The HYLAN M Study: Efficacy of 0.15% High Molecular Weight Hyaluronan Fluid in the Treatment of Severe Dry Eye Disease in a Multicenter Randomized Trial
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Participants
2.3. Efficacy Assessment
2.4. Statistical Analysis
3. Results
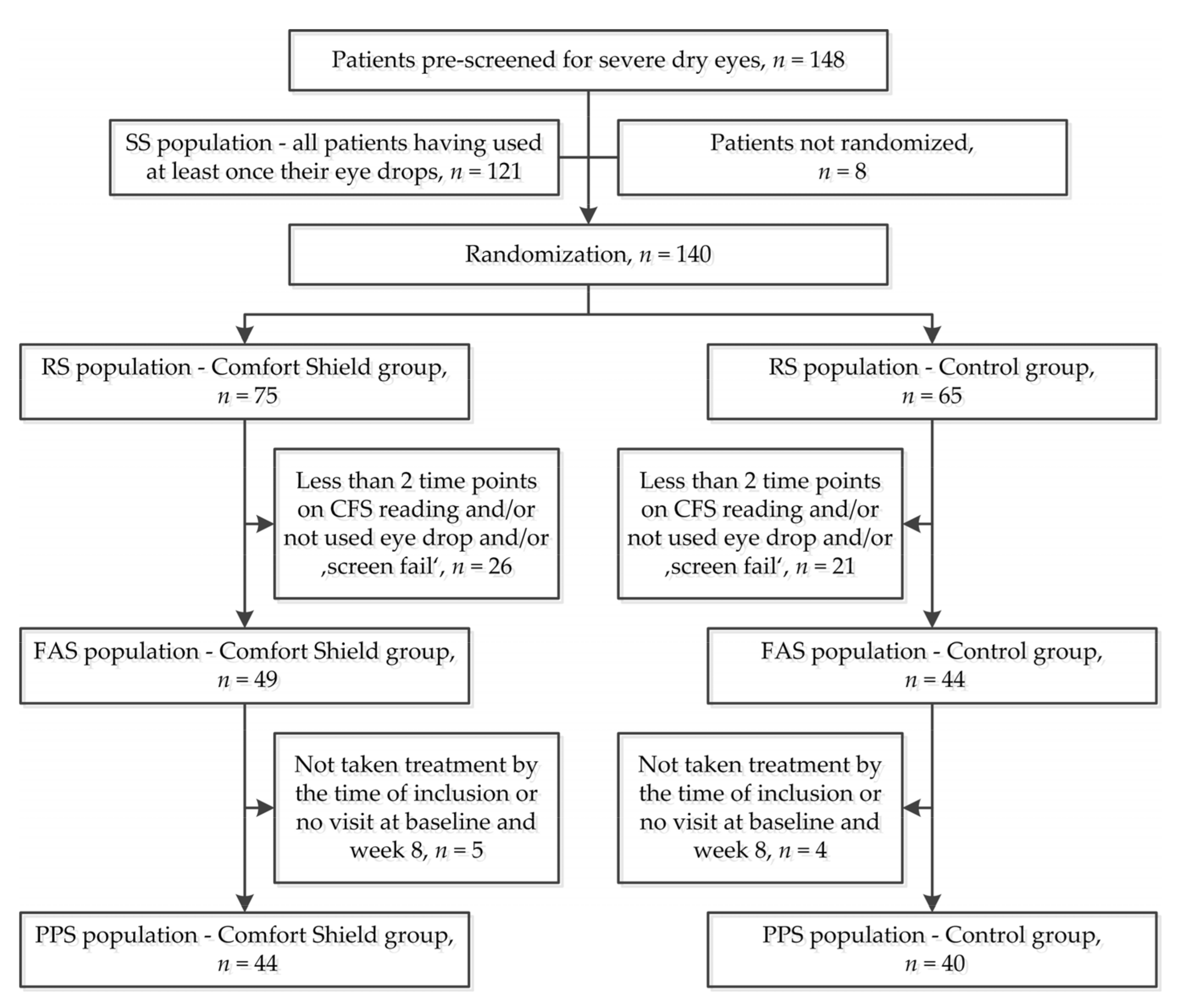
3.1. Participant Flow
3.2. Demographic Data
3.3. Efficacy Results
3.3.1. Corneal Fluorescein Staining
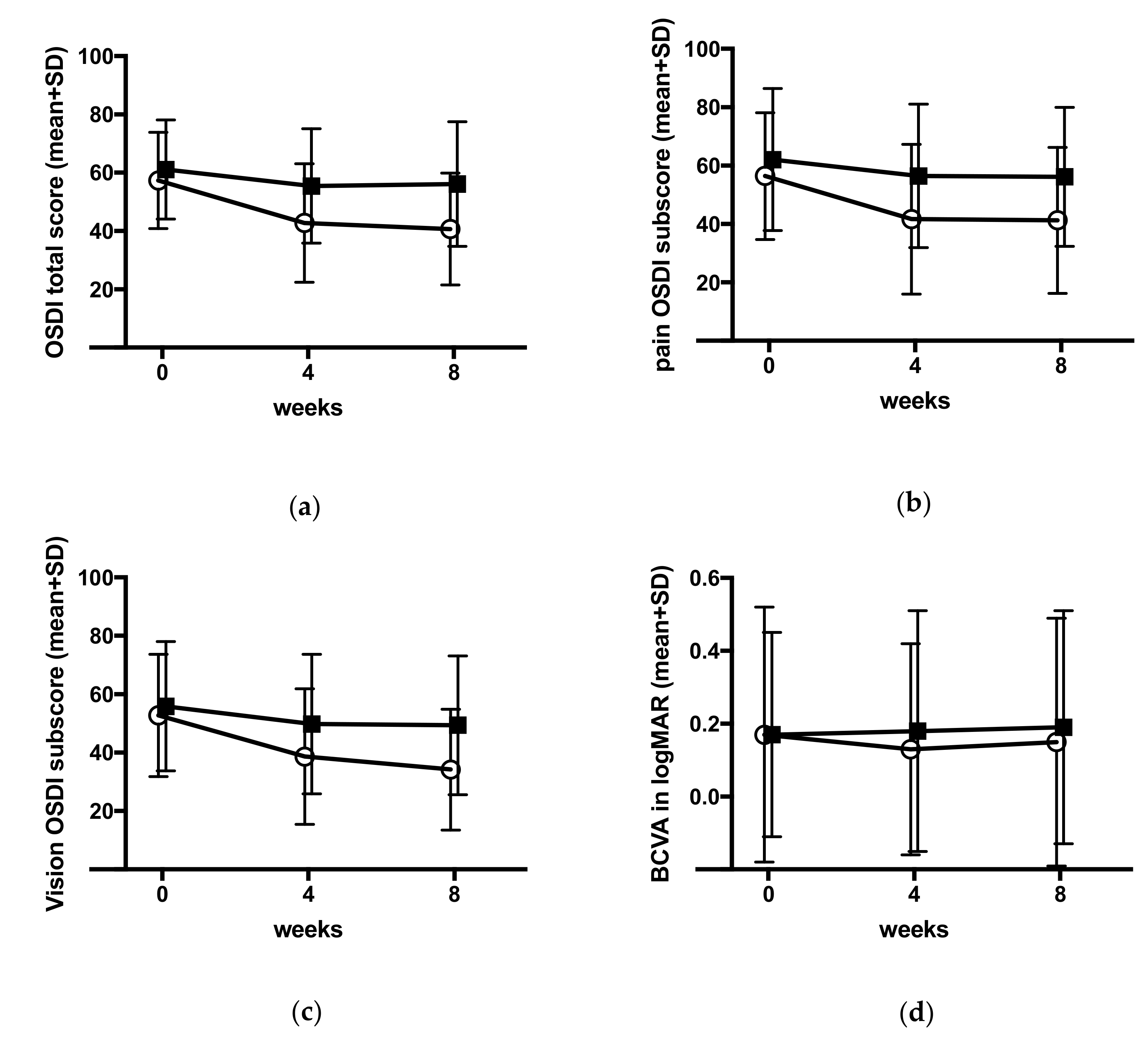
3.3.2. Ocular Surface Disease Index
3.3.3. Best Corrected Visual Acuity
3.3.4. Other Secondary Endpoints
3.3.5. Observation of the Subbasal Nerve Plexus by Confocal Microscopy
3.3.6. Dropping Frequency
3.3.7. Influence of Climate on CFS and OSDI
3.4. Safety Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Study Centers, Administrative Structure of the Study, and Author Contributions
- (1)
- Centre Hospitalier National d’Ophtalmologie—Quinze-Vingts, Paris, France, principle investigator (PI) Christophe Baudouin
- (2)
- St. Erik Eye Hospital, Stockholm, Sweden, PI Gysbert-Botho van Setten
- (3)
- Department of Ophthalmology, University Medical Center Rostock, Rostock, Germany, PI Ria Beck
- (4)
- Department of Ophthalmology, Medical University Graz, Graz, Austria, PI Jutta Horwath-Winter
- (5)
- Department of Ophthalmology, University Clinic Salzburg, Paracelsus Medical University, Salzburg, Austria, PI Herbert A. Reitsamer
- (6)
- Department of Ophthalmology, Saarland University Medical Center, Homburg/Saar, Germany, PI Berthold Seitz
- (7)
- KKESH—King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia, PI Osama Al-Sheikh
- (8)
- Marmara University Pendik Training and Research Hospital, Marmara University Medical School, Department of Ophthalmology, Istanbul, Turkey, PI Ebru Toker
- (9)
- Department of Ophthalmology, PSMMC Prince Sultan Military Medical City, MSD Medical Services Department, MODA Ministry of Defense and Aviation, Riyadh, Saudi Arabia, PI Sultan Al-Zaaidi
- (10)
- Universidad Complutense de Madrid, Hospital Clinico San Carlos, Departamentos de Oftalmologia, Madrid, Spain, PI Jose M. Benitez-del-Castillo
- (11)
- Ocular Surface & Dry Eye Center, Ospedale L. Sacco, University of Milan, Milan, Italy, PI Stefano Barabino
- IPR—International Pharmaceutical Consultancy, Paris, France
- OPIS, Desio, Italy
- Monitor Medikal Araştırma ve Danışmanlık Tic. Ltd. Şti., Istanbul, Turkey
- KKESH Research Department, Riyadh, Kingdom of Saudi Arabia.
Appendix B. Ethics Committee Approval, Compliance with the Declaration of Helsinki, and Registration of the Study
- Austria: Medical University Graz, Ethic Committee, reg. no. 28-458 ex 15/16
- France: Comite de protection des Persones Ile de France V, reg. no. 16138
- Germany: Ärztekammer des Saarlandes, Ethik-Kommission, reg. no. 176/16
- Italy: Comitato Etico Milano Area 1, protocol no. 47068/2018
- Kingdom of Saudi Arabia: King Khaled Eye Specialist Hospital, Research Department, reference RSCH/665/5957-16 and Prince Sultan Military Medical City, Research Ethics Committee, reg. no. HAP-01-R-015
- Spain: CEIC Hospital Clinico San Carlos, reg. no. 18/016-R_P
- Sweden: EPN Regionala etikprövningsnämnden I Stockholm protocol 19 October 2016
- Turkey: Marmara University Hospital, Klinik Arastirmalar Etik Kurulu, form 2013-KAEK-60
Appendix C. Investigational Device
Appendix D. Corneal Fluorescein Staining and Electronic Analysis Method

Appendix E. Medical History
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | ||
|---|---|---|---|---|
| Rheumatoid disease n (%) | n | 43 | 40 | 83 |
| no | 21 (48.8) | 20 (50.0) | 41 (49.4) | |
| yes | 22 (51.2) | 20 (50.0) | 42 (50.6) | |
| missing | 1 | 0 | 1 | |
| Thyroid disease n (%) | n | 43 | 40 | 83 |
| no | 27 (62.8) | 33 (82.5) | 60 (72.3) | |
| yes | 16 (37.2) | 7 (17.5) | 23 (27.7) | |
| missing | 1 | 0 | 1 | |
| Trachoma n (%) | n | 43 | 40 | 83 |
| no | 43 (100.0) | 40 (100.0) | 83 (100.0) | |
| missing | 1 | 0 | 1 | |
| Other disease n (%) | n | 43 | 39 | 82 |
| no | 23 (53.5) | 17 (43.6) | 40 (48.8) | |
| yes | 20 (46.5) | 22 (56.4) | 42 (51.2) | |
| missing | 1 | 1 | 2 | |
| History betablocker n (%) | n | 43 | 40 | 83 |
| no | 33 (76.7) | 33 (82.5) | 66 (79.5) | |
| yes | 10 (23.3) | 7 (17.5) | 17 (20.5) | |
| missing | 1 | 0 | 1 | |
| Antidepressants n (%) | n | 43 | 40 | 83 |
| no | 36 (83.7) | 34 (85.0) | 70 (84.3) | |
| yes | 7 (16.3) | 6 (15.0) | 13 (15.7) | |
| missing | 1 | 0 | 1 | |
| Other drugs n (%) | n | 42 | 38 | 80 |
| no | 20 (47.6) | 11 (28.9) | 31 (38.8) | |
| yes | 22 (52.4) | 27 (71.1) | 49 (61.2) | |
| missing | 2 | 2 | 4 | |
| History contact lenses n (%) | n | 43 | 40 | 83 |
| no | 42 (97.7) | 40 (100.0) | 82 (98.8) | |
| yes | 1 (2.3) | 0 (0.0) | 1 (1.2) | |
| missing | 1 | 0 | 1 | |
| Conjunctival injection n (%) | n | 43 | 39 | 82 |
| no | 25 (58.1) | 20 (51.3) | 45 (54.9) | |
| yes | 18 (41.9) | 19 (48.7) | 37 (45.1) | |
| missing | 1 | 1 | 2 | |
| Inflammation of lid rim n (%) | n | 43 | 39 | 82 |
| no | 30 (69.8) | 28 (71.8) | 58 (70.7) | |
| yes | 13 (30.2) | 11 (28.2) | 24 (29.3) | |
| missing | 1 | 1 | 2 |
Appendix F. Tear Osmolarity Test Results
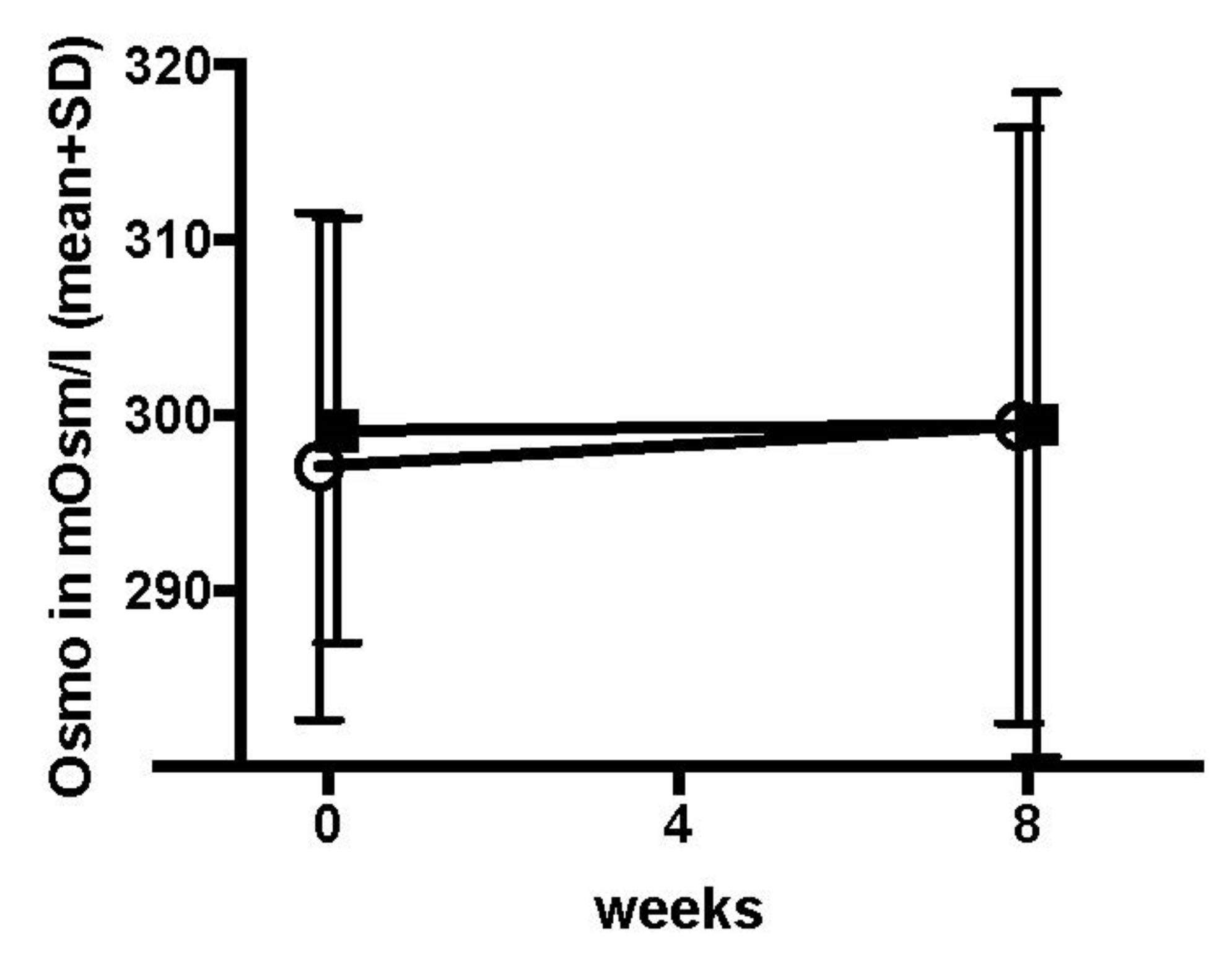
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | ||
|---|---|---|---|---|
| value at baseline | n | 40 | 37 | 77 |
| mean (sd) | 297.12 (14.47) | 299.16 (12.11) | 298.1 (13.34) | |
| median (iqr) | 295.5 (287.75, 304.25) | 299 (292, 307) | 298 (289, 306) | |
| min, max | 277, 337 | 275, 336 | 275, 337 | |
| * <275 mOsm/L | 2 | 2 | 4 | |
| missing | 2 | 1 | 3 | |
| value at week 8 | n | 40 | 37 | |
| mean (sd) | 299.43 (16.99) | 299.49 (18.94) | ||
| median (iqr) | 300 (286, 308) | 299 (291, 304) | ||
| min, max | 275, 353 | 276, 395 | ||
| * <275 mOsm/L | 1 | 3 | ||
| missing | 3 | 0 |
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | ||
|---|---|---|---|---|
| value at baseline | n | 42 | 39 | 81 |
| <275 mOsm/L | 2 (4.8) | 2 (5.1) | 4 (4.9) | |
| ≥275 mOsm/L | 40 (95.2) | 37 (94.9) | 77 (95.1) | |
| missing | 2 | 1 | 3 | |
| value at week 8 | n | 41 | 40 | |
| <275 mOsm/L | 1 (2.4) | 3 (7.5) | ||
| * ≥275 mOsm/L | 40 (97.6) | 37 (92.5) | ||
| missing | 3 | 0 |
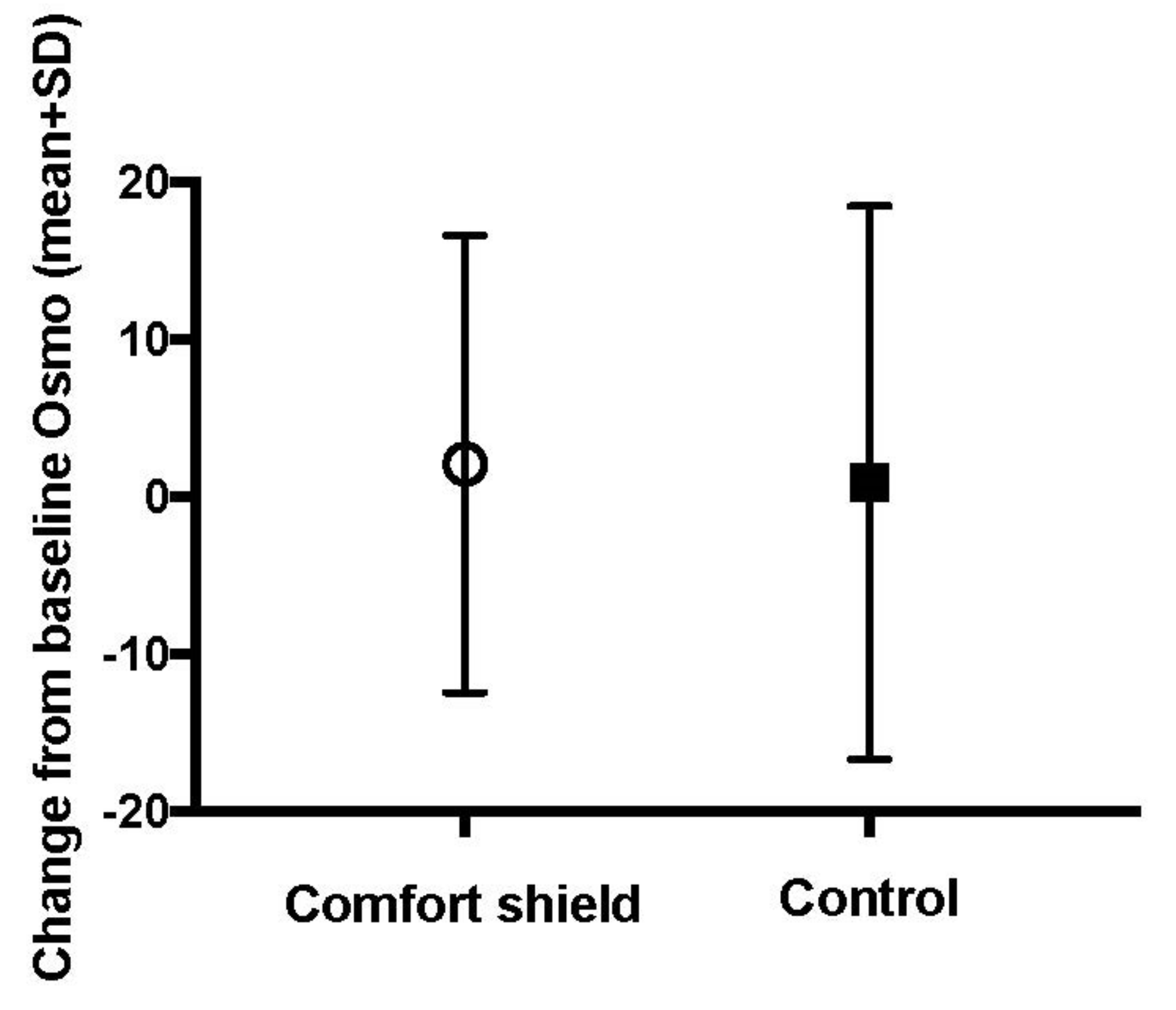
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | p-Value | ||
|---|---|---|---|---|---|
| value at baseline | n | 40 | 37 | 77 | |
| mean (sd) | 297.12 (14.47) | 299.16 (12.11) | 298.1 (13.34) | ||
| median (iqr) | 295.5 (287.75, 304.25) | 299 (292, 307) | 298 (289, 306) | ||
| min, max | 277, 337 | 275, 336 | 275, 337 | ||
| * <275 mOsm/L | 2 | 2 | 4 | ||
| missing | 2 | 1 | 3 | ||
| change from baseline to week 8 | n | 37 | 35 | 0.294 | |
| mean (sd) | 2.11 (14.54) | 0.94 (17.59) | |||
| median (iqr) | 2 (−6, 12) | −1 (−7.0, 7.5) | |||
| min, max | −29, 27 | −30, 80 | |||
| * <275 mOsm/L | 1 | 3 | |||
| ** missing | 6 | 2 |
Appendix G. Influence of Climate on CFS and OSDI
| Comfort Shield | Control | ||||
|---|---|---|---|---|---|
| Desert (n = 6) | Europe (n = 38) | Desert (n = 7) | Europe (n = 33) | ||
| value at baseline | n | 6 | 38 | 7 | 33 |
| mean (sd) | 3.79 (0.71) | 4.04 (0.52) | 4.11 (0.61) | 4.30 (0.55) | |
| median (iqr) | 3.62 (3.37, 4.05) | 3.94 (3.59, 4.39) | 3.95 (3.60, 4.56) | 4.41 (3.80, 4.65) | |
| min, max | 3, 5 | 3, 5 | 3, 5 | 3, 5 | |
| missing | 0 | 0 | 0 | 0 | |
| value at week 4 | n | 5 | 36 | 6 | 32 |
| mean (sd) | 3.37 (0.89) | 3.89 (0.82) | 2.22 (1.53) | 3.72 (1.05) | |
| median (iqr) | 3.74 (2.81, 4.09) | 4.00 (3.43, 4.54) | 2.15 (1.41, 3.36) | 3.93 (3.25, 4.48) | |
| min, max | 2, 4 | 1, 5 | 0, 4 | 0, 5 | |
| missing | 1 | 2 | 1 | 1 | |
| value at week 8 | n | 6 | 35 | 6 | 32 |
| mean (sd) | 3.79 (1.30) | 3.94 (0.97) | 2.21 (1.91) | 3.88 (0.83) | |
| median (iqr) | 4.20 (2.60, 4.84) | 4.31 (3.38, 4.68) | 2.56 (0.58, 3.22) | 3.99 (3.37, 4.45) | |
| min, max | 2, 5 | 0, 5 | 0, 5 | 2, 5 | |
| missing | 0 | 3 | 1 | 1 | |
| Comfort Shield | Control | ||||
|---|---|---|---|---|---|
| Desert (n = 6) | Europe (n = 38) | Desert (n = 7) | Europe (n = 33) | ||
| baseline | n | 6 | 38 | 7 | 33 |
| mean (sd) | 3.79 (0.71) | 4.04 (0.52) | 4.11 (0.61) | 4.30 (0.55) | |
| median (iqr) | 3.62 (3.37, 4.05) | 3.94 (3.59, 4.39) | 3.95 (3.60, 4.56) | 4.41 (3.80, 4.65) | |
| min, max | 3, 5 | 3, 5 | 3, 5 | 3, 5 | |
| missing | 0 | 0 | 0 | 0 | |
| change from baseline to week 4 | n | 5 | 36 | 6 | 32 |
| mean (sd) | −0.43 (0.59) | −0.18 (0.78) | −1.74 (1.16) | −0.57 (0.90) | |
| median (iqr) | −0.70 (−0.91, −0.06) | −0.03 (−0.52, 0.31) | −1.51 (−2.29, −0.82) | −0.35 (−0.92, −0.14) | |
| min, max | −1, 0 | −3, 1 | −4, −1 | −4, 1 | |
| missing | 1 | 2 | 1 | 1 | |
| change from baseline to week 8 | n | 6 | 35 | 6 | 32 |
| mean (sd) | 0.00 (0.98) | −0.14 (1.06) | −1.79 (1.48) | −0.42 (0.82) | |
| median (iqr) | −0.02 (−0.62, 0.64) | −0.07 (−0.56, 0.56) | −1.33 (−3.11, −0.75) | −0.23 (−0.64, 0.12) | |
| min, max | −1, 1 | −4, 1 | −4, 0 | −3, 1 | |
| missing | 0 | 3 | 1 | 1 | |
| Comfort Shield | Control | ||||
|---|---|---|---|---|---|
| Desert (n = 6) | Europe (n = 38) | Desert (n = 7) | Europe (n = 33) | ||
| baseline | n | 6 | 38 | 7 | 33 |
| mean (sd) | 62.34 (18.55) | 56.63 (16.29) | 62.98 (20.34) | 60.73 (16.53) | |
| median (iqr) | 63.63 (51.14, 76.14) | 54.36 (43.32, 68.44) | 70.45 (46.93, 77.71) | 61.11 (52.08, 75.00) | |
| min, max | 35, 84 | 34, 92 | 36, 85 | 34, 95 | |
| missing | 0 | 0 | 0 | 0 | |
| value at week 4 | n | 6 | 38 | 7 | 33 |
| mean (sd) | 36.89 (31.71) | 43.75 (18.39) | 49.74 (15.73) | 56.71 (20.37) | |
| median (iqr) | 20.45 (19.18, 61.36) | 40.91 (32.71, 55.31) | 43.18 (36.82, 64.02) | 55.56 (41.67, 69.44) | |
| min, max | 7, 80 | 9, 85 | 33, 70 | 22, 95 | |
| missing | 0 | 0 | 0 | 0 | |
| value at week 8 | n | 6 | 38 | 7 | 33 |
| mean (sd) | 26.45 (20.34) | 42.95 (18.26) | 65.85 (12.65) | 54.11 (22.42) | |
| median (iqr) | 32.95 (9.38, 40.91) | 41.67 (29.27, 55.94) | 65.91 (58.62, 74.75) | 52.08 (35.42, 75.00) | |
| min, max | 0, 48 | 15, 88 | 46, 82 | 9, 93 | |
| missing | 0 | 0 | 0 | 0 | |
| Comfort Shield | Control | ||||
|---|---|---|---|---|---|
| Desert (n = 6) | Europe (n = 38) | Desert (n = 7) | Europe (n = 33) | ||
| baseline | n | 6 | 38 | 7 | 33 |
| mean (sd) | 62.34 (18.55) | 56.63 (16.29) | 62.98 (20.34) | 60.73 (16.53) | |
| median (iqr) | 63.63 (51.14, 76.14) | 54.36 (43.32, 68.44) | 70.45 (46.93, 77.71) | 61.11 (52.08, 75.00) | |
| min, max | 35, 84 | 34, 92 | 36, 85 | 34, 95 | |
| missing | 0 | 0 | 0 | 0 | |
| change from baseline to week 4 | n | 6 | 38 | 7 | 33 |
| mean (sd) | −25.45 (33.48) | −12.88 (18.03) | −13.24 (12.94) | −4.02 (12.19) | |
| median (iqr) | −34.59 (−45.60, −7.07) | −9.66 (−19.69, −2.61) | −12.50 (−16.32, −5.93) | −2.50 (−8.61, 0.00) | |
| min, max | −64, 27 | −71, 17 | −38, 2 | −31, 26 | |
| missing | 0 | 0 | 0 | 0 | |
| change from baseline to week 8 | n | 6 | 38 | 7 | 33 |
| mean (sd) | −35.89 (27.94) | −13.68 (20.02) | 2.88 (18.26) | −6.63 (16.47) | |
| median (iqr) | −34.95 (−40.34, −23.15) | −12.50 (−20.98, −0.23) | −2.50 (−7.41, 4.52) | −3.86 (−18.75, 6.82) | |
| min, max | −84, 0 | −69, 27 | −12, 41 | −43, 15 | |
| missing | 0 | 0 | 0 | 0 | |
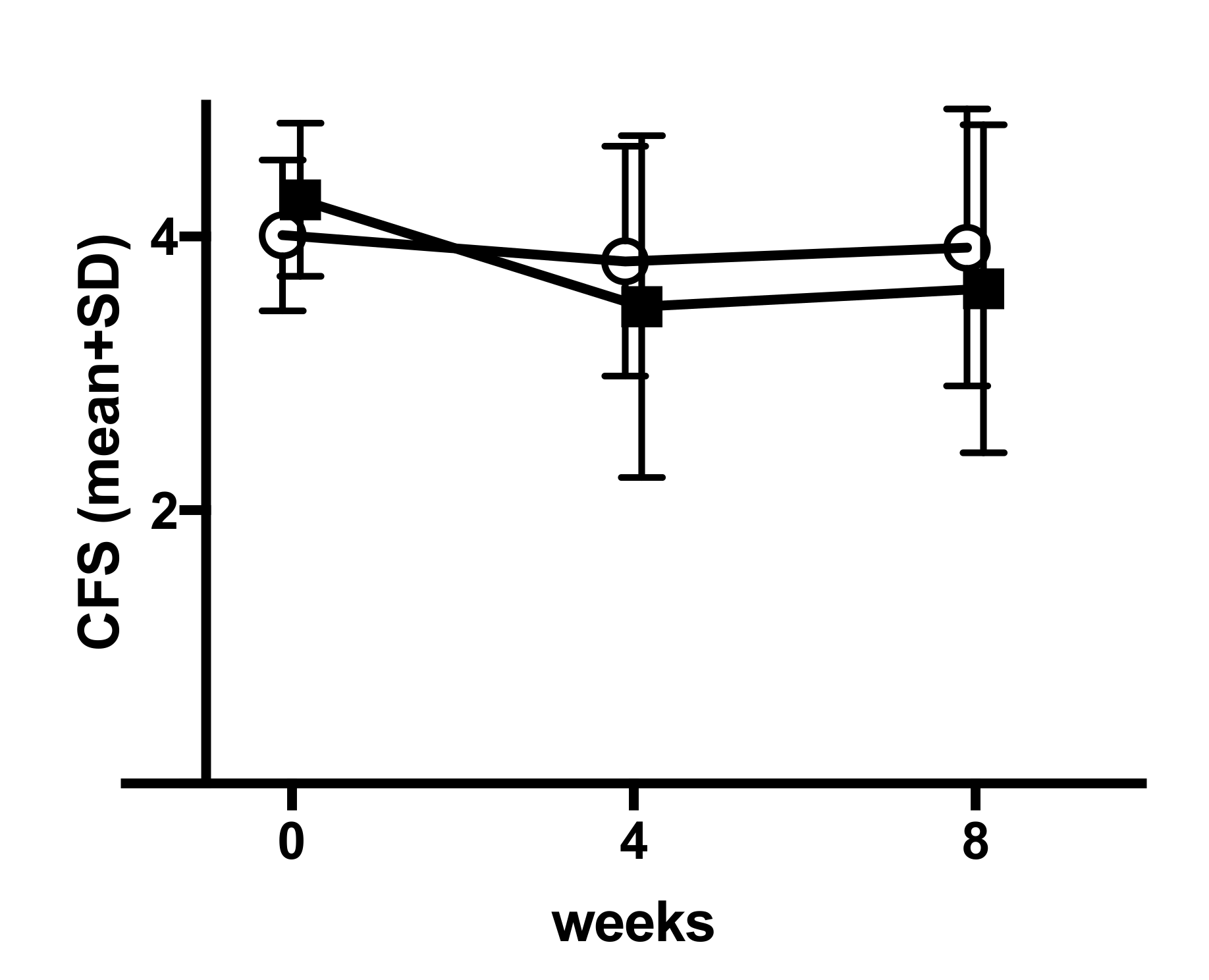
Appendix H. Fluctuation of Corneal Fluorescein Staining in the Control Group
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, I.J.; Petris, R.; Han, G.; Dickersin, K.; Akpek, E.K. Research Questions and Outcomes Prioritized by Patients With Dry Eye. JAMA Ophthalmol. 2018, 136, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Benítez-del-Castillo, J.; Labetoulle, M.; Baudouin, C.; Rolando, M.; Akova, Y.A.; Aragona, P.; Geerling, G.; Merayo-Lloves, J.; Messmer, E.M.; Boboridis, K. Visual acuity and quality of life in dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 2017, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Watanabe, J.; Strauss, J.; Dubey, A. Work productivity loss in patients with dry eye disease: An online survey. Curr. Med Res. Opin. 2011, 27, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Asche, C.V.; Fairchild, C.J. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011, 30, 379–387. [Google Scholar] [CrossRef]
- Uchino, M. What We Know About the Epidemiology of Dry Eye Disease in Japan. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES1–DES6. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. A New Perspective on Dry Eye Classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46 (Suppl. 1), S2–S13. [Google Scholar] [CrossRef]
- van Setten, G.B.; Mueller-Lierheim, W.; Baudouin, C. Dry Eye Etiology: Focus on Friction. Klin. Mon. Augenheilkd. 2019, 237, 1235–1236. [Google Scholar] [CrossRef]
- van Setten, G.-B. Impact of Attrition, Intercellular Shear in Dry Eye Disease: When Cells are Challenged and Neurons are Triggered. Int. J. Mol. Sci. 2020, 21, 4333. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Pflugfelder, S.C. Inflammation in dry eye. Ocul. Surf. 2004, 2, 124–130. [Google Scholar] [CrossRef]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review. Br. J. Ophthalmol. 2020, 32703782. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C. A new approach for better comprehension of diseases of the ocular surface. J. Fr. Ophtalmol. 2007, 30, 239–246. [Google Scholar] [CrossRef]
- Baudouin, C.; Irkec, M.; Messmer, E.M.; Benitez-Del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- van Setten, G.B. Osmokinetics: A new dynamic concept in dry eye disease. J. Fr. Ophtalmol. 2019, 42, 221–225. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- van Setten, G.B. Osmokinetics: Defining the Characteristics of Osmotic Challenge to the Ocular Surface. Klin. Mon. Augenheilkd. 2020, 237, 644–648. [Google Scholar] [CrossRef]
- Belmonte, C. Pain, Dryness, and Itch Sensations in Eye Surface Disorders Are Defined By a Balance Between Inflammation and Sensory Nerve Injury. Cornea 2019, 38 (Suppl. 1), S11–S24. [Google Scholar] [CrossRef]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef] [PubMed]
- Schein, O.D.; Tielsch, J.M.; Munoz, B.; Bandeen-Roche, K.; West, S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology 1997, 104, 1395–1401. [Google Scholar] [CrossRef]
- Nichols, K.K.; Nichols, J.J.; Mitchell, G.L. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004, 23, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.S.; Felix, E.R.; Levitt, R.C.; Feuer, W.J.; Sarantopoulos, C.D.; Galor, A. Epidemiology of discordance between symptoms and signs of dry eye. Br. J. Ophthalmol. 2018, 102, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Keith, M.S.; Sudharshan, L.; Snedecor, S.J. Associations between signs and symptoms of dry eye disease: A systematic review. Clin. Ophthalmol. 2015, 9, 1719–1730. [Google Scholar] [CrossRef]
- Galor, A.; Moein, H.R.; Lee, C.; Rodriguez, A.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef]
- Dua, H.S.; Said, D.G.; Messmer, E.M.; Rolando, M.; Benitez-Del-Castillo, J.M.; Hossain, P.N.; Shortt, A.J.; Geerling, G.; Nubile, M.; Figueiredo, F.C.; et al. Neurotrophic keratopathy. Prog. Retin. Eye Res. 2018, 66, 107–131. [Google Scholar] [CrossRef]
- Galor, A.; Batawi, H.; Felix, E.R.; Margolis, T.P.; Sarantopoulos, K.D.; Martin, E.R.; Levitt, R.C. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br. J. Ophthalmol. 2016, 100, 745–749. [Google Scholar] [CrossRef]
- Galor, A. Painful Dry Eye Symptoms: A Nerve Problem or a Tear Problem? Ophthalmology 2019, 126, 648–651. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Gomis, A.; Pawlak, M.; Balazs, E.A.; Schmidt, R.F.; Belmonte, C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004, 50, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Caires, R.; Luis, E.; Taberner, F.J.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Balazs, E.A.; Gomis, A.; Belmonte, C.; de la Pena, E. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat. Commun. 2015, 6, 8095. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.F.; Khomula, E.V.; Araldi, D.; Levine, J.D. CD44 Signaling Mediates High Molecular Weight Hyaluronan-Induced Antihyperalgesia. J. Neurosci. 2018, 38, 308–321. [Google Scholar] [CrossRef]
- Müller-Lierheim, W.G.K. Why Chain Length of Hyaluronan in Eye Drops Matters. Diagnostics 2020, 10, 511. [Google Scholar] [CrossRef]
- Kojima, T.; Nagata, T.; Kudo, H.; Müller-Lierheim, W.G.K.; van Setten, G.-B.; Dogru, M.; Tsubota, K. The Effects of High Molecular Weight Hyaluronic Acid Eye Drop Application in Environmental Dry Eye Stress Mice. Int. J. Mol. Sci. 2020, 21, 3516. [Google Scholar] [CrossRef] [PubMed]
- Karpecki, P.M. Why dry eye trials often fail: From disease variability to confounding underlying conditions, there are countless reasons why new dry eye drugs have come up short in FDA testing. Rev. Optom. 2013, 2013, 50. [Google Scholar]
- Baudouin, C.; Aragona, P.; Van Setten, G.; Rolando, M.; Irkec, M.; Benitez del Castillo, J.; Geerling, G.; Labetoulle, M.; Bonini, S.; Members, O.E.C.G. Diagnosing the severity of dry eye: A clear and practical algorithm. Br. J. Ophthalmol. 2014, 98, 1168–1176. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Fuller, D.G.; Potts, K.; Kim, J. Noninvasive tear breakup times and ocular surface disease. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2013, 90, 1086–1091. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J. The Effect of instilled fluorescein solution volume on the values and repeatability of TBUT measurements. Cornea 2005, 24, 811–817. [Google Scholar] [CrossRef]
- Murube, J. The Schirmer test: Celebration of its first centenary. Ocul. Surf. 2003, 1, 157–159. [Google Scholar] [CrossRef]
- Han, S.B.; Liu, Y.C.; Mohamed-Noriega, K.; Tong, L.; Mehta, J.S. Objective Imaging Diagnostics for Dry Eye Disease. J. Ophthalmol. 2020, 2020, 3509064. [Google Scholar] [CrossRef]
- Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 108–152. [CrossRef]
- Hamrah, P.; Alipour, F.; Jiang, S.; Sohn, J.H.; Foulks, G.N. Optimizing evaluation of Lissamine Green parameters for ocular surface staining. Eye 2011, 25, 1429–1434. [Google Scholar] [CrossRef]
- Korb, D.R.; Herman, J.P.; Blackie, C.A.; Scaffidi, R.C.; Greiner, J.V.; Exford, J.M.; Finnemore, V.M. Prevalence of lid wiper epitheliopathy in subjects with dry eye signs and symptoms. Cornea 2010, 29, 377–383. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kutsuna, M.; Uno, T.; Zheng, X.; Kodama, T.; Ohashi, Y. Marx line: Fluorescein staining line on the inner lid as indicator of meibomian gland function. Am. J. Ophthalmol. 2006, 141, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kowtharapu, B.S.; Winter, K.; Marfurt, C.; Allgeier, S.; Kohler, B.; Hovakimyan, M.; Stahnke, T.; Wree, A.; Stachs, O.; Guthoff, R.F. Comparative quantitative assessment of the human corneal sub-basal nerve plexus by in vivo confocal microscopy and histological staining. Eye 2017, 31, 481–490. [Google Scholar] [CrossRef] [PubMed]
- van Setten, G.; Labetoulle, M.; Baudouin, C.; Rolando, M. Evidence of seasonality and effects of psychrometry in dry eye disease. Acta Ophthalmol. 2016, 94, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2001. [Google Scholar]
- Farrand, K.F.; Fridman, M.; Stillman, I.O.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef]
- Novack, G.D.; Asbell, P.; Barabino, S.; Bergamini, M.V.W.; Ciolino, J.B.; Foulks, G.N.; Goldstein, M.; Lemp, M.A.; Schrader, S.; Woods, C.; et al. TFOS DEWS II Clinical Trial Design Report. Ocul. Surf. 2017, 15, 629–649. [Google Scholar] [CrossRef] [PubMed]
- Begley, C.; Caffery, B.; Chalmers, R.; Situ, P.; Simpson, T.; Nelson, J.D. Review and analysis of grading scales for ocular surface staining. Ocul. Surf. 2019, 17, 208–220. [Google Scholar] [CrossRef]
- Kim, Y.H.; Graham, A.D.; Li, W.; Radke, C.J.; Lin, M.C. Human Lacrimal Production Rate and Wetted Length of Modified Schirmer’s Tear Test Strips. Transl. Vis. Sci. Technol. 2019, 8, 40. [Google Scholar] [CrossRef]
- Harper, C.L.; Boulton, M.E.; Bennett, D.; Marcyniuk, B.; Jarvis-Evans, J.H.; Tullo, A.B.; Ridgway, A.E. Diurnal variations in human corneal thickness. Br. J. Ophthalmol. 1996, 80, 1068–1072. [Google Scholar] [CrossRef]
- Niimi, J.; Tan, B.; Chang, J.; Zhou, Y.; Ghanekar, A.; Wong, M.; Lee, A.; Lin, M.C. Diurnal Pattern of Tear Osmolarity and Its Relationship to Corneal Thickness and Deswelling. Cornea 2013, 32, 1305–1310. [Google Scholar] [CrossRef]
- Ayaki, M.; Tachi, N.; Hashimoto, Y.; Kawashima, M.; Tsubota, K.; Negishi, K. Diurnal variation of human tear meniscus volume measured with tear strip meniscometry self-examination. PLoS ONE 2019, 14, e0215922. [Google Scholar] [CrossRef]
- Shen, M.; Wang, J.; Tao, A.; Chen, Q.; Lin, S.; Qu, J.; Lu, F. Diurnal variation of upper and lower tear menisci. Am. J. Ophthalmol. 2008, 145, 801–806. [Google Scholar] [CrossRef]
- Nichols, K.K.; Mitchell, G.L.; Zadnik, K. The repeatability of clinical measurements of dry eye. Cornea 2004, 23, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J.; Darvish, M.; Nichols, K.K.; Jones, L.; Karpecki, P.M. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf. 2019, 17, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Tashbayev, B.; Utheim, T.P.; Utheim, O.A.; Raeder, S.; Jensen, J.L.; Yazdani, M.; Lagali, N.; Vitelli, V.; Dartt, D.A.; Chen, X. Utility of Tear Osmolarity Measurement in Diagnosis of Dry Eye Disease. Sci. Rep. 2020, 10, 5542. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Yagi, Y.; Matsumoto, Y.; Tsubota, K. Impaired functional visual acuity of dry eye patients. Am. J. Ophthalmol. 2002, 133, 181–186. [Google Scholar] [CrossRef]
- Kaido, M.; Dogru, M.; Ishida, R.; Tsubota, K. Concept of functional visual acuity and its applications. Cornea 2007, 26 (Suppl. 1), S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Kaido, M. Functional Visual Acuity. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES29–DES35. [Google Scholar] [CrossRef] [PubMed]
| Test | Baseline | Week 4 | Week 8 |
|---|---|---|---|
| OSDI | X | X | X |
| dropping frequency | X | X | X |
| BCVA | X | X | X |
| CFS | X | X | X |
| TBUT | X | X | X |
| Schirmer 1 | X | X | |
| Tear osmolarity | X | X | |
| IOP | X | X | |
| LWE Korb score | (X) | (X) | |
| Yamaguchi score | (X) | (X) | |
| Confocal microscopy | (X) | (X) |
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | ||
|---|---|---|---|---|
| climate zone | n | 44 | 40 | 84 |
| Desert | 6 (13.6) | 7 (17.5) | 13 (15.5) | |
| Europe | 38 (86.4) | 33 (82.5) | 71 (84.5) | |
| missing | 0 | 0 | 0 | |
| age (years) | n | 44 | 40 | 84 |
| mean (sd) | 57.66 (14.39) | 59.45 (12.48) | 58.51 (13.46) | |
| median (iqr) | 61.5 (50.75, 65.25) | 60 (51.5, 69.0) | 61 (50.75, 67.00) | |
| min, max | 26, 81 | 27, 84 | 26, 84 | |
| missing | 0 | 0 | 0 | |
| age (years) | n | 44 | 40 | 84 |
| <40 | 6 (13.6) | 2 (5.0) | 8 (9.5) | |
| [40–65[ | 25 (56.8) | 23 (57.5) | 48 (57.1) | |
| ≥65 | 13 (29.5) | 15 (37.5) | 28 (33.3) | |
| missing | 0 | 0 | 0 | |
| sex n (%) | n | 44 | 40 | 84 |
| female | 35 (79.5) | 34 (85.0) | 69 (82.1) | |
| male | 9 (20.5) | 6 (15.0) | 15 (17.9) | |
| missing | 0 | 0 | 0 | |
| ethnicity n (%) | n | 44 | 40 | 84 |
| Arabian | 7 (15.9) | 8 (20.0) | 15 (17.9) | |
| Caucasian | 36 (81.8) | 31 (77.5) | 67 (79.8) | |
| other | 1 (2.3) | 1 (2.5) | 2 (2.4) | |
| missing | 0 | 0 | 0 |
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | ||
|---|---|---|---|---|
| value at baseline | n | 44 | 40 | 84 |
| mean (sd) | 4.01 (0.55) | 4.27 (0.56) | 4.13 (0.57) | |
| median (iqr) | 3.92 (3.55, 4.37) | 4.39 (3.72, 4.67) | 4.18 (3.6, 4.6) | |
| min, max | 3.0, 5.0 | 3.12, 5.00 | 3.0, 5.0 | |
| missing | 0 | 0 | 0 | |
| value at week 4 | n | 41 | 38 | |
| mean (sd) | 3.82 (0.84) | 3.49 (1.25) | ||
| median (iqr) | 4 (3.26, 4.46) | 3.8 (2.82, 4.36) | ||
| min, max | 1.35, 5.0 | 0.0, 5.0 | ||
| missing | 3 | 2 | ||
| value at week 8 | n | 41 | 38 | |
| mean (sd) | 3.91 (1.04) | 3.62 (1.2) | ||
| median (iqr) | 4.31 (3.38, 4.68) | 3.82 (3.27, 4.43) | ||
| min, max | 0.0, 5.0 | 0.0, 5.0 | ||
| missing | 3 | 2 |
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | ||
|---|---|---|---|---|
| baseline | n | 44 | 40 | 84 |
| mean (sd) | 4.01 (0.55) | 4.27 (0.56) | 4.13 (0.57) | |
| median (iqr) | 3.92 (3.55, 4.37) | 4.39 (3.72, 4.67) | 4.18 (3.6, 4.6) | |
| min,max | 3.0, 5.0 | 3.12, 5.00 | 3.0, 5.0 | |
| missing | 0 | 0 | 0 | |
| change from baseline to week 4 | n | 41 | 38 | |
| mean (sd) | −0.22 (0.76) | −0.76 (1.02) | ||
| median (iqr) | −0.06 (−0.70, 0.28) | −0.68 (−1.02, −0.20) | ||
| min,max | −2.54, 1.13 | −4.41, 0.90 | ||
| missing | 3 | 2 | ||
| change from baseline to week 8 | n | 41 | 38 | |
| mean (sd) | −0.13 (1.08) | −0.63 (1.05) | ||
| median (iqr) | −0.07 (−0.57, 0.58) | −0.33 (−0.99, 0.10) | ||
| min,max | −4.73, 1.45 | −3.60, 0.84 | ||
| missing | 3 | 2 |
| Parameter | Comparison | E(1) | CI 95% Low | CI 95% High | p-Value (2) |
|---|---|---|---|---|---|
| Change from baseline to week 8 | Control vs. Comfort Shield | −0.411 | −0.865 | 0.043 | 0.075 |
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | ||
|---|---|---|---|---|
| value at baseline | n | 44 | 40 | 84 |
| mean (sd) | 57.41 (16.5) | 61.13 (16.99) | 59.18 (16.74) | |
| median (iqr) | 54.55 (43.61, 68.75) | 61.8 (51.56, 75.00) | 57.91 (43.75, 70.45) | |
| min, max | 34.09, 91.67 | 34.09, 95.45 | 34.09, 95.45 | |
| missing | 0 | 0 | 0 | |
| value at week 4 | n | 44 | 40 | |
| mean (sd) | 42.82 (20.34) | 55.49 (19.64) | ||
| median (iqr) | 39.2 (29.79, 56.96) | 54.03 (38.07, 68.50) | ||
| min, max | 7.14, 85.42 | 22.22, 95.45 | ||
| missing | 0 | 0 | ||
| value at week 8 | n | 44 | 40 | |
| mean (sd) | 40.7 (19.18) | 56.16 (21.39) | ||
| median (iqr) | 40.91 (28.13, 52.81) | 57.91 (36.11, 75.00) | ||
| min, max | 0.0, 87.5 | 9.09, 93.18 | ||
| missing | 0 | 0 |
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | ||
|---|---|---|---|---|
| value at baseline | n | 44 | 40 | 84 |
| mean (sd) | 57.41 (16.5) | 61.13 (16.99) | 59.18 (16.74) | |
| median (iqr) | 54.55 (43.61, 68.75) | 61.8 (51.56, 75.00) | 57.91 (43.75, 70.45) | |
| min, max | 34.09, 91.67 | 34.09, 95.45 | 34.09, 95.45 | |
| missing | 0 | 0 | 0 | |
| change from baseline to week 4 | n | 44 | 40 | |
| mean (sd) | −14.6 (20.71) | −5.63 (12.66) | ||
| median (iqr) | −10.21 (−25.80, −2.09) | −3.98 (−12.5, 0.0) | ||
| min, max | −70.83, 27.27 | −37.92, 25.76 | ||
| missing | 0 | 0 | ||
| change from baseline to week 8 | n | 44 | 40 | |
| mean (sd) | −16.71 (22.25) | −4.96 (16.95) | ||
| median (iqr) | −13.41 (−29.66, −0.68) | −3.82 (−13.02, 6.99) | ||
| min, max | −84.09, 27.08 | −43.06, 40.91 | ||
| missing | 0 | 0 |
| Parameter | Comparison | E (1) | CI 95% Low | CI 95% High | p-Value (2) |
|---|---|---|---|---|---|
| Change from baseline to week 8 | Control vs. Comfort Shield | 13.511 | 5.586 | 21.437 | 0.001 |
| Parameter | Comparison | E (1) | 95% CI Low (1) | 95% CI High (1) | p-Value (2) |
|---|---|---|---|---|---|
| Change from baseline to week 8 | Comfort Shield vs. Control | 14.503 | 5.517 | 23.49 | 0.002 |
| Parameter | Comparison | E(1) | 95% CI Low(1) | 95% CI High(1) | p-Value(2) |
|---|---|---|---|---|---|
| Change from baseline to week 8 | Comfort Shield vs. Control | 13.999 | 5.011 | 22.986 | 0.003 |
| Comfort Shield (n = 44) | Control (n = 40) | Total (n = 84) | p-Value | ||
|---|---|---|---|---|---|
| baseline | n | 44 | 40 | 84 | |
| mean (sd) | 0.17 (0.35) | 0.17 (0.28) | 0.17 (0.31) | ||
| median (iqr) | 0 (0.0, 0.2) | 0.1 (0.00, 0.22) | 0 (0.0, 0.2) | ||
| min, max | −0.1, 1.5 | −0.2, 1.3 | −0.2, 1.5 | ||
| missing | 0 | 0 | 0 | ||
| change from baseline to week 4 | n | 41 | 39 | ||
| mean (sd) | 0 (0.11) | 0.02 (0.11) | |||
| median (iqr) | 0 (0, 0) | 0 (0.00, 0.05) | |||
| min, max | −0.4, 0.3 | −0.2, 0.5 | |||
| missing | 3 | 1 | |||
| change from baseline to week 8 | n | 44 | 40 | 0.033 | |
| mean (sd) | −0.02 (0.14) | 0.02 (0.1) | |||
| median (iqr) | 0 (−0.1, 0.0) | 0 (0.0, 0.1) | |||
| min, max | −0.4, 0.6 | −0.2, 0.3 | |||
| missing | 0 | 0 |
| Comfort Shield Group n = 44 | Control Group n = 40 | p-Value | |||
|---|---|---|---|---|---|
| Baseline Mean (SD) | Change at Week 8 Mean (SD) | Baseline Mean (SD) | Change at Week 8 Mean (SD) | Change from Baseline to Week 8 | |
| TBUT (s) | n = 44 2.90 (1.87) | n = 42 0.66 (2.32) | n = 40 2.76 (1.44) | n = 40 0.24 (1.47) | 0.468 |
| Schirmer I (mm/5 min) | n = 44 5.19 (5.99) | n = 43 −0.43 (4.72) | n = 40 6.50 (7.52) | n = 40 0.55 (4.61) | 0.343 |
| LWE Korb score | n = 37 1.22 (1.00) | n = 37 −0.19 (0.92) | n = 37 0.91 (0.96) | n = 37 0.12 (0.71) | 0.153 |
| Yamaguchi score | n = 37 5.05 (2.33) | n = 37 −0.14 (2.42) | n = 37 4.14 (2.41) | n = 37 0.19 (1.97) | 0.498 |
| tear osmolarity * (mOsm/L) | n = 40 297.12 (14.47) | n = 37 2.11 (14.54) | n = 37 299.16 (12.11) | n = 35 0.94 (17.59) | 0.294 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Setten, G.-B.; Baudouin, C.; Horwath-Winter, J.; Böhringer, D.; Stachs, O.; Toker, E.; Al-Zaaidi, S.; Benitez-del-Castillo, J.M.; Beck, R.; Al-Sheikh, O.; et al. The HYLAN M Study: Efficacy of 0.15% High Molecular Weight Hyaluronan Fluid in the Treatment of Severe Dry Eye Disease in a Multicenter Randomized Trial. J. Clin. Med. 2020, 9, 3536. https://doi.org/10.3390/jcm9113536
van Setten G-B, Baudouin C, Horwath-Winter J, Böhringer D, Stachs O, Toker E, Al-Zaaidi S, Benitez-del-Castillo JM, Beck R, Al-Sheikh O, et al. The HYLAN M Study: Efficacy of 0.15% High Molecular Weight Hyaluronan Fluid in the Treatment of Severe Dry Eye Disease in a Multicenter Randomized Trial. Journal of Clinical Medicine. 2020; 9(11):3536. https://doi.org/10.3390/jcm9113536
Chicago/Turabian Stylevan Setten, Gysbert-Botho, Christophe Baudouin, Jutta Horwath-Winter, Daniel Böhringer, Oliver Stachs, Ebru Toker, Sultan Al-Zaaidi, Jose M. Benitez-del-Castillo, Ria Beck, Osama Al-Sheikh, and et al. 2020. "The HYLAN M Study: Efficacy of 0.15% High Molecular Weight Hyaluronan Fluid in the Treatment of Severe Dry Eye Disease in a Multicenter Randomized Trial" Journal of Clinical Medicine 9, no. 11: 3536. https://doi.org/10.3390/jcm9113536
APA Stylevan Setten, G.-B., Baudouin, C., Horwath-Winter, J., Böhringer, D., Stachs, O., Toker, E., Al-Zaaidi, S., Benitez-del-Castillo, J. M., Beck, R., Al-Sheikh, O., Seitz, B., Barabino, S., Reitsamer, H. A., & Müller-Lierheim, W. G. K. (2020). The HYLAN M Study: Efficacy of 0.15% High Molecular Weight Hyaluronan Fluid in the Treatment of Severe Dry Eye Disease in a Multicenter Randomized Trial. Journal of Clinical Medicine, 9(11), 3536. https://doi.org/10.3390/jcm9113536








