The Relationship between Suicide and Oxidative Stress in a Group of Psychiatric Inpatients
Abstract
1. Introduction
2. Experimental Section
2.1. Design
2.2. Means of Assessment
2.2.1. Pro-Oxidant Enzymes
2.2.2. Enzymatic and Non-Enzymatic Antioxidants
2.2.3. Total Antioxidant/Oxidant Status
2.2.4. Oxidative Modification Products
2.2.5. Nitric Oxide
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hołyst, B.; Zapobieganie Samobójstwom. Imperatyw Ogólnoświatowy. World Health Organization. 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/131056/9789241564779-pol.pdf?ua=1 (accessed on 14 April 2020).
- Komenda Główna Policja (KGP). Statystyki Samobójstw. 2019. Available online: http://statystyka.policja.pl/st/wybrane-statystyki (accessed on 14 April 2020).
- Large, M.M. The role of prediction in suicide prevention. Dialogues Clin. Neurosci. 2018, 20, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.O.; Nunes, S.O.V.; De Castro, M.P.; Bortolasci, C.C.; Barbosa, D.S.; Morimoto, H.K.; Venugopal, K.; Dodd, S.; Maes, M.; Berk, M. Oxidative stress and lowered total antioxidant status are associated with a history of suicide attempts. J. Affect. Disord. 2013, 150, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2016, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Colaianna, M.; Curtis, L. Impact of early life stress on the pathogenesis of mental disorders: Relation to brain oxidative stress. Curr. Pharm. Des. 2015, 21, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Sawa, A.; Sedlak, T.W. Oxidative stress and inflammation in schizophrenia. Schizophr. Res. 2016, 176, 1–2. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2016, 76, 197–205. [Google Scholar] [CrossRef]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef]
- Batty, G.D.; Bell, S.; Stamatakis, E.; Kivimaki, M. Association of Systemic Inflammation with risk of completed suicide in the general population. JAMA Psychiatry 2016, 73, 993–995. [Google Scholar] [CrossRef]
- Brent, D.A.; Melhem, N. Familial Transmission of Suicidal Behavior. Psychiatr. Clin. N. Am. 2008, 31, 157–177. [Google Scholar] [CrossRef]
- Dalgleish, T.; Black, M.J.; Johnston, D.; Bevan, A. Transdiagnostic approaches to mental health problems: Current status and future directions. J. Consult. Clin. Psychol. 2020, 88, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Mundt, J.C.; Greist, J.H.; Gelenberg, A.J.; Katzelnick, D.J.; Jefferson, J.W.; Modell, J.G. Feasibility and validation of a computer-automated Columbia-Suicide Severity Rating Scale using interactive voice response technology. J. Psychiatr. Res. 2010, 44, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Mundt, J.C.; Greist, J.H.; Jefferson, J.W.; Federico, M.; Mann, J.J.; Posner, K. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic columbia-suicide Severity rating scale. J. Clin. Psychiatry 2013, 74, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Posner, K.; Brown, G.K.; Stanley, B.; Brent, D.A.; Yershova, K.V.; Oquendo, M.A.; Currier, G.W.; Melvin, G.A.; Greenhill, L.; Shen, S.; et al. The Columbia–Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 2011, 168, 1266–1277. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II Stimulates NADH and NADPH Oxidase Activity in Cultured Vascular Smooth Muscle Cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Erel, O. A Novel Automated Direct Measurement Method for Total Antioxidant Capacity Using a New Generation, More Stable ABTS Radical Cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Falkowski, M.; Maciejczyk, M.; Koprowicz, T.; Mikoluc, B.; Milewska, A.J.; Zalewska, A.; Car, H. Whey Protein Concentrate WPC-80 Improves Antioxidant Defense Systems in the Salivary Glands of 14-Month Wistar Rats. Nutrients 2018, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Skrha, J.; Zima, T. Advanced Glycation End-Products and Advanced Oxidation Protein Products in Patients with Diabetes Mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar] [PubMed]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant Defence, Oxidative Stress and Oxidative Damage in Saliva, Plasma and Erythrocytes of Dementia Patients. Can Salivary AGE be a Marker of Dementia? Int. J. Mol. Sci. 2017, 18, 2205. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Krȩtowski, A.J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jabłońska, E.; Załęski, P.; Waszkiel, D.; Ładny, J.R.; Żukowski, P.; et al. The Redox Balance in Erythrocytes, Plasma, and Periosteum of Patients with Titanium Fixation of the Jaw. Front. Physiol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Gęgotek, A.; Rybałtowska-Kawałko, P.; Skrzydlewska, E. Rutin as a Mediator of Lipid Metabolism and Cellular Signaling Pathways Interactions in Fibroblasts Altered by UVA and UVB Radiation. Oxidative Med. Cell. Longev. 2017, 2017, 1–20. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of Nitrate and Nitrite in Extracellular Fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar] [CrossRef]
- O’Connor, R.C. The integrated motivational-volitional model of suicidal behavior. Crisis 2011, 32, 295–298. [Google Scholar] [CrossRef]
- Carmo, M.B.O.D.; Mendes-Ribeiro, A.C.; Matsuura, C.; Pinto, V.L.; Mury, W.V.; Pinto, N.O.; Moss, M.B.; Ferraz, M.R.; Brunini, T.M.C. Major depression induces oxidative stress and platelet hyperaggregability. J. Psychiatr. Res. 2015, 61, 19–24. [Google Scholar] [CrossRef]
- Maes, M.; Bonifacio, K.L.; Morelli, N.R.; Vargas, H.O.; Barbosa, D.S.; Carvalho, A.F.; Nunes, S.O.V. Major Differences in Neurooxidative and Neuronitrosative Stress Pathways Between Major Depressive Disorder and Types I and II Bipolar Disorder. Mol. Neurobiol. 2018, 56, 141–156. [Google Scholar] [CrossRef]
- Cumurcu, B.E.; Ozyurt, H.; Etikan, I.; Demir, S.; Karlidag, R. Total antioxidant capacity and total oxidant status in patients with major depression: Impact of antidepressant treatment. Psychiatry Clin. Neurosci. 2009, 63, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and Psychological Disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef]
- Anwar, A.; Abruzzo, P.; Pasha, S.; Rajpoot, K.; Bolotta, A.; Ghezzo, A.; Marini, M.; Posar, A.; Visconti, P.; Thornalley, P.J.; et al. Advanced glycation endproducts, dityrosine and arginine transporter dysfunction in autism—A source of biomarkers for clinical diagnosis. Mol. Autism 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Pan, T.-L.; Lan, W.-H.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Wei, H.-T.; Chen, T.-J.; et al. Risk of Suicide Attempts Among Adolescents and Young Adults With Autism Spectrum Disorder: A Nationwide Longitudinal Follow-Up Study. J. Clin. Psychiatry 2017, 78, e1174–e1179. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Abu-Akel, A.; Chisholm, K.; Lin, A.; Zahid, S.; Pelton, M.; Apperly, I.A.; Hansen, P.C.; Wood, S.J. Autism and psychosis: Clinical implications for depression and suicide. Schizophr. Res. 2017, 195, 80–85. [Google Scholar] [CrossRef]
- Strang, J.F.; Kenworthy, L.; Daniolos, P.; Case, L.; Wills, M.C.; Martin, A.; Wallace, G.L. Depression and anxiety symptoms in children and adolescents with autism spectrum disorders without intellectual disability. Res. Autism Spectr. Disord. 2012, 6, 406–412. [Google Scholar] [CrossRef]
- Richa, S.; Fahed, M.; Khoury, E.; Mishara, B. Suicide in autism spectrum disorders. Arch. Suicide Res. 2014, 18, 327–339. [Google Scholar] [CrossRef]
- Kato, K.; Mikami, K.; Akama, F.; Yamada, K.; Maehara, M.; Kimoto, K.; Kimoto, K.; Sato, R.; Takahashi, Y.; Fukushima, R.; et al. Clinical features of suicide attempts in adults with autism spectrum disorders. Gen. Hosp. Psychiatry 2013, 35, 50–53. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Ringel, E. The Presuicidal Syndrome. Suicide Life Threat. Behav. 1976, 6, 131–149. [Google Scholar] [CrossRef]
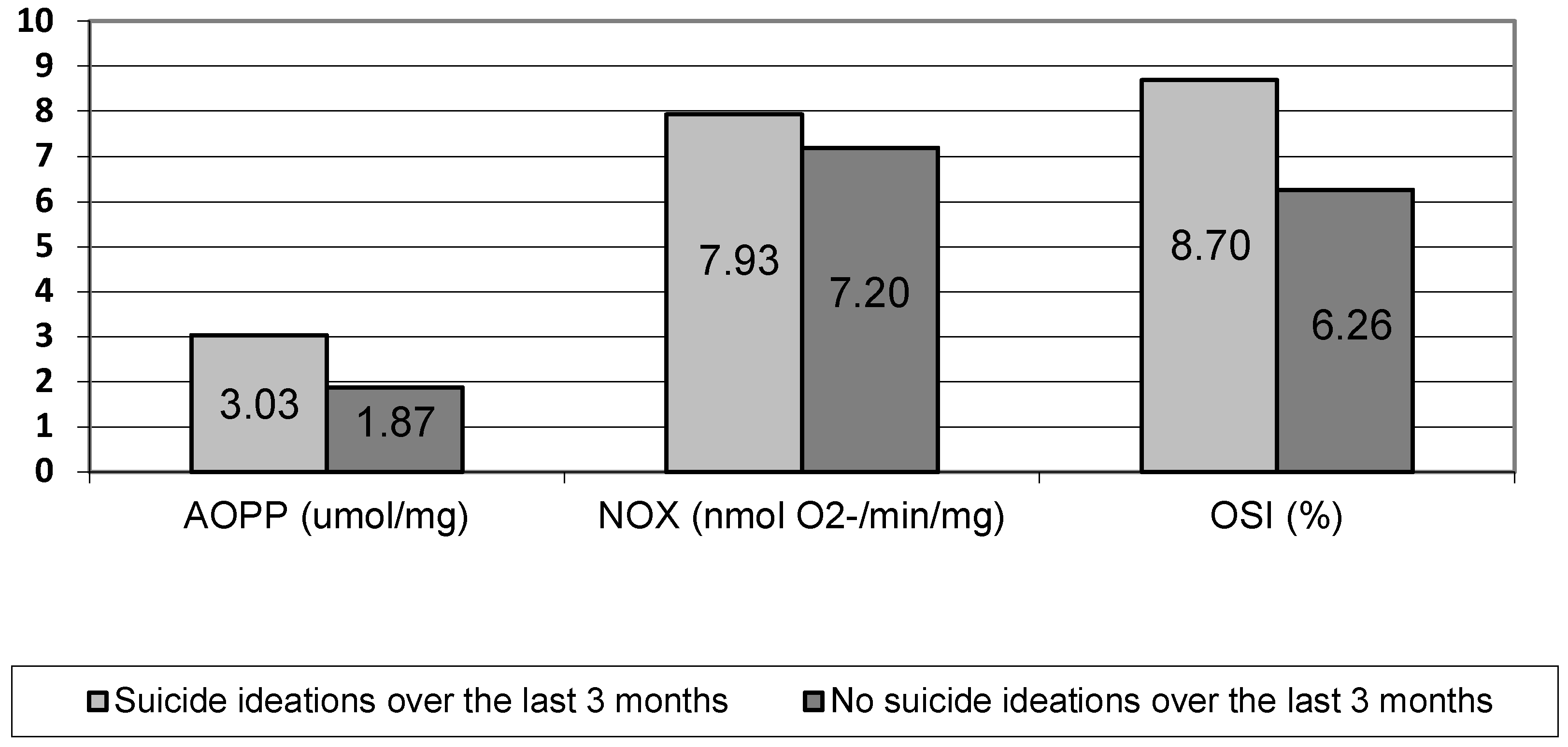
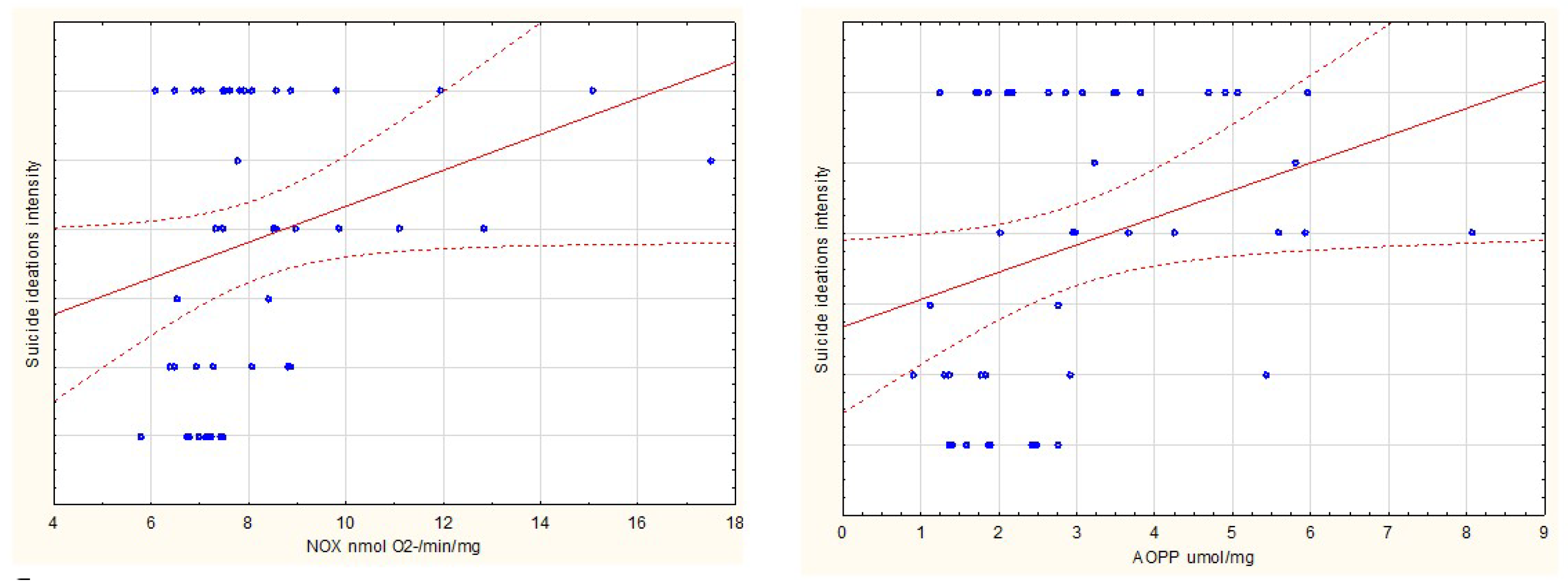

| Suicidality | n | SI in Last 3 Months | SH in Last 3 Months | SA in Last 3 Months | Number of Lifetime SA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | Present | None | Present | None | Present | Median | Min | Max | ||
| Total | 48 | 17 | 31 | 27 | 21 | 32 | 16 | 1 | 0 | 4 |
| Gender | ||||||||||
| Woman | 23 | 10 | 13 | 13 | 10 | 16 | 7 | 1 | 0 | 3 |
| Man | 25 | 7 | 18 | 14 | 11 | 16 | 9 | 1 | 0 | 4 |
| Marital status | ||||||||||
| Single | 24 | 9 | 15 | 10 | 14 | 15 | 9 | 1 | 0 | 3 |
| Married | 19 | 7 | 12 | 13 | 6 | 13 | 6 | 1 | 0 | 4 |
| Widowed | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 3 | 3 |
| Divorced | 4 | 0 | 4 | 3 | 1 | 3 | 1 | 1 | 1 | 2 |
| Education | ||||||||||
| Primary | 7 | 0 | 7 | 2 | 5 | 3 | 4 | 2 | 1 | 4 |
| Vocational | 7 | 4 | 3 | 4 | 3 | 5 | 2 | 1 | 0 | 3 |
| Secondary | 26 | 12 | 14 | 14 | 12 | 16 | 10 | 1 | 0 | 3 |
| Higher | 8 | 1 | 7 | 7 | 1 | 8 | 0 | 0.5 | 0 | 2 |
| Place of residence | ||||||||||
| Village | 11 | 4 | 7 | 6 | 5 | 8 | 3 | 1 | 0 | 3 |
| Small town (population <100,000) | 20 | 5 | 15 | 12 | 8 | 12 | 8 | 1 | 0 | 4 |
| Big town (population >100,000) | 17 | 8 | 9 | 9 | 8 | 12 | 5 | 1 | 0 | 3 |
| Source of income | ||||||||||
| Social benefits | 7 | 4 | 3 | 5 | 2 | 5 | 2 | 1 | 0 | 3 |
| Family support | 9 | 4 | 5 | 6 | 3 | 8 | 1 | 1 | 0 | 2 |
| Job | 32 | 9 | 23 | 16 | 16 | 19 | 13 | 1 | 0 | 4 |
| Diagnosis | ||||||||||
| Substance-related and addictive disorders | 13 | 5 | 8 | 7 | 6 | 8 | 5 | 2 | 0 | 4 |
| Schizophrenia spectrum and other psychotic disorders | 9 | 6 | 3 | 7 | 2 | 9 | 0 | 1 | 0 | 3 |
| Depressive disorders | 11 | 2 | 9 | 7 | 4 | 7 | 4 | 1 | 0 | 3 |
| Anxiety disorders | 10 | 3 | 7 | 5 | 5 | 5 | 5 | 1 | 0 | 2 |
| Personality disorders | 5 | 1 | 4 | 1 | 4 | 3 | 2 | 1 | 1 | 3 |
| Lifetime suicide attempt | ||||||||||
| None | 15 | 9 | 6 | 15 | 0 | 15 | 0 | |||
| At least one | 33 | 8 | 25 | 12 | 21 | 17 | 16 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koweszko, T.; Gierus, J.; Zalewska, A.; Maciejczyk, M.; Waszkiewicz, N.; Szulc, A. The Relationship between Suicide and Oxidative Stress in a Group of Psychiatric Inpatients. J. Clin. Med. 2020, 9, 3462. https://doi.org/10.3390/jcm9113462
Koweszko T, Gierus J, Zalewska A, Maciejczyk M, Waszkiewicz N, Szulc A. The Relationship between Suicide and Oxidative Stress in a Group of Psychiatric Inpatients. Journal of Clinical Medicine. 2020; 9(11):3462. https://doi.org/10.3390/jcm9113462
Chicago/Turabian StyleKoweszko, Tytus, Jacek Gierus, Anna Zalewska, Mateusz Maciejczyk, Napoleon Waszkiewicz, and Agata Szulc. 2020. "The Relationship between Suicide and Oxidative Stress in a Group of Psychiatric Inpatients" Journal of Clinical Medicine 9, no. 11: 3462. https://doi.org/10.3390/jcm9113462
APA StyleKoweszko, T., Gierus, J., Zalewska, A., Maciejczyk, M., Waszkiewicz, N., & Szulc, A. (2020). The Relationship between Suicide and Oxidative Stress in a Group of Psychiatric Inpatients. Journal of Clinical Medicine, 9(11), 3462. https://doi.org/10.3390/jcm9113462








