Impact of Diabetes Mellitus on Outcomes after High-Risk Interventional Coronary Procedures
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. Baseline and Procedural Characteristics
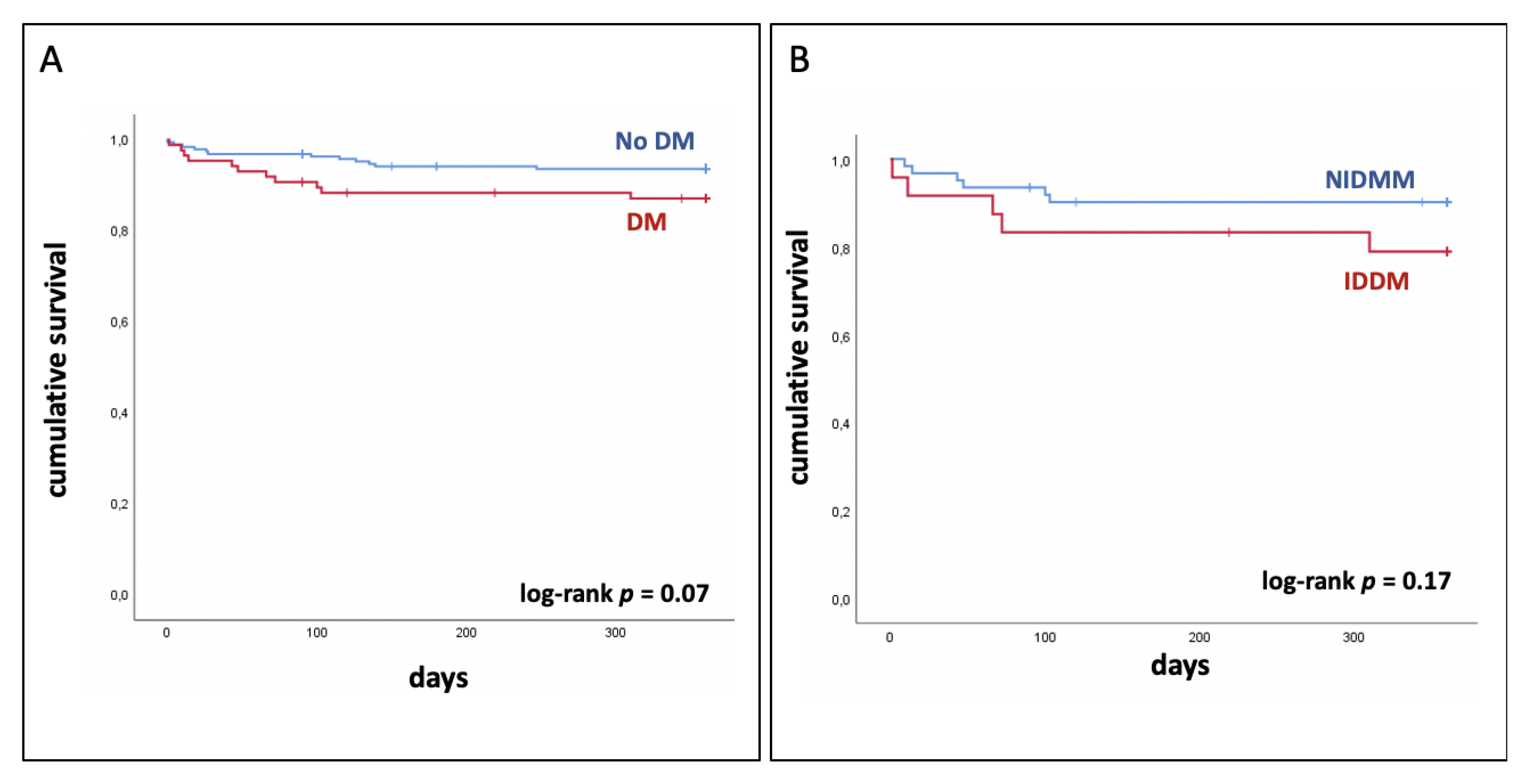
3.2. No Significant Difference between Patients with and without Diabetes Mellitus
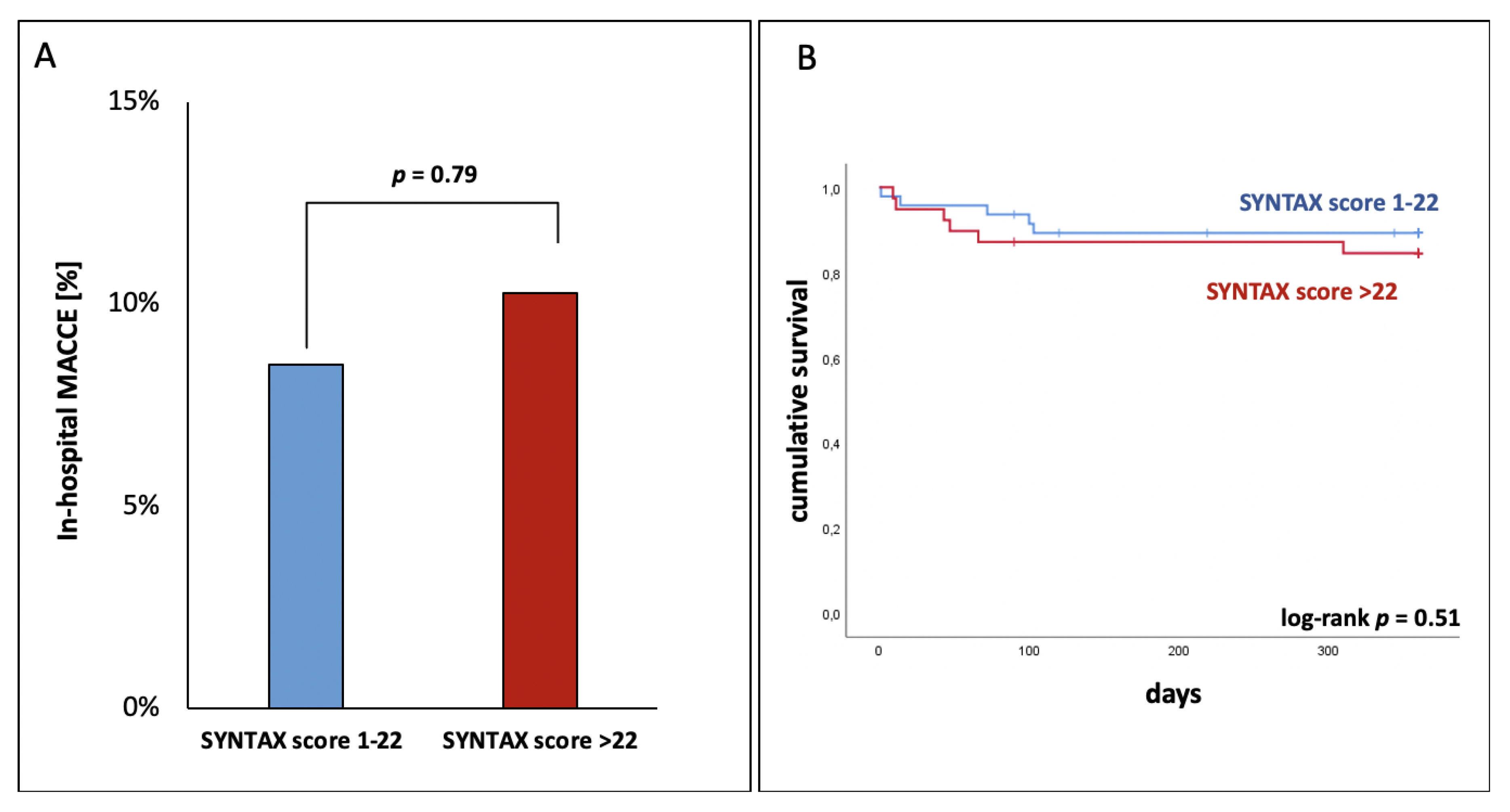
3.3. Complex CAD Had No Influence on In-Hospital MACCE
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Donahoe, S.M.; Stewart, G.C.; McCabe, C.H.; Mohanavelu, S.; Murphy, S.A.; Cannon, C.P.; Antman, E.M. Diabetes and mortality following acute coronary syndromes. JAMA 2007, 298, 765–775. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Tuzcu, E.M.; Kalidindi, S.; Wolski, K.; Moon, K.-W.; Sipahi, I.; Schoenhagen, P.; Nissen, S.E. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: A pooled analysis of 5 intravascular ultrasound trials. J. Am. Coll. Cardiol. 2008, 52, 255–262. [Google Scholar] [CrossRef]
- Flaherty, J.D.; Davidson, C.J. Diabetes and coronary revascularization. JAMA 2005, 293, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.; Costa, M.A.; Centemero, M.P.; Abizaid, A.S.; Legrand, V.M.; Limet, R.V.; Schuler, G.; Mohr, F.W.; Lindeboom, W.; Sousa, A.; et al. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: Insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation 2001, 104, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Marcheix, B.; Eynden, F.V.; Demers, P.; Bouchard, D.; Cartier, R. Influence of diabetes mellitus on long-term survival in systematic off-pump coronary artery bypass surgery. Ann. Thorac. Surg. 2008, 86, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Hear. J. 2018, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Banning, A.P.; Serruys, P.W.; Morice, M.-C.; Taeymans, Y.; Van Nooten, G.; Possati, G.; Crea, F.; Hood, K.L.; Leadley, K.; et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann. Thorac. Surg. 2011, 92, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Di Salvo, M.E.; Cincotta, G.; Miano, M.; Tamburino, C. Usefulness of the SYNTAX score for predicting clinical outcome after percutaneous coronary intervention of unprotected left main coronary artery disease. Circ. Cardiovasc. Interv. 2009, 2, 302–308. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Feldman, T.E.; Mack, M.J.; Morice, M.-C.; Holmes, D.R.; Ståhle, E.; Dawkins, K.D.; Mohr, F.W.; Serruys, P.W.; Colombo, A. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur. Heart J. 2011, 32, 2125–2134. [Google Scholar] [CrossRef]
- Kirtane, A.J.; Doshi, D.; Leon, M.B.; LaSala, J.M.; Ohman, E.M.; O’Neill, W.W.; Shroff, A.; Cohen, M.G.; Palacios, I.F.; Beohar, N.; et al. Treatment of higher-risk patients with an indication for revascularization: Evolution within the field of contemporary percutaneous coronary intervention. Circulation 2016, 134, 422–431. [Google Scholar] [CrossRef]
- Al-Rashid, F.; Totzeck, M.; Mahabadi, A.A.; Johannsen, L.; Luedike, P.; Lind, A.; Krueger, A.; Kamler, M.; Kahlert, P.; Jánosi, R.A.; et al. Safety and efficacy of a novel algorithm to guide decision-making in high-risk interventional coronary procedures. Int. J. Cardiol. 2020, 299, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Baumann, S.; Eder, F.; Perschka, S.; Loßnitzer, D.; Fastner, C.; Behnes, M.; Doesch, C.; Borggrefe, M.; Akin, I. Comparison of peri and post-procedural complications in patients undergoing revascularisation of coronary artery multivessel disease by coronary artery bypass grafting or protected percutaneous coronary intervention with the Impella 2.5 device. Eur. Heart J. Acute Cardiovasc. Care 2017, 8, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Rihal, C.S.; Naidu, S.S.; Givertz, M.M.; Szeto, W.Y.; Burke, J.A.; Kapur, N.K.; Kern, M.; Garratt, K.N.; Goldstein, J.A.; Dimas, V.; et al. 2015 SCAI/ACC/HFSA/STS Clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J. Am. Coll. Cardiol. 2015, 65, e7–e26. [Google Scholar] [CrossRef] [PubMed]
- Myat, A.; Patel, N.; Tehrani, S.; Banning, A.P.; Redwood, S.R.; Bhatt, D.L. Percutaneous circulatory assist devices for high-risk coronary intervention. JACC Cardiovasc. Interv. 2015, 8, 229–244. [Google Scholar] [CrossRef]
- Généreux, P.; Palmerini, T.; Caixeta, A.; Rosner, G.; Green, P.; Dressler, O.; Xu, K.; Parise, H.; Mehran, R.; Serruys, P.W.; et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: The residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J. Am. Coll. Cardiol. 2012, 59, 2165–2174. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. In Circulation; American Heart Association, Inc.: Dallas, TX, USA, 2012; pp. 2020–2035. [Google Scholar]
- Serruys, P.W.; Morice, M.-C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; Brand, M.V.D.; Bass, E.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef]
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B. Kidney disease: Improving global outcomes acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012, 1–138. [Google Scholar]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; Van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; Van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardio-Thorac. Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef]
- Means, G.; End, C.; Kaul, P. Management of percutaneous coronary intervention complications. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 2666. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. In Catheterization and Cardiovascular Interventions; Wiley: Hoboken, NJ, USA, 2013; Volume 124, pp. E266–E355. [Google Scholar]
- Hee, L.; Mussap, C.J.; Yang, L.; Dignan, R.; Kadappu, K.K.; Juergens, C.P.; Thomas, L.; French, J.K. Outcomes of coronary revascularization (percutaneous or bypass) in patients with diabetes mellitus and multivessel coronary disease. Am. J. Cardiol. 2012, 110, 643–648. [Google Scholar] [CrossRef]
- Cohen, M.G.; Matthews, R.; Maini, B.; Dixon, S.; Vetrovec, G.; Wohns, D.; Palacios, I.; Popma, J.; Ohman, E.M.; Schreiber, T.; et al. Percutaneous left ventricular assist device for high-risk percutaneous coronary interventions: Real-world versus clinical trial experience. Am. Heart J. 2015, 170, 872–879. [Google Scholar] [CrossRef]
- Melina, G.; Angeloni, E.; Benedetto, U.; Monti, F.; Roscitano, A.; Serdoz, R.; Sinatra, R. Complexity of coronary artery disease affects outcome of patients undergoing coronary artery bypass grafting with impaired left ventricular function. J. Thorac. Cardiovasc. Surg. 2013, 146, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Escaned, J.; Collet, C.; Ryan, N.; De Maria, G.L.; Walsh, S.; Sabate, M.; Davies, J.; Lesiak, M.; Moreno, R.; Cruz-Gonzalez, I.; et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur. Heart J. 2017, 38, 3124–3134. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Morice, M.-C.; Banning, A.P.; Serruys, P.W.; Mohr, F.-W.; Dawkins, K.D.; Mack, M.J.; on behalf of the SYNTAX Investigators. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur. J. Cardio-Thorac. Surg. 2013, 43, 1006–1013. [Google Scholar] [CrossRef]
- Mohr, F.W.; Morice, M.-C.; Kappetein, A.P.; Feldman, T.E.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Morel, M.-A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Farkouh, M.E.; Domanski, M.; Sleeper, L.A.; Siami, F.S.; Dangas, G.; Mack, M.; Yang, M.; Cohen, D.J.; Rosenberg, Y.; Solomon, S.D.; et al. Strategies for multivessel revascularization in patients with diabetes. N. Engl. J. Med. 2012, 367, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Toklu, B.; Bangalore, S. Comparison of coronary artery bypass graft surgery and percutaneous coronary intervention in patients with diabetes. Curr. Treat. Options Cardiovasc. Med. 2015, 17, 377. [Google Scholar] [CrossRef]
- Farkouh, M.E.; Dangas, G.; Leon, M.B.; Smith, C.; Nesto, R.; Buse, J.B.; Cohen, D.J.; Mahoney, E.; Sleeper, L.A.; King, S.; et al. Design of the future revascularization evaluation in patients with diabetes mellitus: Optimal management of multivessel disease (FREEDOM) trial. Am. Heart J. 2008, 155, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Petursson, P.; Lagerqvist, B.; Skúladóttir, H.; Svensson, A.; Eliasson, B.; Gudbjörnsdottir, S.; Eeg-Olofsson, K.; Norhammar, A. Long-term mortality in patients with type 2 diabetes undergoing coronary angiography: The impact of glucose-lowering treatment. Diabetologia 2012, 55, 2109–2117. [Google Scholar] [CrossRef][Green Version]
- Akin, I.; Bufe, A.; Eckardt, L.; Reinecke, H.; Senges, J.; Richardt, G.; Kuck, K.-H.; Schneider, S.; Nienaber, C.A. Comparison of outcomes in patients with insulin-dependent versus non-insulin dependent diabetes mellitus receiving drug-eluting stents (from the first phase of the prospective multicenter German DES.DE registry). Am. J. Cardiol. 2010, 106, 1201–1207. [Google Scholar] [CrossRef]
- Luciani, N.; Nasso, G.; Gaudino, M.; Abbate, A.; Glieca, F.; Alessandrini, F.; Girola, F.; Santarelli, F.; Possati, G. Coronary artery bypass grafting in type II diabetic patients: A comparison between insulin-dependent and non-insulin-dependent patients at short-and mid-term follow-up. Ann. Thorac. Surg. 2003, 76, 1149–1154. [Google Scholar] [CrossRef]
- Doshi, D.; Ben-Yehuda, O.; Bonafede, M.; Josephy, N.; Karmpaliotis, D.; Parikh, M.A.; Moses, J.W.; Stone, G.W.; Leon, M.B.; Schwartz, A.; et al. Underutilization of coronary artery disease testing among patients hospitalized with new-onset heart failure. J. Am. Coll. Cardiol. 2016, 68, 450–458. [Google Scholar] [CrossRef]
| All (n = 276) | No DM (n = 190) | DM (n = 86) | p-Value | |
|---|---|---|---|---|
| age (yrs.), mean ± SD | 70 ± 11 | 70 ± 11 | 70 ± 11 | 0.76 |
| male sex, n (%) | 203 (74) | 138 (73) | 65 (76) | 0.66 |
| body mass index (kg/m2), mean ± SD | 27 ± 5 | 27 ± 5 | 29 ± 5 | 0.001 |
| logistic EuroSCORE (%), mean ± SD | 10 ± 12 | 9 ± 11 | 11 ±15 | 0.07 |
| SYNTAX I score (%), mean ± SD | 21 ± 10 | 21 ± 10 | 22 ± 11 | 0.31 |
| LVEF (%), mean ± SD | 47 ± 10 | 48 ± 10 | 46 ± 11 | 0.06 |
| chronic obstructive pulmonary disease, n (%) | 20 (7) | 13 (7) | 7 (8) | 0.8 |
| peripheral artery disease stage IV, n (%) | 7 (3) | 4 (2) | 3 (4) | 0.68 |
| pulmonary hypertension, n (%) | 36 (13) | 24 (13) | 12 (14) | 0.85 |
| CAD with prior PCI, n (%) | 134 (49) | 87 (46) | 47 (55) | 0.19 |
| coronary artery bypass grafting, n (%) | 31 (11) | 22 (12) | 9 (11) | 0.84 |
| prior cardiac surgery, n (%) | 37 (13) | 25 (13) | 12 (14) | 0.85 |
| atrial fibrillation, n (%) | 53 (19) | 30 (16) | 23 (27) | <0.05 |
| hypertension, n (%) | 234 (85) | 153 (81) | 81 (94) | 0.03 |
| baseline creatinine (mg/dL), mean ± SD | 1.32 ± 0.84 | 1.27 ± 0.82 | 1.45 ± 0.86 | 0.09 |
| All (n = 276) | No DM (n = 190) | DM (n = 86) | p-Value | |
|---|---|---|---|---|
| acute coronary syndrome, n (%) | 129 (46) | 86 (45) | 43 (50) | 0.52 |
| MCS, n (%) | 61 (22) | 38 (20) | 23 (27) | 0.25 |
| multivessel PCI | 265 (96) | 181 (95) | 84 (98) | 0.51 |
| residual SYNTAX I score < 8%, n (%) | 247 (90) | 170 (90) | 77 (90) | 0.98 |
| PCI left main artery, n (%) | 103 (37) | 65 (34) | 38 (44) | 0.14 |
| PCI left anterior descending coronary artery, n (%) | 169 (61) | 112 (59) | 57 (66) | 0.29 |
| PCI left circumflex coronary artery, n (%) | 127 (46) | 86 (45) | 41 (48) | 0.8 |
| PCI right coronary artery, n (%) | 81 (29) | 60 (32) | 21 (24) | 0.25 |
| PCI bypass graft, n (%) | 15 (5) | 11 (6) | 4 (5) | 0.78 |
| last remaining vessel, n (%) | 3 (1) | 2 (1) | 1 (1) | 0.94 |
| contrast agent (mL), mean ± SD | 247 ± 114 | 249 ± 121 | 241 ± 101 | 0.61 |
| number of implanted stents per patient, median (IQR) | 2 (1–7) | 2 (1–7) | 2 (1–7) | 0.37 |
| stent length per patient (mm), median (IQR) | 42 (9–143) | 42 (9–143) | 44 (9–111) | 0.99 |
| All (n = 276) | No DM (n = 190) | DM (n = 86) | p-Value | |
|---|---|---|---|---|
| MACCE, n (%) | 9 (3) | 4 (2) | 5 (6) | 0.24 |
| stroke, n (%) | 1 (1) | 0 | 1 (1) | 0.31 |
| new myocardial infarction, n (%) | 0 | 0 | 0 | |
| death, n (%) | 8 (3) | 4 (2) | 4 (5) | 0.26 |
| acute kidney injury, n (%) | 24 (9) | 11 (6) | 13 (16) | 0.02 |
| vascular complications, n (%) | 13 (5) | 9 (5) | 4 (5) | 0.98 |
| coronary complications, n (%) | 9 (3) | 5 (3) | 4 (5) | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johannsen, L.; Soldat, J.; Krueger, A.; Mahabadi, A.A.; Dykun, I.; Totzeck, M.; Jánosi, R.A.; Rassaf, T.; Al-Rashid, F. Impact of Diabetes Mellitus on Outcomes after High-Risk Interventional Coronary Procedures. J. Clin. Med. 2020, 9, 3414. https://doi.org/10.3390/jcm9113414
Johannsen L, Soldat J, Krueger A, Mahabadi AA, Dykun I, Totzeck M, Jánosi RA, Rassaf T, Al-Rashid F. Impact of Diabetes Mellitus on Outcomes after High-Risk Interventional Coronary Procedures. Journal of Clinical Medicine. 2020; 9(11):3414. https://doi.org/10.3390/jcm9113414
Chicago/Turabian StyleJohannsen, Laura, Julian Soldat, Andrea Krueger, Amir A. Mahabadi, Iryna Dykun, Matthias Totzeck, Rolf Alexander Jánosi, Tienush Rassaf, and Fadi Al-Rashid. 2020. "Impact of Diabetes Mellitus on Outcomes after High-Risk Interventional Coronary Procedures" Journal of Clinical Medicine 9, no. 11: 3414. https://doi.org/10.3390/jcm9113414
APA StyleJohannsen, L., Soldat, J., Krueger, A., Mahabadi, A. A., Dykun, I., Totzeck, M., Jánosi, R. A., Rassaf, T., & Al-Rashid, F. (2020). Impact of Diabetes Mellitus on Outcomes after High-Risk Interventional Coronary Procedures. Journal of Clinical Medicine, 9(11), 3414. https://doi.org/10.3390/jcm9113414







