Daytime Neurophysiological Hyperarousal in Chronic Insomnia: A Study of qEEG
Abstract
1. Introduction
2. Experimental Section
2.1. Participants
2.2. EEG Recording and Analysis
2.3. ECG Recording and Analysis
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. HRV of Insomniacs and Normal Sleepers
3.3. EEG Activity
3.4. Brain Source Localization of INS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edinger, J.D.; Bonnet, M.H.; Bootzin, R.R.; Doghramji, K.; Dorsey, C.M.; Espie, C.A.; Jamieson, A.O.; McCall, W.V.; Morin, C.M.; Stepanski, E.J. Derivation of Research Diagnostic Criteria for Insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep 2004, 27, 1567–1596. [Google Scholar] [CrossRef]
- DSM-5 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- Sateia, M.J. International Classification of Sleep Disorders-Third Edition. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A Behavioral Perspective on Insomnia Treatment. Psychiatr. Clin. North Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Green, M.J.; Espie, C.A.; Hunt, K.; Benzeval, M. The Longitudinal Course of Insomnia Symptoms: Inequalities by Sex and Occupational Class Among Two Different Age Cohorts Followed for 20 Years in the West of Scotland. Sleep 2012, 35, 815–823. [Google Scholar] [CrossRef]
- Morin, C.M.; Bélanger, L.; Leblanc, M.; Ivers, H.; Savard, J.; Espie, C.A.; Mérette, C.; Baillargeon, L.; Grégoire, J.-P. The Natural History of Insomnia. Arch. Intern. Med. 2009, 169, 447–453. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Espie, C.; Pollmächer, T.; Léger, D.; Bassetti, C.; Van Someren, E. Chronic Insomnia: Clinical and Research Challenges—An Agenda. Pharmacopsychiatry 2010, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Arand, D.L. Heart Rate Variability in Insomniacs and Matched Normal Sleepers. Psychosom. Med. 1998, 60, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Gau, S.-F. Core Body Temperature is Elevated During Constant Wakefulness in Elderly Poor Sleepers. Sleep 2000, 23, 1–7. [Google Scholar] [CrossRef]
- Adam, K.; Tomeny, M.; Oswald, I. Physiological and psychological differences between good and poor sleepers. J. Psychiatr. Res. 1986, 20, 301–316. [Google Scholar] [CrossRef]
- Bonnet, P.M.H.; Arand, D.L. 24-Hour Metabolic Rate in Insomniacs and Matched Normal Sleepers. Sleep 1995, 18, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.M.; Prolo, P.; Mastorakos, G.; Vela-Bueno, A.; Chrousos, G.P. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: Clinical implications. J. Clin. Endocrinol. Metab. 2001, 86, 3787–3794. [Google Scholar] [CrossRef]
- Nofzinger, E.A. Functional Neuroimaging Evidence for Hyperarousal in Insomnia. Am. J. Psychiatry 2004, 161, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Haynes, S.N.; Follingstad, D.R.; McGowan, W.T. Insomnia: Sleep patterns and anxiety level. J. Psychosom. Res. 1974, 18, 69–74. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and insomnia: State of the science. Sleep Med. Rev. 2010, 14, 9–15. [Google Scholar] [CrossRef]
- Perlis, M.L.; Smith, M.T.; Andrews, P.J.; Orff, H.; Giles, D.E. Beta/Gamma EEG Activity in Patients with Primary and Secondary Insomnia and Good Sleeper Controls. Sleep 2001, 24, 110–117. [Google Scholar] [CrossRef]
- Perlis, M.L.; Mericab, H.; Smith, M.T.; Giles, D.E. Beta EEG activity and insomnia. Sleep Med. Rev. 2001, 5, 365–376. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Regen, W.; Feige, B.; Holz, J.; Piosczyk, H.; Baglioni, C.; Riemann, D.; Nissen, C. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol. Psychol. 2012, 91, 329–333. [Google Scholar] [CrossRef]
- Israel, B.; Buysse, D.J.; Krafty, R.T.; Begley, A.; Miewald, J.; Hall, M. Short-Term Stability of Sleep and Heart Rate Variability in Good Sleepers and Patients with Insomnia: For Some Measures, One Night is Enough. Sleep 2012, 35, 1285–1291. [Google Scholar] [CrossRef]
- Merica, H.; Blois, R.; Gaillard, J.-M. Spectral characteristics of sleep EEG in chronic insomnia. Eur. J. Neurosci. 1998, 10, 1826–1834. [Google Scholar] [CrossRef]
- Krystal, A.D.; Edinger, J.D.; Wohlgemuth, W.K.; Marsh, G.R. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep 2002, 25, 626–636. [Google Scholar] [CrossRef]
- Wołyńczyk-Gmaj, D.; Szelenberger, W. Waking EEG in primary insomnia. Acta Neurobiol. Exp. 2011, 71, 387–392. [Google Scholar]
- Buysse, D.J.; Germain, A.; Hall, M.L.; Moul, D.E.; Nofzinger, E.A.; Begley, A.; Ehlers, C.L.; Thompson, W.; Kupfer, D.J. EEG Spectral Analysis in Primary Insomnia: NREM Period Effects and Sex Differences. Sleep 2008, 31, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.R.; Bonnet, M.H. Reported Chronic Insomnia Is Independent of Poor Sleep as Measured by Electroencephalography. Psychosom. Med. 2000, 62, 474–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stepanski, E.; Zorick, F.; Roehrs, T.; Young, D.; Roth, T.; Edward, S.; Frank, Z.; Timothy, R.; David, Y.; Thomas, R. Daytime Alertness in Patients with Chronic Insomnia Compared with Asymptomatic Control Subjects. Sleep 1988, 11, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lichstein, K.L.; Wilson, N.M.; Noe, S.L.; Aguillard, R.N.; Bellur, S.N. Daytime Sleepiness in Insomnia: Behavioral, Biological and Subjective Indices. Sleep 1994, 17, 693–702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérusse, A.D.; Turcotte, I.; St-Jean, G.; Ellis, J.; Hudon, C.; Bastien, C.H. Types of Primary Insomnia: Is Hyperarousal Also Present during Napping? J. Clin. Sleep Med. 2013, 9, 1273–1280. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Figueredo-Rodríguez, P.; Del Río-Portilla, Y.; Sánchez-Romero, J.; Galán, L.; Bosch-Bayard, J. Enhanced Frontoparietal Synchronized Activation During the Wake-Sleep Transition in Patients with Primary Insomnia. Sleep 2012, 35, 501–511. [Google Scholar] [CrossRef]
- Colombo, M.A.; Ramautar, J.R.; Wei, Y.; Gomez-Herrero, G.; Stoffers, D.; Wassing, R.; Benjamins, J.S.; Tagliazucchi, E.; Van Der Werf, Y.D.; Cajochen, C.; et al. Wake High-Density Electroencephalographic Spatiospectral Signatures of Insomnia. Sleep 2016, 39, 1015–1027. [Google Scholar] [CrossRef]
- Han, O.S.; Hong, J.P. SCID-I. Structured Clinical Interview for Axis I Disorder of DSM-IV; Hana Medical: Seoul, Korea, 2000. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Morin, C.M. Insomnia Severity Index. PsycTESTS Dataset 1993. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire To Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Marx, E.; Deutschländer, A.; Stephan, T.; Dieterich, M.; Wiesmann, M.; Brandt, T. Eyes open and eyes closed as rest conditions: Impact on brain activation patterns. NeuroImage 2004, 21, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, L.; Chen, L. Differences of EEG between eyes-open and eyes-closed states based on autoregressive method. J. Electron. Sci. Technol. 2009, 7, 175–179. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef]
- Thatcher, R.W. Handbook of Quantitative Electroencephalography and EEG Biofeedback, 2nd ed.; Anipublishing Company: Petersburg, FL, USA, 2016. [Google Scholar]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Nicassio, P.M.; Mendlowitz, D.R.; Fussell, J.J.; Petras, L. The phenomenology of the pre-sleep state: The development of the pre-sleep arousal scale. Behav. Res. Ther. 1985, 23, 263–271. [Google Scholar] [CrossRef]
- Freedman, R.R. EEG power spectra in sleep-onset insomnia. Electroencephalogr. Clin. Neurophysiol. 1986, 63, 408–413. [Google Scholar] [CrossRef]
- Merica, H.; Gaillard, J.-M. The EEG of the sleep onset period in insomnia: A discriminant analysis. Physiol. Behav. 1992, 52, 199–204. [Google Scholar] [CrossRef]
- Staner, L.; Cornette, F.; Maurice, D.; Viardot, G.; Le Bon, O.; Haba, J.; Staner, C.; Luthringer, R.; Muzet, A.; Macher, J.-P. Sleep microstructure around sleep onset differentiates major depressive insomnia from primary insomnia. J. Sleep Res. 2003, 12, 319–330. [Google Scholar] [CrossRef]
- Christensen, J.A.E.; Wassing, R.; Wei, Y.; Ramautar, J.R.; Lakbila-Kamal, O.; Jennum, P.J.; Van Someren, E.J.W. Data-Driven Analysis of EEG Reveals Concomitant Superficial Sleep During Deep Sleep in Insomnia Disorder. Front. Neurosci. 2019, 13, 598. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Rojas-Ramos, O.A.; Del Río-Portilla, Y. Waking EEG signs of non-restoring sleep in primary insomnia patients. Clin. Neurophysiol. 2016, 127, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Schutter, D.J.; Leitner, C.; Kenemans, J.L.; Van Honk, J. Electrophysiological correlates of cortico-subcortical interaction: A cross-frequency spectral EEG analysis. Clin. Neurophysiol. 2006, 117, 381–387. [Google Scholar] [CrossRef]
- Putman, P.; Arias-Garcia, E.; Pantazi, I.; Van Schie, C. Emotional Stroop interference for threatening words is related to reduced EEG delta–beta coupling and low attentional control. Int. J. Psychophysiol. 2012, 84, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Schutter, D.J.; Van Honk, J. Salivary cortisol levels and the coupling of midfrontal delta–beta oscillations. Int. J. Psychophysiol. 2005, 55, 127–129. [Google Scholar] [CrossRef]
- Yan, C.-Q.; Liu, C.-Z.; Wang, X.; Huo, J.-W.; Zhou, P.; Zhang, S.; Fu, Q.-N.; Zhang, J.; Wang, Z.-Y.; Liu, Q.-Q. Abnormal Functional Connectivity of Anterior Cingulate Cortex in Patients with Primary Insomnia: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Aging Neurosci. 2018, 10, 167. [Google Scholar] [CrossRef]
- Li, Z.; Chen, R.; Guan, M.; Wang, E.; Qian, T.; Zhao, C.; Zou, Z.; Beck, T.; Shi, D.; Wang, M.; et al. Disrupted brain network topology in chronic insomnia disorder: A resting-state fMRI study. NeuroImage Clin. 2018, 18, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, J.; Brzezicka, A.; Gola, M.; Wróbel, A. Beta band oscillations engagement in human alertness process. Int. J. Psychophysiol. 2012, 85, 125–128. [Google Scholar] [CrossRef]
- Marco-Pallares, J.; Münte, T.F.; Rodríguez-Fornells, A. The role of high-frequency oscillatory activity in reward processing and learning. Neurosci. Biobehav. Rev. 2015, 49, 1–7. [Google Scholar] [CrossRef]
- Harvey, A.G.; Payne, S. The management of unwanted pre-sleep thoughts in insomnia: Distraction with imagery versus general distraction. Behav. Res. Ther. 2002, 40, 267–277. [Google Scholar] [CrossRef]
- Hellberg, S.N.; Buchholz, J.L.; Abramowitz, J.S. Insomnia and obsessive-compulsive symptom dimensions: The mediating role of anxiety and depression. J. Obs. Compuls. Relat. Disord. 2019, 23, 100482. [Google Scholar] [CrossRef]
- Stepanski, E.; Glinn, M.; Zorick, F.; Roehrs, T.; Roth, T. Heart rate changes in chronic insomnia. Stress Med. 1994, 10, 261–266. [Google Scholar] [CrossRef]
- Covassin, N.; De Zambotti, M.; Sarlo, M.; Tona, G.D.M.; Russo, S.; Stegagno, L. Cognitive performance and cardiovascular markers of hyperarousal in primary insomnia. Int. J. Psychophysiol. 2011, 80, 79–86. [Google Scholar] [CrossRef]
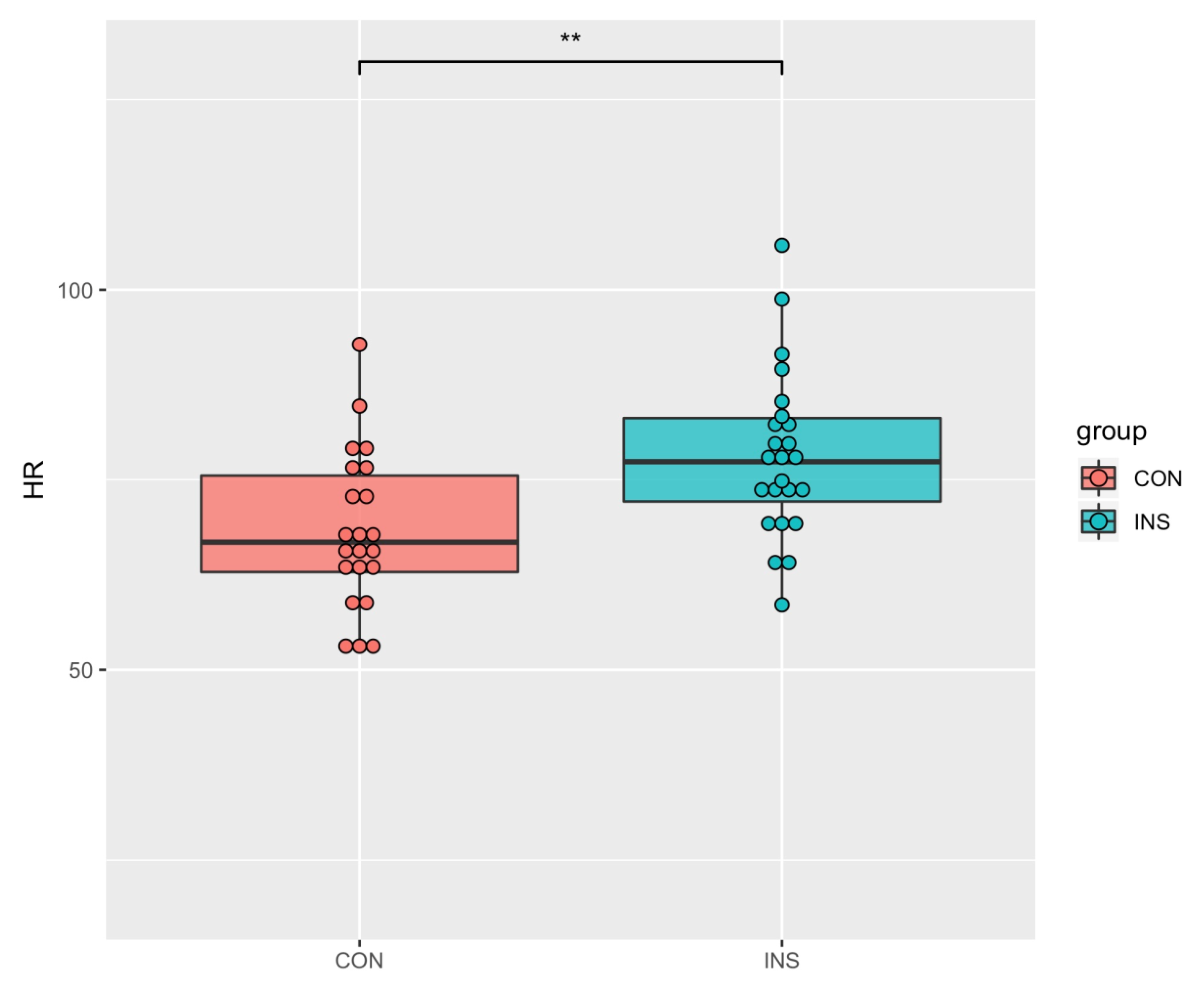
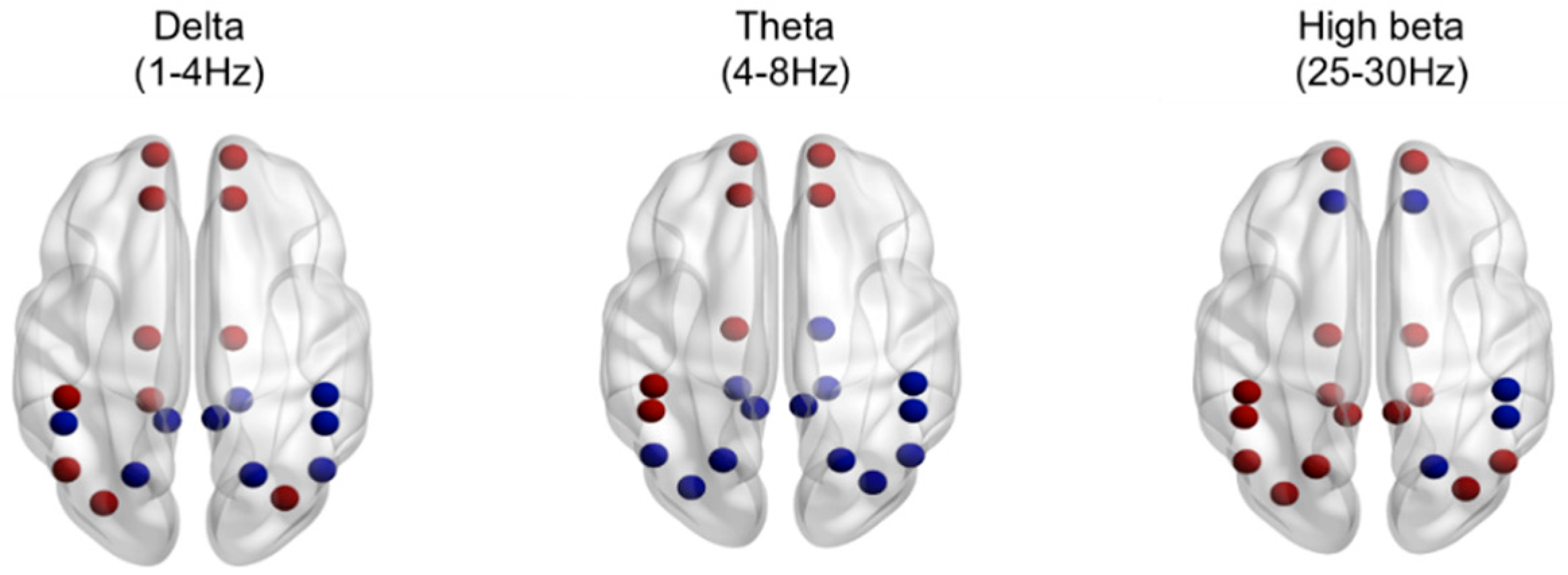
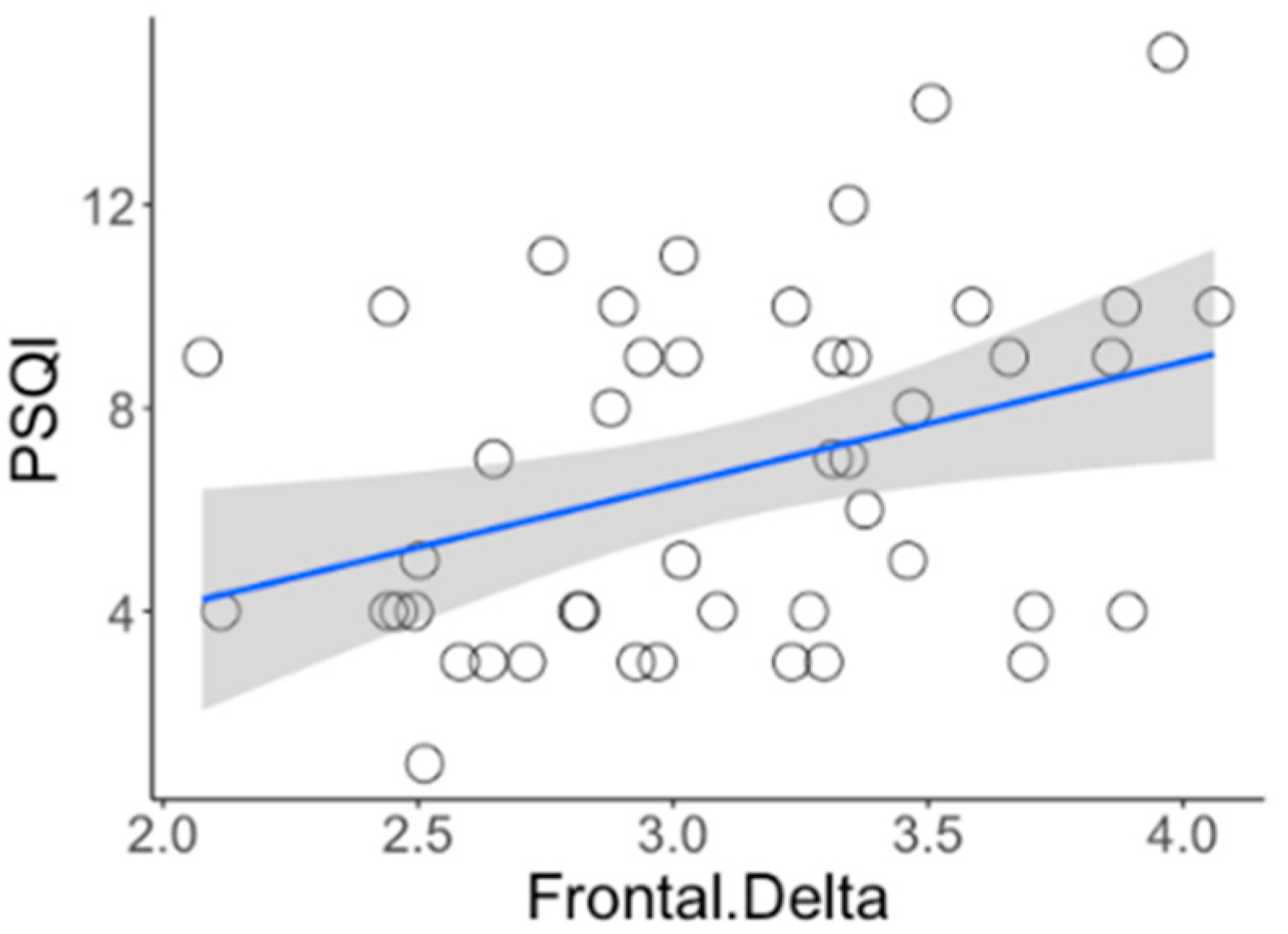
| Variables | INS (n = 24) | NSC (n = 22) | t | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 3 (12.5%) | 4 (18.18%) | ||
| Female | 21 (87.5%) | 18 (81.81%) | ||
| Age | 22.58 (2.18) | 24.72 (6.34) | −1.22 | 0.226 |
| Education | 15.33 (0.91) | 15.68 (1.42) | −0.99 | 0.325 |
| BMI | 20.30 (2.04) | 21.07 (2.38) | −1.18 | 0.243 |
| PSQI | 9.50 (2.16) | 3.68 (0.99) | 11.52 | <0.0001 *** |
| ISI | 14.13 (4.27) | 2.18 (1.53) | 12.38 | <0.001 *** |
| ESS | 8.50 (3.28) | 6.32 (3.56) | 2.16 | 0.036 * |
| PSAS | 46.46 (9.28) | 30.18 (8.27) | 6.25 | <0.001 *** |
| BDI | 10.38 (6.27) | 5.55 (4.48) | 2.97 | 0.004 * |
| BAI | 6.96 (3.99) | 4.05 (3.74) | 2.54 | 0.014 * |
| HR (beats/min) | 78.17 (10.88) | 68.24 (10.39) | 3.15 | 0.002 * |
| HF (%) | 40.68 (14.69) | 42.28 (18.90) | −0.32 | 0.748 |
| LF (%) | 33.60 (11.25) | 29.71 (10.48) | 1.2 | 0.233 |
| LF/HF ratio | 1.39 (1.31) | 1.22 (1.14) | 0.48 | 0.626 |
| HR | HF (%) | LF (%) | LF/HF Ratio | |
|---|---|---|---|---|
| PSQI | 0.270 *** | −0.128 *** | 0.282 *** | 0.111 * |
| (0.007 to 0.556) | (−0.391 to 0.17) | (0.05 to 0.48) | (−0.182 to 0.388) | |
| ISI | 0.319 *** | −0.033 | 0.224 *** | 0.003 |
| (0.085 to 0.554) | (−0.279 to −0.139) | (−0.020 to 0.446) | (−0.248 to 0.268) | |
| ESS | 0.082 * | −0.139 *** | 0.047 | 0.046 |
| (0.001 to 0.138) | (−0.496 to 0.191) | (−0.247 to 0.334) | (−0.279 to 0.371) | |
| PSAS | 0.292 *** | −0.141 *** | 0.089 * | 0.019 |
| (0.042 to 0.524) | (−0.372 to 0.126) | (−0.172 to 0.336) | (−0.262 to 0.288) | |
| BDI | 0.235 *** | −0.263 *** | 0.263 *** | 0.215 *** |
| (0.049 to 0.513) | (−0.487 to −0.015) | (−0.008 to 0.503) | (0.006 to 0.404) | |
| BAI | 0.078 * | −0.160 *** | 0.026 | 0.026 |
| (−0.193 to 0.349) | (−0.369 to 0.092) | (−0.247 to 0.288) | (0.211 to 0.273) |
| INS (n = 24) | NSC (n = 22) | Group χ2 | Group*Region χ2 | PFDR | |
|---|---|---|---|---|---|
| Delta | 461.895 *** | 25.509 *** | |||
| F | 27.17 (18.23) | 21.52 (15.17) | 0.001 ** | ||
| C | 13.98 (10.41) | 14.45 (11.11) | 0.559 | ||
| P | 13.18 (5.81) | 13.93 (6.85) | 0.774 | ||
| Theta | 0.159 | 10.979 * | |||
| F | 10.48 (5.35) | 8.43 (3.91) | <0.0001 *** | ||
| C | 8.31 (4.60) | 7.85 (4.80) | 0.698 | ||
| P | 8.45 (3.95) | 8.28 (4.39) | 0.491 | ||
| Alpha | 0.435 | 6.929 * | |||
| F | 7.19 (5.68) | 8.74 (7.32) | |||
| C | 8.85 (9.44) | 11.23 (10.62) | |||
| P | 15.61 (19.5) | 22.13 (24.88) | |||
| Beta | 0.991 | 7.300 * | |||
| F | 7.14 (4.90) | 7.49 (8.71) | 0.950 | ||
| C | 8.23 (6.48) | 7.24 (4.44) | 0.375 | ||
| P | 7.83 (6.35) | 8.77 (6.41) | 0.950 | ||
| High beta | 3.934 * | 8.295 * | |||
| F | 1.98 (2.42) | 1.89 (3.78) | |||
| C | 1.92 (2.36) | 1.17 (1.06) | |||
| P | 1.10 (0.61) | 0.90 (0.49) | |||
| Gamma | 5.095 * | 13.190 ** | |||
| F | 2.72 (3.73) | 2.7 (5.36) | 0.057 | ||
| C | 3.2 (5.33) | 1.86 (2.46) | 0.886 | ||
| P | 1.26 (0.69) | 1.21 (0.99) | 0.886 |
| INS (n = 24) | NSC (n = 22) | Group χ2 | Group*Region χ2 | PFDR | |
|---|---|---|---|---|---|
| Delta | 0.003 | 5.043 | |||
| F | 22.02 (13.74) | 20.24 (12.74) | |||
| C | 14.42 (8.76) | 14.38 (8.61) | |||
| P | 15.62 (7.02) | 15.62 (8.27) | |||
| Theta | 0.518 | 2.515 | |||
| F | 12.18 (5.50) | 10.46 (4.91) | |||
| C | 10.83 (5.77) | 9.91 (5.85) | |||
| P | 12.96 (6.01) | 11.82 (5.65) | |||
| Alpha | 0.082 | 1.042 | |||
| F | 17.46 (16.73) | 17.01 (12.67) | |||
| C | 21.63 (23.63) | 21.18 (20.54) | |||
| P | 50.07 (52.98) | 48.25 (36.31) | |||
| Beta | 0.037 | 1.579 | |||
| F | 7.06 (3.17) | 6.65 (3.00) | |||
| C | 8.71 (4.77) | 8.24 (4.56) | |||
| P | 13.29 (7.99) | 13.29 (7.7) | |||
| High beta | 2019.460 *** | 829.583 *** | |||
| F | 1.27 (0.68) | 1.10 (0.67) | 0.011 * | ||
| C | 1.39 (1.04) | 1.04 (0.62) | 0.003 * | ||
| P | 1.27 (0.64) | 1.15 (0.60) | 0.047 * | ||
| Gamma | 8.892 | 1.434 | |||
| F | 1.52 (0.93) | 1.45 (0.98) | |||
| C | 1.69 (1.66) | 1.44 (1.46) | |||
| P | 1.31 (0.57) | 1.39 (1.10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, D.Y.; Park, S.M.; Choi, S.W. Daytime Neurophysiological Hyperarousal in Chronic Insomnia: A Study of qEEG. J. Clin. Med. 2020, 9, 3425. https://doi.org/10.3390/jcm9113425
Oh DY, Park SM, Choi SW. Daytime Neurophysiological Hyperarousal in Chronic Insomnia: A Study of qEEG. Journal of Clinical Medicine. 2020; 9(11):3425. https://doi.org/10.3390/jcm9113425
Chicago/Turabian StyleOh, Da Young, Su Mi Park, and Sung Won Choi. 2020. "Daytime Neurophysiological Hyperarousal in Chronic Insomnia: A Study of qEEG" Journal of Clinical Medicine 9, no. 11: 3425. https://doi.org/10.3390/jcm9113425
APA StyleOh, D. Y., Park, S. M., & Choi, S. W. (2020). Daytime Neurophysiological Hyperarousal in Chronic Insomnia: A Study of qEEG. Journal of Clinical Medicine, 9(11), 3425. https://doi.org/10.3390/jcm9113425




