The Wearable VOSTARS System for Augmented Reality-Guided Surgery: Preclinical Phantom Evaluation for High-Precision Maxillofacial Tasks
Abstract
1. Introduction
2. Materials and Methods
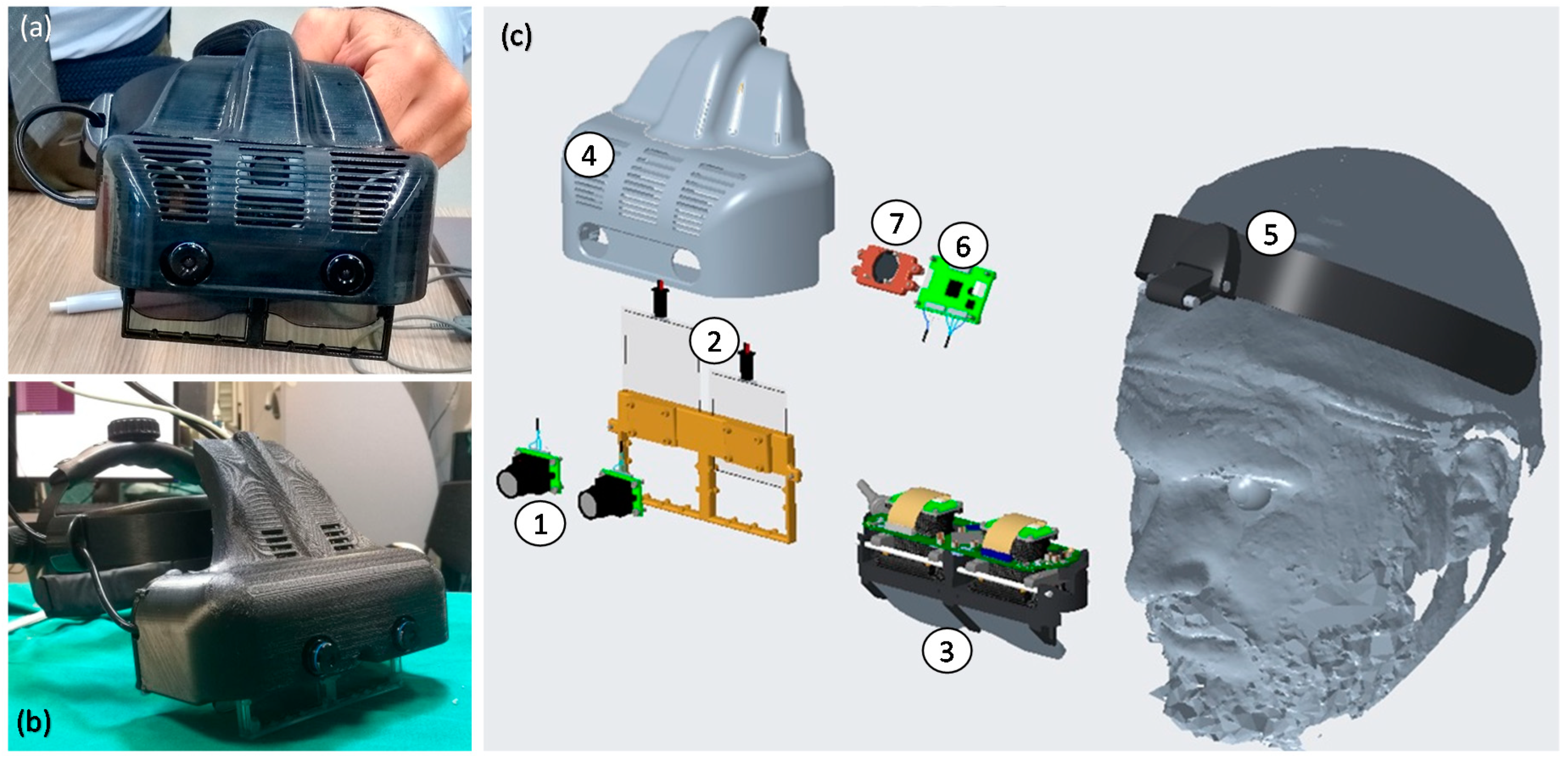
2.1. VOSTARS Head-Mounted Display
- (1)
- A dedicated optical self-tracking mechanism: the head-anchored RGB cameras used for implementing the VST augmentation also provide the stereo localization of three spherical markers (Ø = 11 mm) conveniently placed on the patient’s body and/or around the working area, thus, without requiring obtrusive external trackers or additional tracking cameras [33].
- (2)
- An automatic image-to-patient registration strategy: based on the use of an occlusal splint that embeds the three optical markers. The positions of the optical markers are dictated in the reference system of the Computed Tomography (CT) dataset. By determining their position with respect to the tracking camera (point 1), the pose of the 3D virtual planning can be directly computed in a closed-form fashion by solving a standard absolute orientation problem with three points (i.e., estimating the rigid transformation that aligns the two sets of corresponding triplets of 3D points).
- (3)
- Scene augmentation in both OST and VST modalities: under OST mode, only the computer-generated elements are rendered onto the two microdisplays of the visor, whereas under VST mode, the real views of the world are grabbed by the external RGB cameras and the virtual elements are digitally added to them before the augmented frames are rendered on the two microdisplays.
2.2. Experimental Set-Up
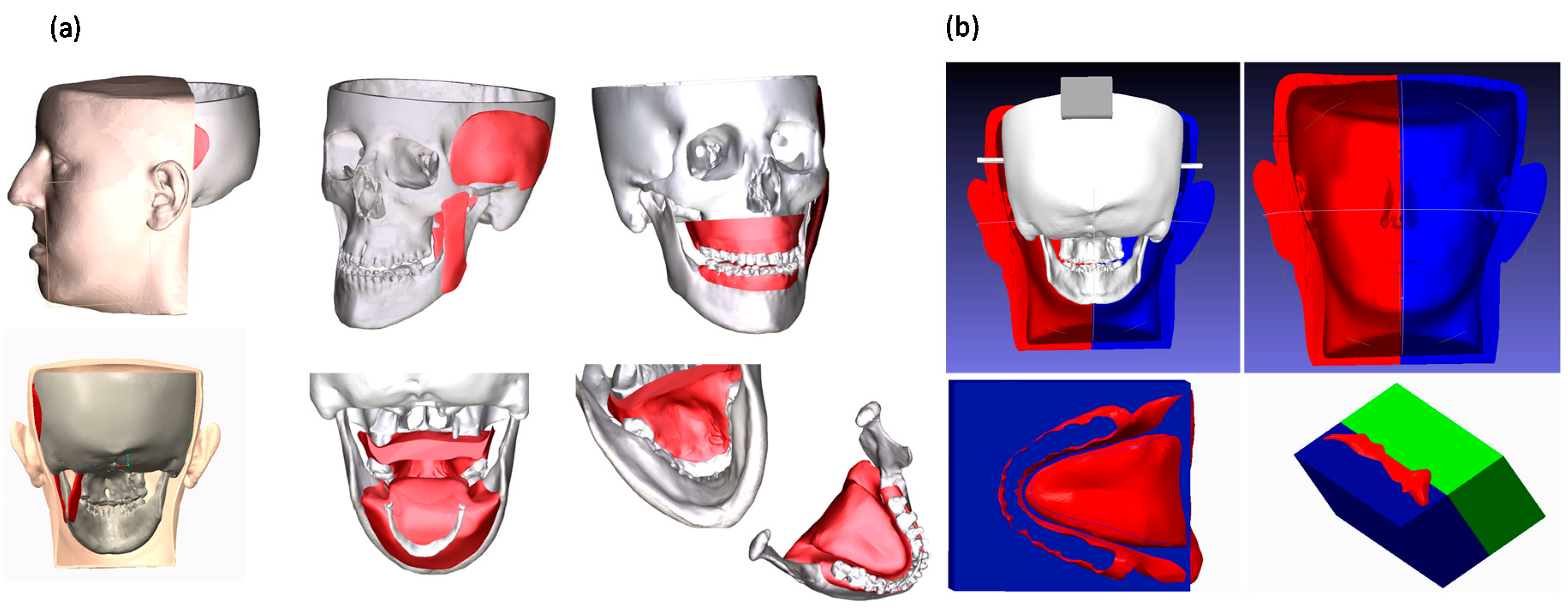
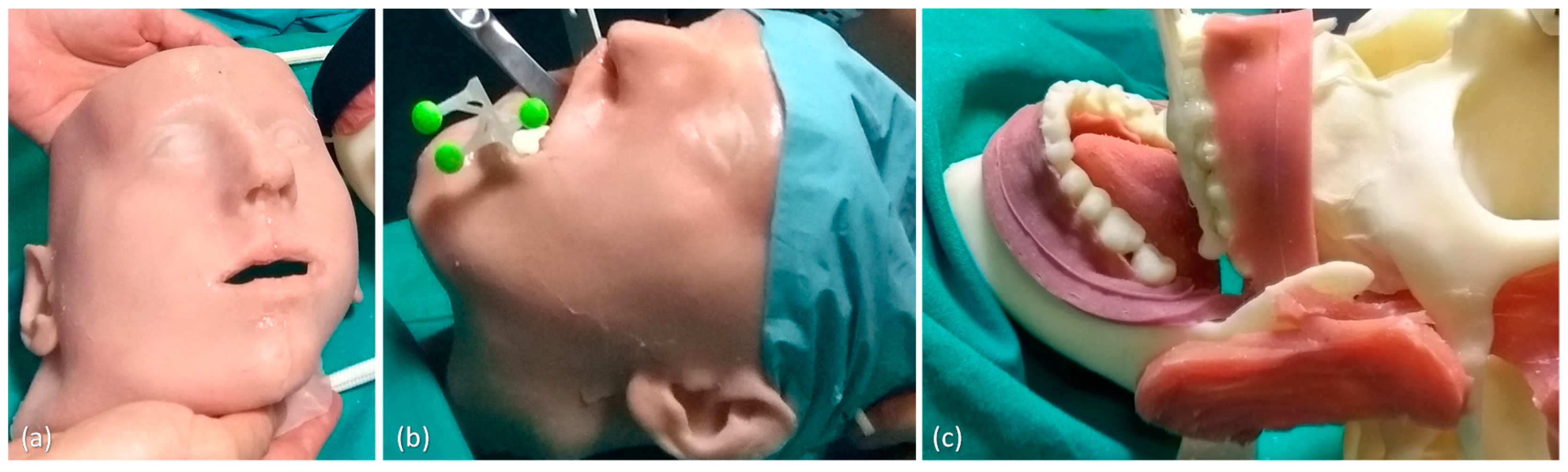
2.2.1. Maxillofacial Phantom
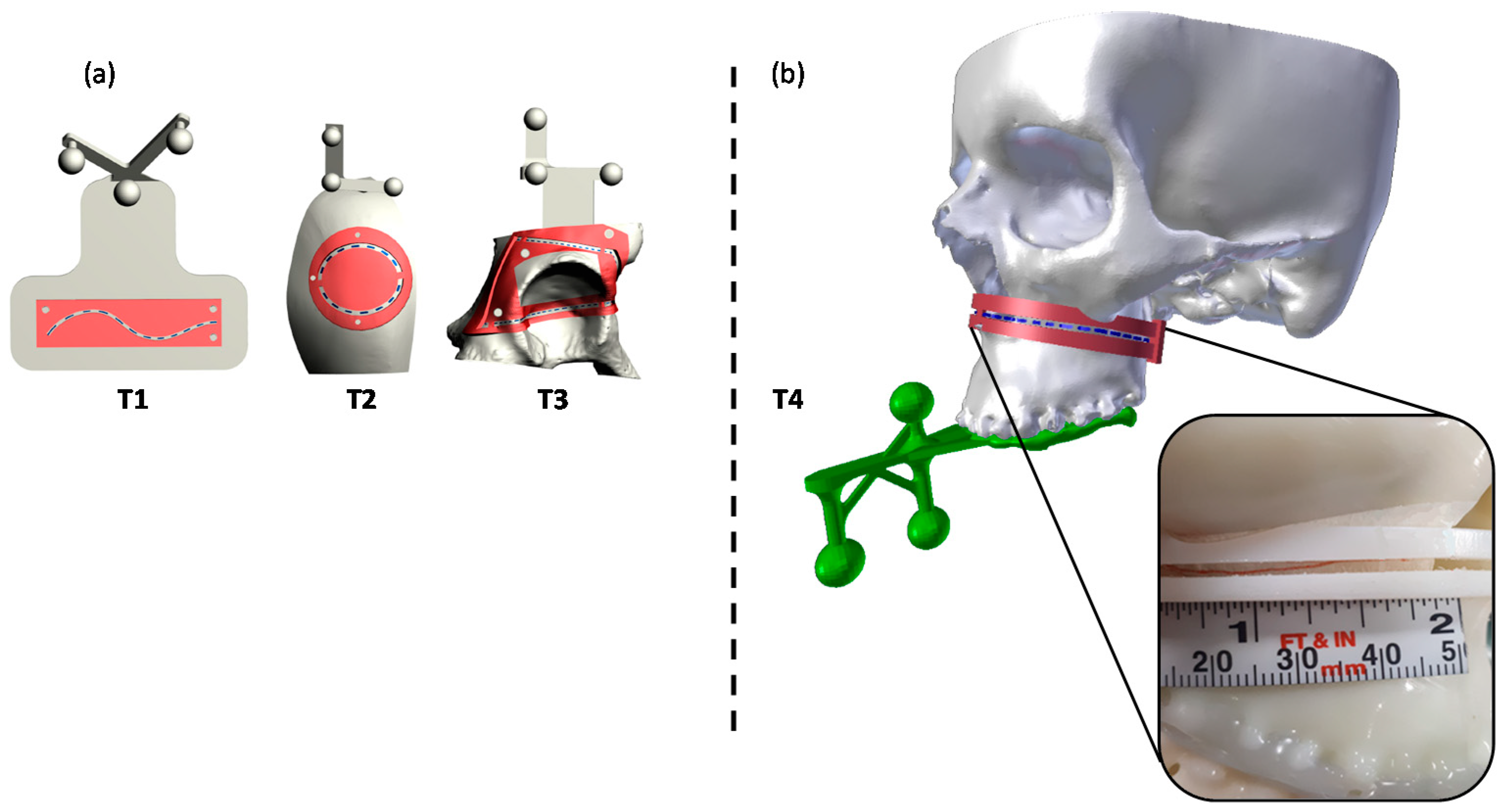
2.2.2. Task-Oriented Tracker
2.3. Test Execution
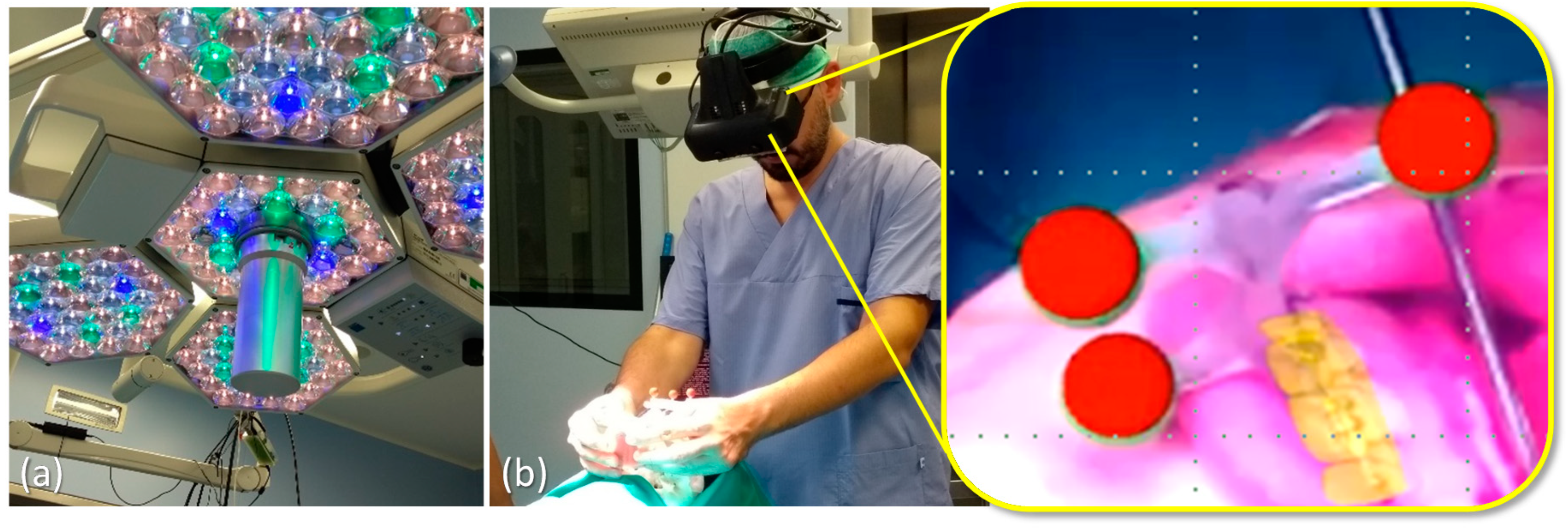
2.3.1. VOSTARS Set-Up and Basic Functionality
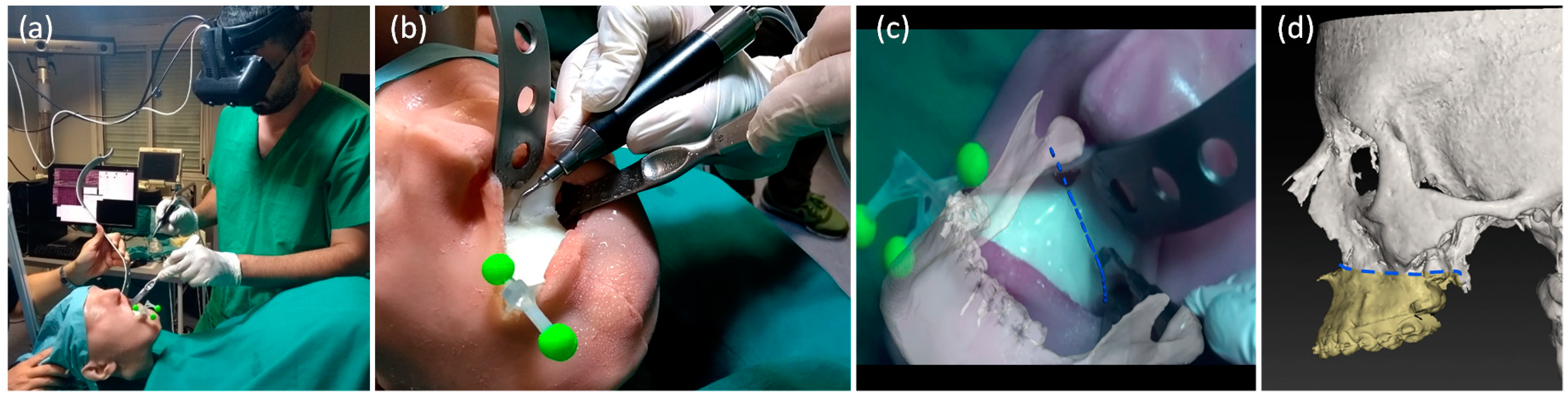
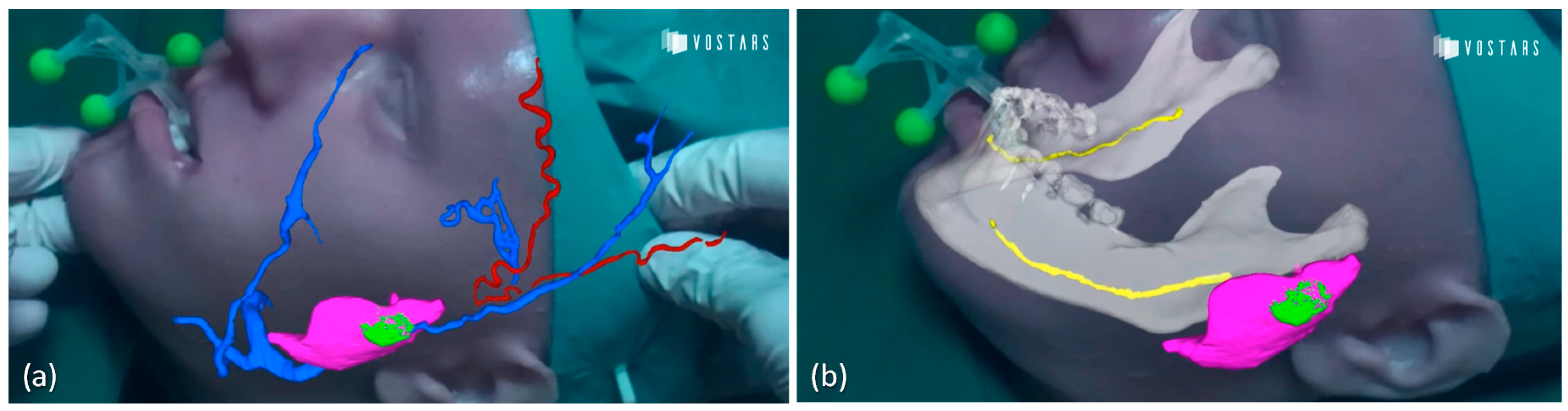
2.3.2. AR-Guided Le Fort 1 Osteotomy Task
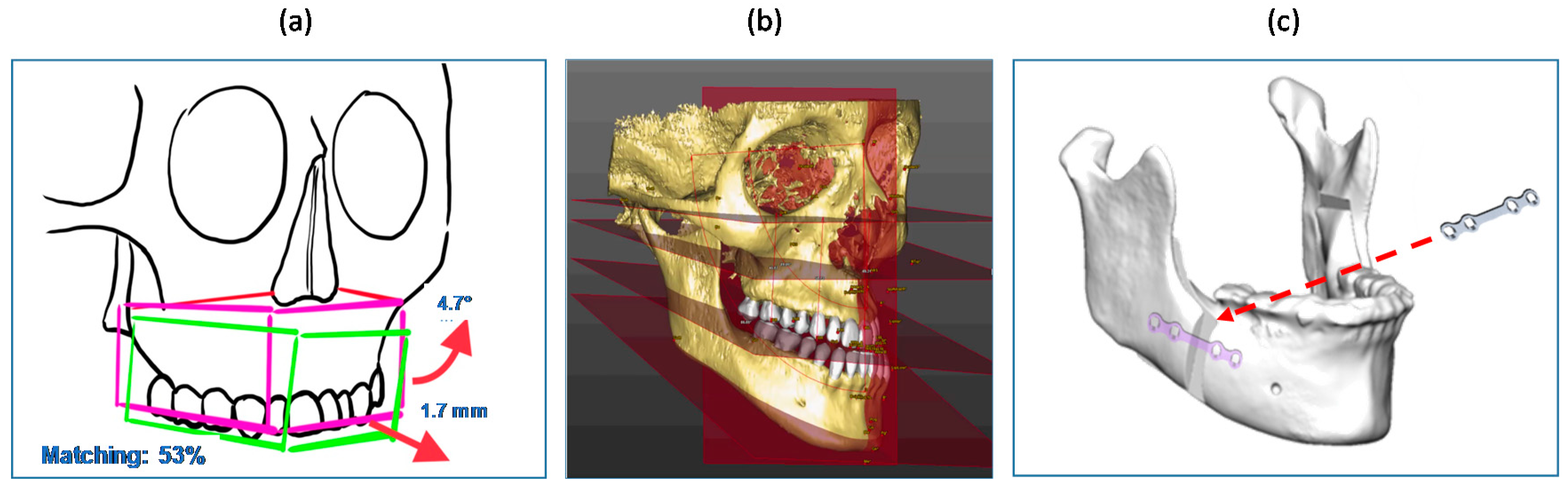
2.3.3. Accuracy Tests
2.3.4. Statistics
3. Results
3.1. Results of Functionality/Usability Tests
3.2. Results of AR-Guided Le Fort 1 Osteotomy
3.3. Results of Accuracy Tests
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olszewski, R.; Reychler, H. Les limites de la chirurgie des modèles en chirurgie orthognathique: Implications théoriques et pratiques. Rev. Stomatol. Chir. Maxillofac. 2004, 105, 165–169. [Google Scholar] [CrossRef]
- Schneider, M.; Tzscharnke, O.; Pilling, E.; Lauer, G.; Eckelt, U. Comparison of the predicted surgical results following virtual planning with those actually achieved following bimaxillary operation of dysgnathia. J. Cranio Maxillofac. Surg. 2005, 33, 8–12. [Google Scholar] [CrossRef]
- Gil, J.; Claus, J.; Manfro, R.; Lima, S. Predictability of maxillary repositioning during bimaxillary surgery: Accuracy of a new technique. Int. J. Oral Maxillofac. Surg. 2007, 36, 296–300. [Google Scholar] [CrossRef]
- Zinser, M.J.; Sailer, H.F.; Ritter, L.; Braumann, B.; Maegele, M.; Zöller, J.E. A Paradigm Shift in Orthognathic Surgery? A Comparison of Navigation, Computer-Aided Designed/Computer-Aided Manufactured Splints, and “Classic” Intermaxillary Splints to Surgical Transfer of Virtual Orthognathic Planning. J. Oral Maxillofac. Surg. 2013, 71, 2151.e1–2151.e21. [Google Scholar] [CrossRef] [PubMed]
- Swennen, G.R.; Mollemans, W.; Schutyser, F. Three-Dimensional Treatment Planning of Orthognathic Surgery in the Era of Virtual Imaging. J. Oral Maxillofac. Surg. 2009, 67, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Badiali, G.; Ferrari, V.; Cutolo, F.; Freschi, C.; Caramella, D.; Bianchi, A.; Marchetti, C. Augmented reality as an aid in maxillofacial surgery: Validation of a wearable system allowing maxillary repositioning. J. Cranio Maxillofac. Surg. 2014, 42, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Badiali, G.; Cercenelli, L.; Bortolani, B.; Marcelli, E.; Cipriani, R.; Contedini, F.; Marchetti, C.; Tarsitano, A. Combination of CAD/CAM and Augmented Reality in Free Fibula Bone Harvest. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2510. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.; Suarez-Cunqueiro, M.M.; Barth, E.L.; Essig, H.; Bormann, K.-H.; Kokemueller, H.; Rücker, M.; Gellrich, N.-C. Computer-Assisted Navigation in Craniomaxillofacial Tumors. J. Craniofacial Surg. 2008, 19, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Essig, H.; Eckardt, A.M.; Tavassol, F.; Ruecker, M.; Schramm, A.; Gellrich, N.-C. Advances and Innovations in Computer-Assisted Head and Neck Oncologic Surgery. J. Craniofacial Surg. 2012, 23, 272–278. [Google Scholar] [CrossRef]
- Badiali, G.; Marcelli, E.; Bortolani, B.; Marchetti, C.; Cercenelli, L. An average three-dimensional virtual human skull for a template-assisted maxillofacial surgery. Int. J. Artif. Organs 2019, 42, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, F.; Cercenelli, L.; Battaglia, S.; Bortolani, B.; Savastio, G.; Marcelli, E.; Marchetti, C.; Tarsitano, A. Navigation-guided resection of maxillary tumors: Can a new volumetric virtual planning method improve outcomes in terms of control of resection margins? J. Cranio Maxillofac. Surg. 2018, 46, 2240–2247. [Google Scholar] [CrossRef]
- Tarsitano, A.; Ricotta, F.; Cercenelli, L.; Bortolani, B.; Battaglia, S.; Lucchi, E.; Marchetti, C.; Marcelli, E. Pretreatment tumor volume and tumor sphericity as prognostic factors in patients with oral cavity squamous cell carcinoma. J. Cranio Maxillofac. Surg. 2019, 47, 510–515. [Google Scholar] [CrossRef]
- Marti, P.; Lampus, F.; Benevento, D.; Setacci, C. Trends in use of 3D printing in vascular surgery: A survey. Int. Angiol. 2019, 38, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, G.; Coles-Black, J.; Chuen, J.; Hardidge, A. Three-dimensional printing in orthopaedic preoperative planning improves intraoperative metrics: A systematic review. ANZ J. Surg. 2019, 90, 243–250. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Barbaresi, U.; Angiolini, A.; Pultrone, C.V.; Manferrari, F.; Bortolani, B.; Cercenelli, L.; Borghesi, M.; Chessa, F.; et al. 3D Reconstruction and physical renal model to improve percutaneous punture during PNL. Int. Braz. J. Urol. 2019, 45, 1281–1282. [Google Scholar] [CrossRef]
- Bianchi, L.; Barbaresi, U.; Cercenelli, L.; Bortolani, B.; Gaudiano, C.; Chessa, F.; Angiolini, A.; Lodi, S.; Porreca, A.; Bianchi, F.M.; et al. The Impact of 3D Digital Reconstruction on the Surgical Planning of Partial Nephrectomy: A Case-control Study. Still Time for a Novel Surgical Trend? Clin. Genitourin. Cancer 2020. [Google Scholar] [CrossRef]
- Schiavina, R.; Bianchi, L.; Borghesi, M.; Chessa, F.; Cercenelli, L.; Marcelli, E.; Brunocilla, E. Three-dimensional digital reconstruction of renal model to guide preoperative planning of robot-assisted partial nephrectomy. Int. J. Urol. 2019, 26, 931–932. [Google Scholar] [CrossRef]
- Bai, S.; Bo, B.; Bi, Y.; Wang, B.; Zhao, J.; Liu, Y.; Feng, Z.; Shang, H.; Zhao, Y. CAD/CAM surface templates as an alternative to the intermediate wafer in orthognathic surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, e1–e7. [Google Scholar] [CrossRef]
- Mazzoni, S.; Bianchi, A.; Schiariti, G.; Badiali, G.; Marchetti, C. Computer-Aided Design and Computer-Aided Manufacturing Cutting Guides and Customized Titanium Plates Are Useful in Upper Maxilla Waferless Repositioning. J. Oral Maxillofac. Surg. 2015, 73, 701–707. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Ricotta, F.; Bortolani, B.; Cercernelli, L.; Marcelli, E.; Cipriani, R.; Marchetti, C. Accuracy of CAD/CAM mandibular reconstruction: A three-dimensional, fully virtual outcome evaluation method. J. Cranio Maxillofac. Surg. 2018, 46, 1121–1125. [Google Scholar] [CrossRef]
- Tarsitano, A.; Ricotta, F.; Baldino, G.; Badiali, G.; Pizzigallo, A.; Ramieri, V.; Cascone, P.; Marchetti, C. Navigation-guided resection of maxillary tumours: The accuracy of computer-assisted surgery in terms of control of resection margins—A feasibility study. J. Cranio Maxillofac. Surg. 2017, 45, 2109–2114. [Google Scholar] [CrossRef]
- Stella, F.; Dolci, G.; Dell’Amore, A.; Badiali, G.; De Matteis, M.; Asadi, N.; Marchetti, C.; Bini, A. Three-dimensional surgical simulation-guided navigation in thoracic surgery: A new approach to improve results in chest wall resection and reconstruction for malignant diseases. Interact. Cardiovasc. Thorac. Surg. 2013, 18, 7–12. [Google Scholar] [CrossRef]
- Roncari, A.; Bianchi, A.; Taddei, F.; Marchetti, C.; Schileo, E.; Badiali, G. Navigation in Orthognathic Surgery: 3D Accuracy. Facial Plast. Surg. 2015, 31, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Badiali, G.; Cutolo, F.; Roncari, A.; Marchetti, C.; Bianchi, A. Simulation-guided navigation for vector control in pediatric mandibular distraction osteogenesis. J. Cranio Maxillofac. Surg. 2017, 45, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.J.; Shevchenko, L.; Gateno, J.; Teichgraeber, J.F.; Taylor, T.D.; Lasky, R.E.; English, J.D.; Kau, C.H.; McGrory, K.R. Outcome Study of Computer-Aided Surgical Simulation in the Treatment of Patients with Craniomaxillofacial Deformities. J. Oral Maxillofac. Surg. 2011, 69, 2014–2024. [Google Scholar] [CrossRef]
- Hsu, S.S.-P.; Gateno, J.; Bell, R.B.; Hirsch, D.L.; Markiewicz, M.R.; Teichgraeber, J.F.; Zhou, X.; Xia, J.J. Accuracy of a Computer-Aided Surgical Simulation Protocol for Orthognathic Surgery: A Prospective Multicenter Study. J. Oral Maxillofac. Surg. 2013, 71, 128–142. [Google Scholar] [CrossRef]
- Zinser, M.J.; Mischkowski, R.A.; Dreiseidler, T.; Thamm, O.C.; Rothamel, D.; Zöller, J.E. Computer-assisted orthognathic surgery: Waferless maxillary positioning, versatility, and accuracy of an image-guided visualisation display. Br. J. Oral Maxillofac. Surg. 2013, 51, 827–833. [Google Scholar] [CrossRef]
- Vostars Horizon 2020 Project. Available online: http://www.vostars.eu/ (accessed on 1 October 2020).
- Badiali, G.; Cutolo, F.; Cercenelli, L.; Carbone, M.; D’Amato, R.; Ferrari, V.; Marchetti, C. The VOSTARS project: A new wearable hybrid video and optical see-through augmented reality surgical system for maxillofacial surgery. Int. J. Oral Maxillofac. Surg. 2019, 48, 153. [Google Scholar] [CrossRef]
- Badiali, G.; Cutolo, F.; Cercenelli, L.; Marchetti, C.; Ferrari, V. The VOSTARS project: Hybrid video and optical see through augmented reality surgical system for maxillofacial surgery. In Proceedings of the 24th Congress of the European Association for Cranio Maxillo Facial Surgery, Munich, Germany, 18–21 September 2018. [Google Scholar]
- Cutolo, F.; Meola, A.; Carbone, M.; Sinceri, S.; Cagnazzo, F.; Denaro, E.; Esposito, N.; Ferrari, M.; Ferrari, V. A new head-mounted display-based augmented reality system in neurosurgical oncology: A study on phantom. Comput. Assist. Surg. 2017, 22, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, F.; Fida, B.; Cattari, N.; Ferrari, V. Software Framework for Customized Augmented Reality Headsets in Medicine. IEEE Access 2020, 8, 706–720. [Google Scholar] [CrossRef]
- Cutolo, F.; Freschi, C.; Mascioli, S.; Parchi, P.D.; Ferrari, M.; Ferrari, V. Robust and Accurate Algorithm for Wearable Stereoscopic Augmented Reality with Three Indistinguishable Markers. Electronics 2016, 5, 59. [Google Scholar] [CrossRef]
- Ferrari, V.; Carbone, M.; Cappelli, C.; Boni, L.; Melfi, F.M.; Ferrari, M.; Mosca, F.; Pietrabissa, A. Value of multidetector computed tomography image segmentation for preoperative planning in general surgery. Surg. Endosc. 2011, 26, 616–626. [Google Scholar] [CrossRef]
- Condino, S.; Fida, B.; Carbone, M.; Cercenelli, L.; Badiali, G.; Ferrari, V.; Cutolo, F. Wearable Augmented Reality Platform for Aiding Complex 3D Trajectory Tracing. Sensors 2020, 20, 1612. [Google Scholar] [CrossRef]
- Badiali, G.; Cercenelli, L.; Battaglia, S.; Marcelli, E.; Marchetti, C.; Ferrari, V.; Cutolo, F. Review on Augmented Reality in Oral and Cranio-Maxillofacial Surgery: Toward “Surgery-Specific” Head-Up Displays. IEEE Access 2020, 8, 59015–59028. [Google Scholar] [CrossRef]
- Fida, B.; Cutolo, F.; Di Franco, G.; Ferrari, M.; Ferrari, V. Augmented reality in open surgery. Updat. Surg. 2018, 70, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Mischkowski, R.A.; Zinser, M.; Kübler, A.C.; Krug, B.; Seifert, U.; Zöller, J.E. Application of an augmented reality tool for maxillary positioning in orthognathic surgery—A feasibility study. J. Cranio Maxillofac. Surg. 2006, 34, 478–483. [Google Scholar] [CrossRef]
- Ferrari, V.; Carbone, M.; Condino, S.; Cutolo, F. Are augmented reality headsets in surgery a dead end? Expert Rev. Med. Devices 2019, 16, 999–1001. [Google Scholar] [CrossRef]
- Grubert, J.; Itoh, Y.; Moser, K.; Swan, J.E.; Ii, J.E.S. A Survey of Calibration Methods for Optical See-Through Head-Mounted Displays. IEEE Trans. Vis. Comput. Graph. 2018, 24, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Lalande, A.; Guigou, C.; Grayeli, A.B. Contribution of Augmented Reality to Minimally Invasive Computer-Assisted Cranial Base Surgery. IEEE J. Biomed. Health Inform. 2019, 1. [Google Scholar] [CrossRef]
- Battaglia, S.; Ratti, S.; Manzoli, L.; Marchetti, C.; Cercenelli, L.; Marcelli, E.; Tarsitano, A.; Ruggeri, A. Augmented Reality-Assisted Periosteum Pedicled Flap Harvesting for Head and Neck Reconstruction: An Anatomical and Clinical Viability Study of a Galeo-Pericranial Flap. J. Clin. Med. 2020, 9, 2211. [Google Scholar] [CrossRef]

| Subject | T4 | |
|---|---|---|
| IW1 (±1 mm) | IW2 (±0.5 mm) | |
| 1 | 100.0% | 81.4% |
| 2 | 100.0% | 93.8% |
| 3 | 100.0% | 100.0% |
| 4 | 100.0% | 100.0% |
| 5 | 100.0% | 87.6% |
| 6 | 100.0% | 49.5% |
| 7 | 100.0% | 93.8% |
| 8 | 100.0% | 86.6% |
| 9 | 100.0% | 92.8% |
| 10 | 100.0% | 90.7% |
| Mean | 100.0% | 87.6% |
| SD | 0.0% | 14.6% |
| Accuracy Level. | T1 vs. T2 | T1 vs. T3 | T1 vs. T4 | T2 vs. T3 | T2 vs. T4 | T3 vs. T4 |
|---|---|---|---|---|---|---|
| ±1.0 mm | 0.465 | 0.326 | 0.109 | 0.115 | 0.042 | 0.042 |
| ±0.5 mm | 0.214 | 0.386 | 0.507 | 0.069 | 0.508 | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cercenelli, L.; Carbone, M.; Condino, S.; Cutolo, F.; Marcelli, E.; Tarsitano, A.; Marchetti, C.; Ferrari, V.; Badiali, G. The Wearable VOSTARS System for Augmented Reality-Guided Surgery: Preclinical Phantom Evaluation for High-Precision Maxillofacial Tasks. J. Clin. Med. 2020, 9, 3562. https://doi.org/10.3390/jcm9113562
Cercenelli L, Carbone M, Condino S, Cutolo F, Marcelli E, Tarsitano A, Marchetti C, Ferrari V, Badiali G. The Wearable VOSTARS System for Augmented Reality-Guided Surgery: Preclinical Phantom Evaluation for High-Precision Maxillofacial Tasks. Journal of Clinical Medicine. 2020; 9(11):3562. https://doi.org/10.3390/jcm9113562
Chicago/Turabian StyleCercenelli, Laura, Marina Carbone, Sara Condino, Fabrizio Cutolo, Emanuela Marcelli, Achille Tarsitano, Claudio Marchetti, Vincenzo Ferrari, and Giovanni Badiali. 2020. "The Wearable VOSTARS System for Augmented Reality-Guided Surgery: Preclinical Phantom Evaluation for High-Precision Maxillofacial Tasks" Journal of Clinical Medicine 9, no. 11: 3562. https://doi.org/10.3390/jcm9113562
APA StyleCercenelli, L., Carbone, M., Condino, S., Cutolo, F., Marcelli, E., Tarsitano, A., Marchetti, C., Ferrari, V., & Badiali, G. (2020). The Wearable VOSTARS System for Augmented Reality-Guided Surgery: Preclinical Phantom Evaluation for High-Precision Maxillofacial Tasks. Journal of Clinical Medicine, 9(11), 3562. https://doi.org/10.3390/jcm9113562











