Two-Year Reproducibility of Axial Length Measurements after Combined Phacovitrectomy for Epiretinal Membrane, and Refractive Outcomes
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Axial Length Measurements and IOL Power Calculations
2.3. Surgical Methods
2.4. Statistical Analyses
2.5. Data Availability
3. Results
3.1. Participants
3.2. Visual Acuity and Central Macular Thickness (CMT)
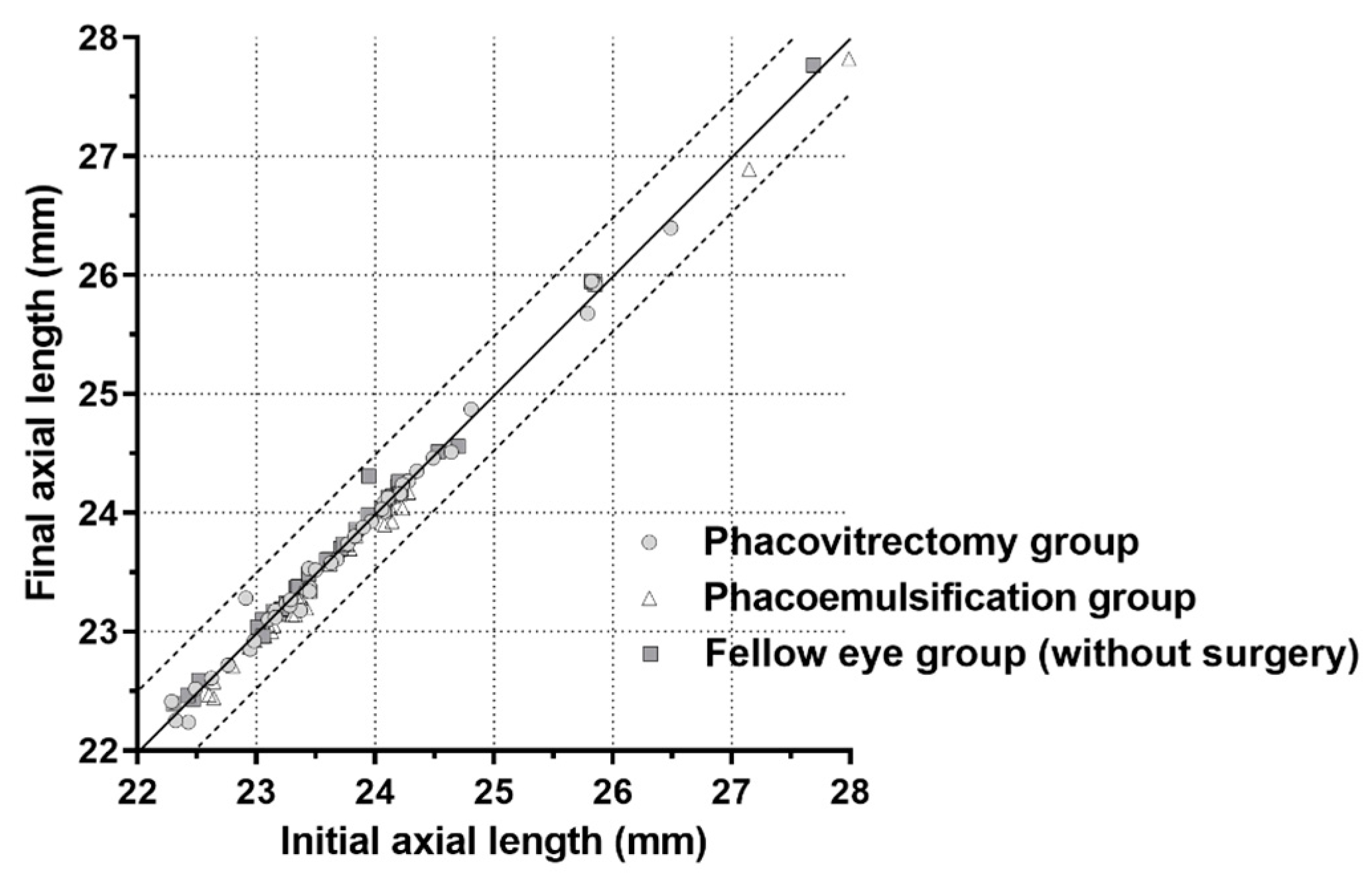
3.3. Reproducibility of Axial Length Measurements and Keratometry Using Partial Interferometry
3.4. Postoperative Prediction Errors
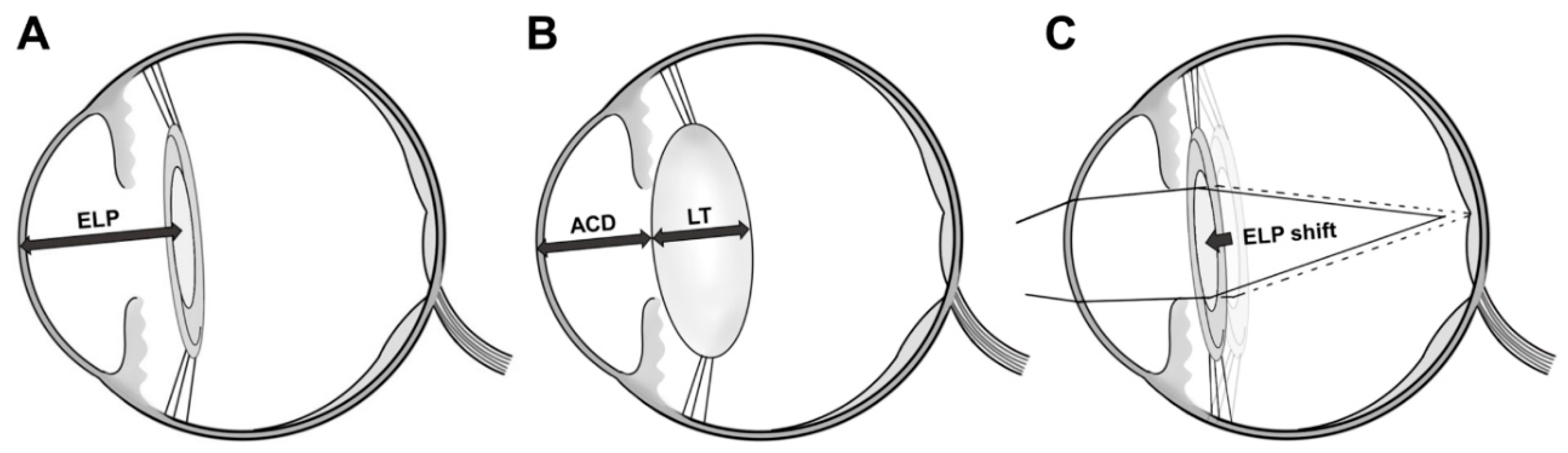
3.5. Changes in Preoperative and Postoperative Anterior Chamber Depth
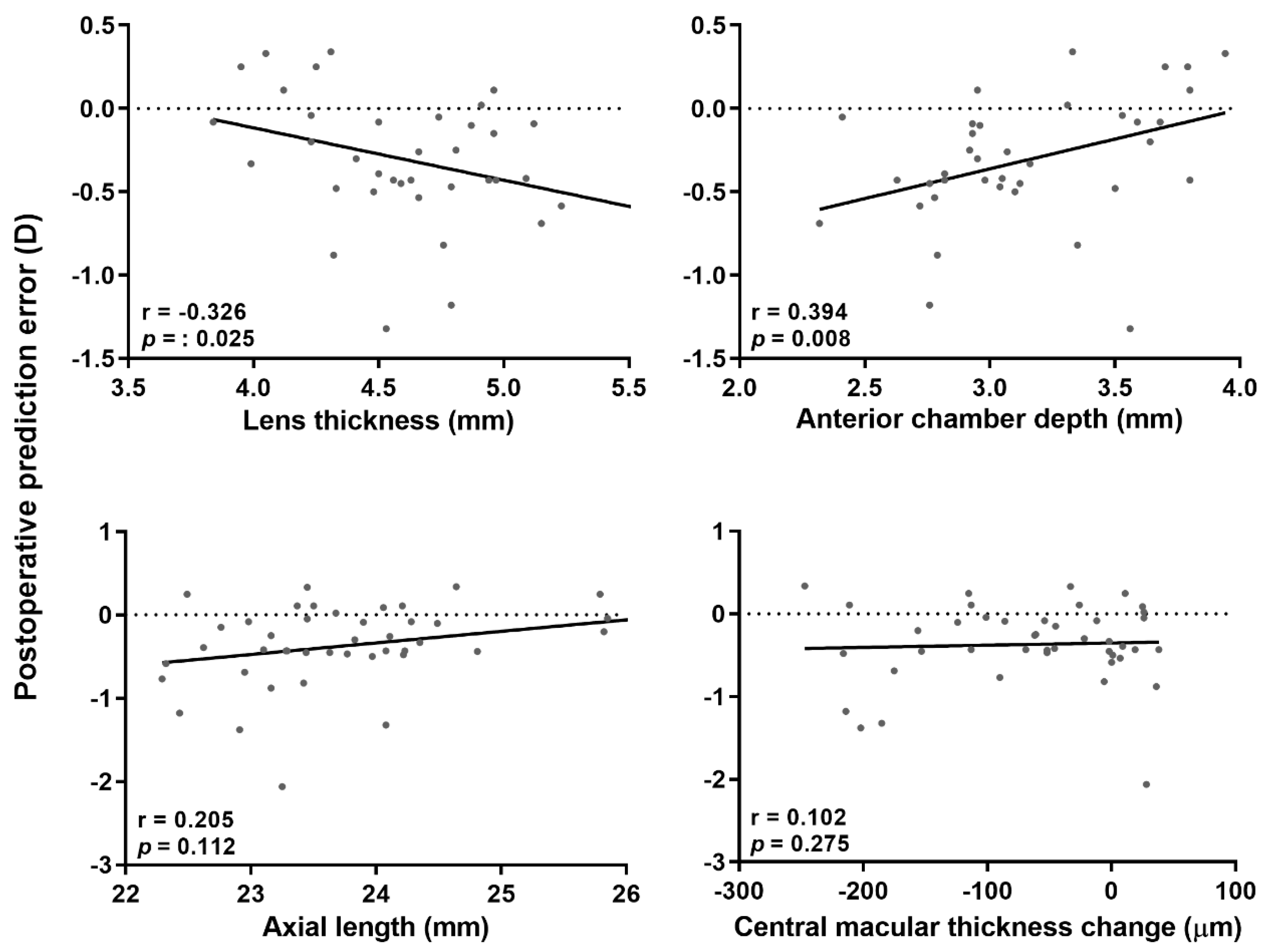
3.6. Univariate and Multivariate Linear Regression Analyses for Postoperative Prediction Error
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wise, G.N. Clinical features of idiopathic preretinal macular fibrosis. Schoenberg Lecture. Am. J. Ophthalmol. 1975, 79, 349–357. [Google Scholar] [CrossRef]
- McCarty, D.J.; Mukesh, B.N.; Chikani, V.; Wang, J.J.; Mitchell, P.; Taylor, H.R.; McCarty, C.A. Prevalence and associations of epiretinal membranes in the visual impairment project. Am. J. Ophthalmol. 2005, 140, 288–294. [Google Scholar] [CrossRef]
- Margherio, R.R.; Cox, M.S., Jr.; Trese, M.T.; Murphy, P.L.; Johnson, J.; Minor, L.A. Removal of epimacular membranes. Ophthalmology 1985, 92, 1075–1083. [Google Scholar] [CrossRef]
- Vedantham, V. Indocyanine green-assisted internal limiting membrane removal in epiretinal membrane surgery. Am. J. Ophthalmol. 2005, 139, 389. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ozaki, N.; Murakami, K. Triamcinolone acetonide facilitates removal of the epiretinal membrane and separation of the residual vitreous cortex in highly myopic eyes with retinal detachment due to a macular hole. Ophthalmologica 2004, 218, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Reibaldi, M.; Longo, A.; Avitabile, T.; Bonfiglio, V.; Toro, M.D.; Russo, A.; Viti, F.; Nicolai, M.; Saitta, A.; Giovannini, A. Transconjunctival nonvitrectomizing vitreous surgery versus 25-gauge vitrectomy in patients with epiretinal membrane: A prospective randomized study. Retina 2015, 35, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, J.D.; Brown, N.A.; Bron, A.J.; Patel, C.K.; Rosen, P.H. Posterior subcapsular and nuclear cataract after vitrectomy. J. Cataract Refract. Surg. 2001, 27, 437–444. [Google Scholar] [CrossRef]
- Hurley, C.; Barry, P. Combined endocapsular phacoemulsification, pars plana vitrectomy, and intraocular lens implantation. J. Cataract Refract. Surg. 1996, 22, 462–466. [Google Scholar] [CrossRef]
- Jeoung, J.W.; Chung, H.; Yu, H.G. Factors influencing refractive outcomes after combined phacoemulsification and pars plana vitrectomy: Results of a prospective study. J. Cataract Refract. Surg. 2007, 33, 108–114. [Google Scholar] [CrossRef]
- Falkner-Radler, C.I.; Benesch, T.; Binder, S. Accuracy of preoperative biometry in vitrectomy combined with cataract surgery for patients with epiretinal membranes and macular holes: Results of a prospective controlled clinical trial. J. Cataract Refract. Surg. 2008, 34, 1754–1760. [Google Scholar] [CrossRef]
- Hamoudi, H.; La Cour, M. Refractive changes after vitrectomy and phacovitrectomy for macular hole and epiretinal membrane. J. Cataract Refract. Surg. 2013, 39, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, I.; Ferencz, M.; Nemes, J.; Somfai, G.; Salacz, G.; Recsan, Z. Intraocular lens power calculation for combined cataract surgery, vitrectomy and peeling of epiretinal membranes for macular oedema. Acta Ophthalmol. Scand. 2007, 85, 88–91. [Google Scholar] [CrossRef]
- Cooke, D.L.; Cooke, T.L. Comparison of 9 intraocular lens power calculation formulas. J. Cataract Refract. Surg. 2016, 42, 1157–1164. [Google Scholar] [CrossRef]
- Olsen, T. Sources of error in intraocular lens power calculation. J. Cataract Refract. Surg. 1992, 18, 125–129. [Google Scholar] [CrossRef]
- Norrby, S. Sources of error in intraocular lens power calculation. J. Cataract Refract. Surg. 2008, 34, 368–376. [Google Scholar] [CrossRef]
- Vogel, A.; Dick, H.B.; Krummenauer, F. Reproducibility of optical biometry using partial coherence interferometry: Intraobserver and interobserver reliability. J. Cataract Refract. Surg. 2001, 27, 1961–1968. [Google Scholar] [CrossRef]
- Sun, H.J.; Choi, K.S. Improving intraocular lens power prediction in combined phacoemulsification and vitrectomy in eyes with macular oedema. Acta Ophthalmol. 2011, 89, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Nawa, Y.; Hara, Y. Relationship between the retinal thickness of the macula and the difference in axial length. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Rahman, R.; Kumarasamy, M. Accuracy of intraocular lens power estimation in eyes having phacovitrectomy for macular holes. J. Cataract Refract. Surg. 2007, 33, 1760–1762. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jee, D. Effects of the intraocular lens type on refractive error following phacovitrectomy with gas tamponade. Curr. Eye Res. 2011, 36, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K.D.; Garcia, R. Myopic shift after combined phacoemulsification and vitrectomy with gas tamponade. Can. J. Ophthalmol. 2008, 43, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Shioya, M.; Ogino, N.; Shinjo, U. Change in postoperative refractive error when vitrectomy is added to intraocular lens implantation. J. Cataract Refract. Surg. 1997, 23, 1217–1220. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sakuraba, T.; Mizutani, H.; Matsuhashi, H.; Nakazawa, M. Postoperative refractive error after simultaneous vitrectomy and cataract surgery. Ophthalmic Surg. Lasers 2000, 31, 271–275. [Google Scholar] [PubMed]
- Byrne, S.; Ng, J.; Hildreth, A.; Danjoux, J.P.; Steel, D.H. Refractive change following pseudophakic vitrectomy. BMC Ophthalmol. 2008, 8, 19. [Google Scholar] [CrossRef]
- Hamoudi, H.; Kofod, M.; La Cour, M. Refractive change after vitrectomy for epiretinal membrane in pseudophakic eyes. Acta Ophthalmol. 2013, 91, 434–436. [Google Scholar] [CrossRef]
- Senn, P.; Schipper, I.; Perren, B. Combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation in the capsular bag: A comparison to vitrectomy and subsequent cataract surgery as a two-step procedure. Ophthalmic Surg. Lasers 1995, 26, 420–428. [Google Scholar]
- Manvikar, S.R.; Allen, D.; Steel, D.H. Optical biometry in combined phacovitrectomy. J. Cataract Refract. Surg. 2009, 35, 64–69. [Google Scholar] [CrossRef]
- Kang, T.S.; Park, H.J.; Jo, Y.J.; Kim, J.Y. Long-Term Reproducibility of Axial Length after Combined Phacovitrectomy in Macula-sparing Rhegmatogenous Retinal Detachment. Sci. Rep. 2018, 8, 15856. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.E.; Lee, D.H.; Koh, H.J.; Lee, S.C.; Kim, S.S. Intraocular lens power estimation in combined phacoemulsification and pars plana vitrectomy in eyes with epiretinal membranes: A case-control study. Yonsei Med. J. 2015, 56, 805–811. [Google Scholar] [CrossRef]
- Olsen, T. Calculation of intraocular lens power: A review. Acta Ophthalmol. Scand. 2007, 85, 472–485. [Google Scholar] [CrossRef]
- Olsen, T.; Hoffmann, P. C constant: New concept for ray tracing-assisted intraocular lens power calculation. J. Cataract Refract. Surg. 2014, 40, 764–773. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, T.; Liu, J.; Ji, Q.; Tan, R. Changes in axial length, central cornea thickness, and anterior chamber depth after rhegmatogenous retinal detachment repair. BMC Ophthalmol. 2016, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tranos, P.G.; Allan, B.; Balidis, M.; Vakalis, A.; Asteriades, S.; Anogeianakis, G.; Triantafilla, M.; Kozeis, N.; Stavrakas, P. Comparison of postoperative refractive outcome in eyes undergoing combined phacovitrectomy vs cataract surgery following vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.; Chan, R.; Pang, P.C. The repeatability and accuracy of axial length and anterior chamber depth measurements from the IOLMaster™. Ophthalmic Physiol. Opt. 2001, 21, 477–483. [Google Scholar] [CrossRef] [PubMed]
| Baseline | Postoperative 1 Year | Postoperative 2 Years | p-Value * | |
|---|---|---|---|---|
| Phacovitrectomy group | ||||
| BCVA (logMAR) | 0.19 ± 0.22 | 0.06 ± 0.09 | 0.05 ± 0.07 | <0.001 |
| Refraction (D) | −0.03 ± 1.93 | −0.85 ± 0.65 | −0.90 ± 0.55 | 0.002 |
| Keratometry (D) | 44.13 ± 1.46 | 44.23 ± 1.43 | 44.34 ± 1.50 | 0.804 |
| Axial length (mm) | 23.77 ± 0.96 | 23.73 ± 0.96 | 23.75 ± 0.97 | 0.987 |
| Phacoemulsification group | ||||
| BCVA (logMAR) | 0.54 ± 0.48 | 0.20 ± 0.39 | 0.16 ± 0.34 | 0.001 |
| Refraction (D) | −2.12 ± 4.77 | −0.92 ± 0.77 | −0.66 ± 0.97 | 0.150 |
| Keratometry (D) | 44.34 ± 1.24 | 44.42 ± 1.26 | 44.38 ± 1.27 | 0.972 |
| Axial length (mm) | 23.60 ± 1.29 | 23.50 ± 1.26 | 23.49 ± 1.24 | 0.944 |
| Fellow-eye group (without surgery) | ||||
| BCVA (logMAR) | 0.11 ± 0.28 | 0.11 ± 0.27 | 0.08 ± 0.24 | 0.888 |
| Refraction (D) | −0.44 ± 2.01 | −0.53 ± 2.26 | −0.55 ± 2.34 | 0.985 |
| Keratometry (D) | 44.45 ± 1.07 | 44.41 ± 1.01 | 44.50 ± 1.03 | 0.954 |
| Axial length (mm) | 23.65 ± 1.03 | 23.69 ± 1.05 | 23.67 ± 1.04 | 0.987 |
| ICC | CV (%) * | TRTSD † | |
|---|---|---|---|
| Phacovitrectomy group | 0.997 | 0.24 | 0.056 |
| Phacoemulsification group | 0.999 | 0.26 | 0.061 |
| Fellow-eye group (without surgery) | 0.999 | 0.23 | 0.054 |
| Phacovitrectomy Group | Phacoemulsification Group | p-Value | |
|---|---|---|---|
| Postoperative prediction error * | −0.37 ± 0.48 | 0.11 ± 0.90 | 0.006 |
| Changes in anterior chamber depth † | 1.10 ± 0.66 | 1.53 ± 0.67 | 0.010 |
| Univariate Regression | Multivariate Regression | |||
|---|---|---|---|---|
| β ± SE | p-Value | β ± SE | p-Value | |
| Surgery (0 = phacoemulsification, 1 = phacovitrectomy) | −0.296 ± 0.096 | 0.003 | −0.228 ± 0.100 | 0.026 |
| Axial length | 0.026 ± 0.047 | 0.581 | ||
| ACD (Preoperative) | −0.014 ± 0.125 | 0.909 | ||
| Preoperative lens thickness | −0.021 ± 0.109 | 0.847 | ||
| Changes in ACD * | 0.187 ± 0.074 | 0.014 | 0.138 ± 0.074 | 0.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.S.; Shin, Y.-I.; Ryu, C.K.; Kim, J.Y. Two-Year Reproducibility of Axial Length Measurements after Combined Phacovitrectomy for Epiretinal Membrane, and Refractive Outcomes. J. Clin. Med. 2020, 9, 3493. https://doi.org/10.3390/jcm9113493
Kang TS, Shin Y-I, Ryu CK, Kim JY. Two-Year Reproducibility of Axial Length Measurements after Combined Phacovitrectomy for Epiretinal Membrane, and Refractive Outcomes. Journal of Clinical Medicine. 2020; 9(11):3493. https://doi.org/10.3390/jcm9113493
Chicago/Turabian StyleKang, Tae Seen, Yong-Il Shin, Cheon Kuk Ryu, and Jung Yeul Kim. 2020. "Two-Year Reproducibility of Axial Length Measurements after Combined Phacovitrectomy for Epiretinal Membrane, and Refractive Outcomes" Journal of Clinical Medicine 9, no. 11: 3493. https://doi.org/10.3390/jcm9113493
APA StyleKang, T. S., Shin, Y.-I., Ryu, C. K., & Kim, J. Y. (2020). Two-Year Reproducibility of Axial Length Measurements after Combined Phacovitrectomy for Epiretinal Membrane, and Refractive Outcomes. Journal of Clinical Medicine, 9(11), 3493. https://doi.org/10.3390/jcm9113493




