, 33, 101141. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Barnett, B.E.; Hermanson, D.; He, J.; Zhang, Z.; Rengarajan, S.; Smith, J. B-Cell Maturation Antigen (BCMA)-Specific, CentyrinTM-Based, PiggyBacTM-Transposed CAR-T Memory Stem Cells Are Effective Against p53-/- and Patient-Derived Multiple Myeloma Tumors. Blood 2017, 130 (Suppl. 1), 3068. [Google Scholar] [CrossRef]
- Costello, C.L.; Gregory, T.K.; Ali, S.A.; Berdeja, J.G.; Patel, M.K.K.; Shah, N.D.; Ostertag, E.; Martin, C.; Ghoddusi, D.M.; Shedlock, D.J.; et al. Phase 2 Study of the Response and Safety of P-Bcma-101 CAR-T Cells in Patients with Relapsed/Refractory (r/r) Multiple Myeloma (MM) (PRIME). Blood 2019, 134, 3184. [Google Scholar] [CrossRef]
- Gregory, T.; Cohen, A.D.; Costello, C.L.; Ali, S.A.; Berdeja, J.G.; Ostertag, E.M.; Martin, C.; Shedlock, D.J.; Resler, B.M.L.; Spear, M.A.; et al. Efficacy and Safety of P-Bcma-101 CAR-T Cells in Patients with Relapsed/Refractory (r/r) Multiple Myeloma (MM). Blood 2018, 132, 1012. [Google Scholar] [CrossRef]
- Galetto, R.; Chion-Sotinel, I.; Gouble, A.; Smith, J. Bypassing the Constraint for Chimeric Antigen Receptor (CAR) Development in T-Cells Expressing the Targeted Antigen: Improvement of Anti-CS1 CAR Activity in Allogenic TCRa/CS1 Double Knockout T-Cells for the Treatment of Multiple Myeloma (MM). Blood 2015, 126, 116. [Google Scholar] [CrossRef]
- Dar, H.; Henderson, D.; Padalia, Z.; Porras, A.; Mu, D.; Kyungah, M.; Police, S.; Kalaitzidis, D.; Terrett, J.; Sagert, J. Preclinical Development of CTX120, an Allogeneic CAR-T Cell Targeting Bcma. Blood 2018, 132, 1921. [Google Scholar] [CrossRef]
- Buechner, J.; Kersten, M.J.; Fuchs, M.; Salmon, F.; Jäger, U. Chimeric Antigen Receptor-T Cell Therapy. HemaSphere 2018, 2, e18. [Google Scholar] [CrossRef]
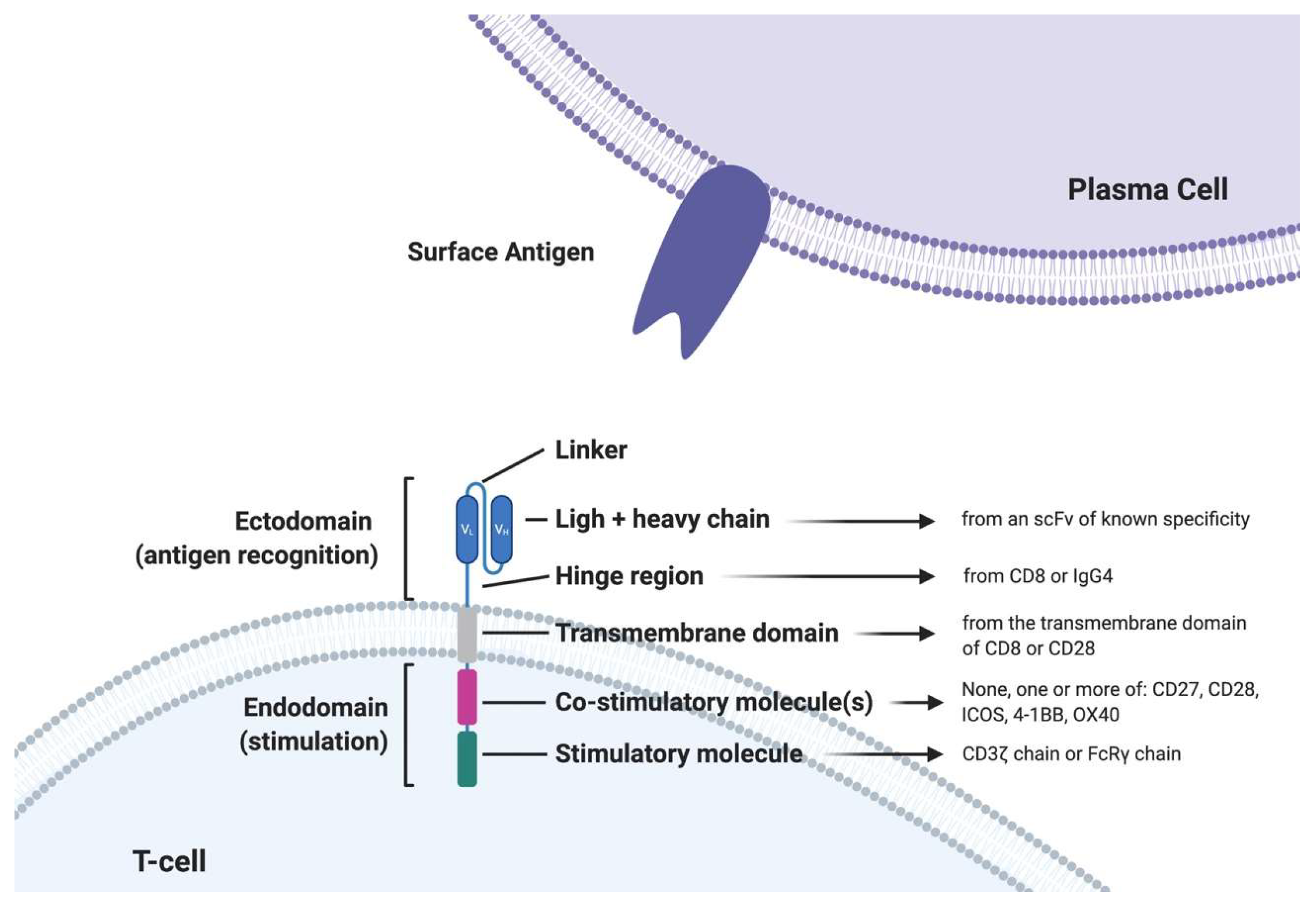
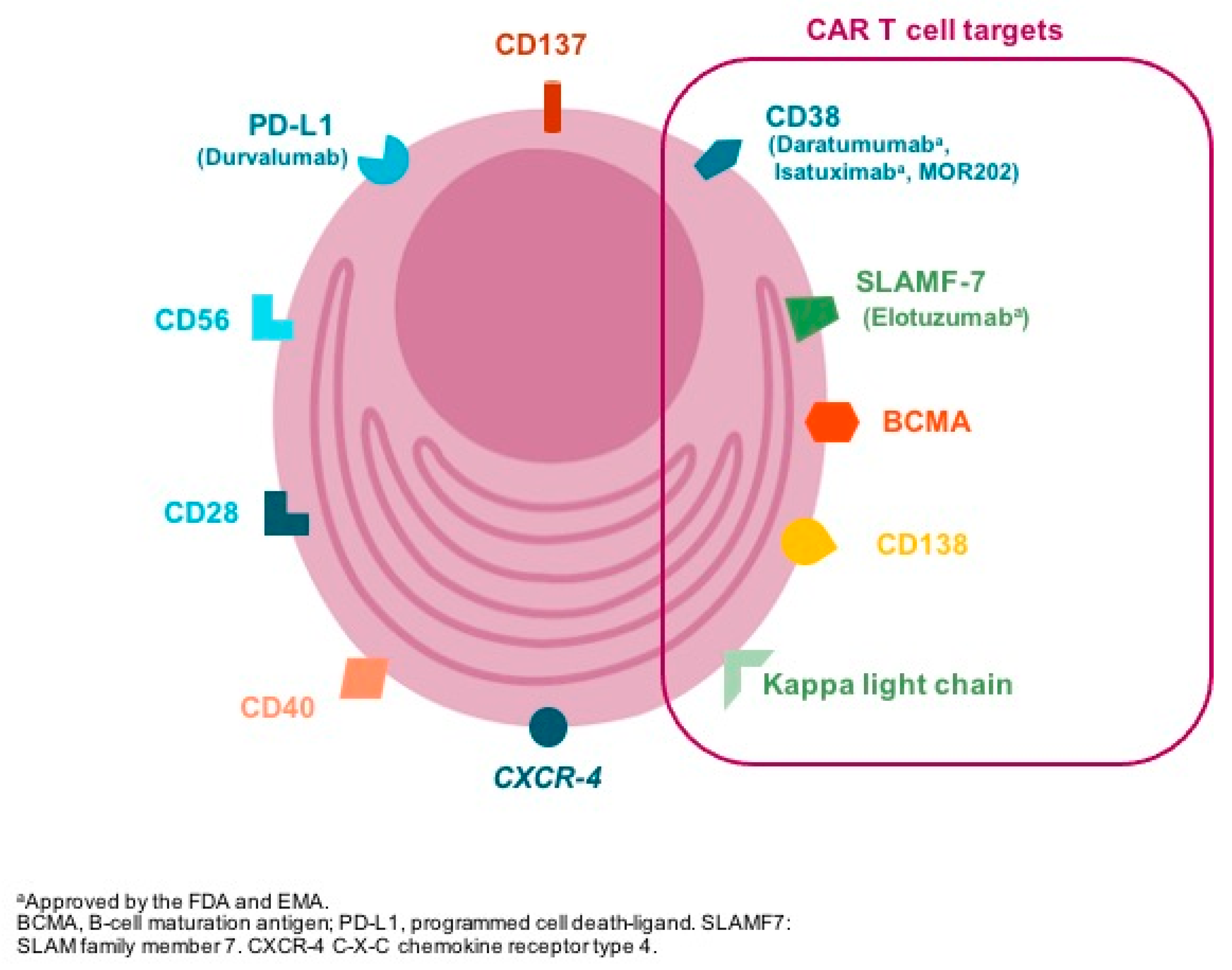
| Antigen | Expression in Plasma Cells | Function | Off-Target Expression | Ongoing Trials |
|---|---|---|---|---|
| BCMA | Universal in plasma cells | Membrane bound of the TNFR superfamily | Restricted to B-cell compartment | NCT04309981 NCT03430011 NCT03288493 NCT04181827 NCT04133636 NCT04093596 NCT04196491 NCT03601078 NCT03651128 NCT04244656 NCT04394650 |
| CD38 (Syndecan 1) | Overexpressed in multiple myeloma cells | As a receptor, CD38 can bind CD31 in T cells, activating them. As an enzyme, it catalyzes the synthesis and hydrolysis of cyclic ADP-ribose. | Normal hematopoietic cells: red blood cells, NK cells | NCT03464916 NCT03473496 NCT03767751 |
| GPRC5D | Universal in plasma cells | Not yet been determined | Hair follicle and lung tissue | NCT04555551 |
| SLAMF7 | Overexpressed in multiple myeloma cells | Mediates activating or inhibitory effects in NK cells | Normal B and T-cells, NK-cells, monocytes, and dendritic cells | NCT04499339 |
| CD19 | Rarely detected in plasma cells | Involved in B-cell maturation | All B-lineage cells | NCT04194931 NCT04182581 NCT03767725 NCT04236011 NCT03455972 NCT04162353 * |
| NKG2D | Not expressed in plasma cells | Important role in the NK, γδ+, and CD8+ T-cell-mediated immune response to tumors | Rarely detectable on healthy cells and tissues | Under development |
| Idecabtagene Vicleucel (Ide-Cel) KARMMA Study [23] | bb21217 [24] | Orvacabtagene-Autoleucel (Orva-Cel) EVOLVE Ph 1/2 Trial [25] | Ciltacabtagene Autoleucel (Cilta-Cel) CARTITUDE 1 [26] | LCAR-B38M LEGEND-2 [27] | |
|---|---|---|---|---|---|
| CAR Design | Autologous, lentiviral vector 4-1BB | Ide-cel cultured with PI3Ki, to enrich memory-like T cells | Fully human (CD28/41BB). 1:1 CD4:CD8 ratio | 2 BCMA-targeting single chain antibody | 2 BCMA-targeting single chain antibody (same as JNJ 4528) |
| Population | 128 (Ph 2) | 38 (Ph 1) | 62 (Ph 1/2) | 29 (Ph 1b/2) | 57 (Ph 2) |
| Number of Prior lines | 6 | 6 | 6 | 5 | 2 |
| CAR T-cell Dose | 150–450 × 106 CAR T-cell | 150–450 × 106 CAR T-cell | 300–600 × 106 CAR T-cell | 0.73 × 106 CAR T-cells/kg | 0.5 × 106 CAR T + Cells/kg |
| Refractory to CD38 MoAb | 94% | 76% | NA | 93% | 1 patients |
| Triple-class Refractory | 84% | 63% | 94% | 86% | -- |
| Extramedullar disease | 39% | NA | 23% | 10% | NA |
| ORR (CR) | 82%* (39%) | NA (33%) | 92% #. (29%) | 100% (86%) | 88% (74%) |
| DOR/PFS/ OS months | 11.3 */12.1 */19.4 | 11.1 &/NA/NA | NA/NA/ NA | NA/86%@9m/NA | 19.9m In CR: mPFS 28.2 m |
| CRS (G 3/4) | 96% * (6%) | 66% (6%) | 88% # (4%) | 93% (7%) | 90% (7%) |
| Neurotox(G3) | 20% * (6%) | 24% (8%) | 13% # (0%) | 10% (3%) | 2% |
| Reference | (1) Munshi NC, et al. Initial KarMMa results. J Clin Oncol. 2020;38 (suppl; abstr 8503). | (2) Berdeja JG, et al. Blood (Internet). 2019 Nov 13;134 (Supplement_1):927. | (3) Mailankody S, et al. J Clin Oncol. 2020;30 (suppl; abstr 8504). | (4) Usmani SZ, et al. EHA Library. 2020. p. EP926. | (5) Chen L, et al. Blood (Internet) 2019 Nov 13;134 (Supplement_1):1858. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Otero, P.; Prósper, F.; Alfonso, A.; Paiva, B.; San Miguel, J.F. CAR T-Cells in Multiple Myeloma Are Ready for Prime Time. J. Clin. Med. 2020, 9, 3577. https://doi.org/10.3390/jcm9113577
Rodríguez-Otero P, Prósper F, Alfonso A, Paiva B, San Miguel JF. CAR T-Cells in Multiple Myeloma Are Ready for Prime Time. Journal of Clinical Medicine. 2020; 9(11):3577. https://doi.org/10.3390/jcm9113577
Chicago/Turabian StyleRodríguez-Otero, Paula, Felipe Prósper, Ana Alfonso, Bruno Paiva, and Jesús F. San Miguel. 2020. "CAR T-Cells in Multiple Myeloma Are Ready for Prime Time" Journal of Clinical Medicine 9, no. 11: 3577. https://doi.org/10.3390/jcm9113577
APA StyleRodríguez-Otero, P., Prósper, F., Alfonso, A., Paiva, B., & San Miguel, J. F. (2020). CAR T-Cells in Multiple Myeloma Are Ready for Prime Time. Journal of Clinical Medicine, 9(11), 3577. https://doi.org/10.3390/jcm9113577






