Occurrence and Severity of Pain in Patients with Venous Leg Ulcers: A 12-Week Longitudinal Study
Abstract
1. Introduction
2. Experimental Section
2.1. Patients
2.2. Ethical Aspects
2.3. Analyzed Parameters
2.4. Statistical Analysis
3. Results
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gonçalves, M.L.; de Gouveia Santos, V.L.C.; de Mattos Pimenta, C.A.; Suzuki, E.; Komegae, K.M. Pain in chronic leg ulcers. J. Wound Ostomy Cont. Nurs. 2004, 31, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.E. Venous ulceration: Compression as the mainstay of therapy. J. Wound Ostomy Cont. Nurs. 1999, 26, 39–47. [Google Scholar] [CrossRef]
- Phillips, T.; Stanton, B.; Provan, A.; Lew, R. A study of the impact of leg ulcers on quality of life: Financial, social, and psychologic implications. J. Am. Acad. Dermatol. 1994, 31, 49–53. [Google Scholar] [CrossRef]
- Kunimoto, B.; Cooling, M.; Gulliver, W.; Houghton, P.; Orsted, H.; Sibbald, R.G. Best practices for the prevention and treatment of venous leg ulcers. Ostomy Wound Manag. 2001, 47, 34–50. [Google Scholar]
- Eklöf, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef]
- Moffatt, C.; Franks, P.; Hollinworth, H. Understanding wound pain and trauma: An international perspective. In Pain at Wound Dressing Changes European Wound Management Association (EWMA) Position Document; Medical Education Partnership, Ltd.: London, UK, 2002; pp. 2–7. [Google Scholar]
- Nemeth, K.A.; Harrison, M.B.; Graham, I.D.; Burke, S. Understanding venous leg ulcer pain: Results of a longitudinal study. Ostomy Wound Manag. 2004, 50, 34–46. [Google Scholar]
- Walshe, C. Living with a venous leg ulcer: A descriptive study of patients’ experiences. J. Adv. Nurs. 1995, 22, 1092–1100. [Google Scholar] [CrossRef]
- Ryan, S.; Eager, C.; Sibbald, R.G. Venous leg ulcer pain. Ostomy Wound Manag. 2003, 49, 16–23. [Google Scholar]
- Moffatt, C.; Harper, P. Leg Ulcer; Churchill Livingstone: London, UK, 1997. [Google Scholar]
- Charles, H. Venous leg ulcer pain and its characteristics. J. Tissue Viability 2002, 12, 154–158. [Google Scholar] [CrossRef]
- Vandenkerkhof, E.G.; Hopman, W.M.; Carley, M.E.; Kuhnke, J.L.; Harrison, M.B. Leg ulcer nursing care in the community: A prospective cohort study of the symptom of pain. BMC Nurs. 2013, 12, 3. [Google Scholar] [CrossRef]
- Franks, P.J.; Moffatt, C.J. Who suffers most from leg ulceration? J. Wound Care 1998, 7, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.A.; Harrison, M.B.; Graham, I.D.; Burke, S. Pain in pure and mixed aetiology venous leg ulcers: A three-phase point prevalence study. J. Wound Care 2003, 12, 336–340. [Google Scholar] [CrossRef]
- Hofman, D.; Ryan, T.J.; Arnold, F.; Cherry, G.W.; Lindholm, C.; Bjellerup, M.; Glynn, C. Pain in venous leg ulcers. J. Wound Care 1997, 6, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Chase, S.K.; Whittemore, R.; Crosby, N.; Freney, D.; Howes, P.; Phillips, T.J. Living with chronic venous leg ulcers: A descriptive study of knowledge and functional health status. J. Community Health Nurs. 2000, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Nilsson, C.; Nilsson, A.; Fagerström, C. Leg ulcers in older people: A national study addressing variation in diagnosis, pain and sleep disturbance. BMC Geriatr. 2016, 16, 25. [Google Scholar] [CrossRef]
- Imbernon-Moya, A.; Ortiz-de Frutos, F.J.; Sanjuan-Alvarez, M.; Portero-Sanchez, I.; Merinero-Palomares, R.; Alcazar, V. Pain, Quality of Life, and Functional Capacity With Topical Sevoflurane Application for Chronic Venous Ulcers: A Retrospective Clinical Study. EJVES Short Rep. 2017, 36, 9–12. [Google Scholar] [CrossRef]
- Herber, O.R.; Schnepp, W.; Rieger, M.A. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual. Life Outcomes 2007, 5, 44. [Google Scholar] [CrossRef]
- Hareendran, A.; Doll, H.; Wild, D.J.; Moffatt, C.J.; Musgrove, E.; Wheatley, C.; Franks, P.J. The venous leg ulcer quality of life (VLU-QoL) questionnaire: Development and psychometric validation. Wound Repair Regen. 2007, 15, 465–473. [Google Scholar] [CrossRef]
- Hareendran, A.; Bradbury, A.; Budd, J.; Geroulakos, G.; Hobbs, R.; Kenkre, J.; Symonds, T. Measuring the impact of venous leg ulcers on quality of life. J. Wound Care 2005, 14, 53–57. [Google Scholar] [CrossRef]
- Flett, R.; Harcourt, B.; Alpass, F. Psychosocial aspects of chronic lower leg ulceration in the elderly. West. J. Nurs. Res. 1994, 16, 183–192. [Google Scholar] [CrossRef]
- Cullum, N.; Roe, B. Leg Ulcers: Nursing Management—A Research Based Guide; Scutari Press: London, UK, 1995. [Google Scholar]
- Pieper, B.; Rossi, R.; Templin, T. Pain associated with venous ulcers in injecting drug users. Ostomy Wound Manag. 1998, 44, 54–67. [Google Scholar]
- Walters, S.J.; Morrell, C.J.; Dixon, S. Measuring health-related quality of life in patients with venous leg ulcers. Qual. Life Res. 1999, 8, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Guarnera, G.; Tinelli, G.; Abeni, D.; Di Pietro, C.; Sampogna, F.; Tabolli, S. Pain and quality of life in patients with vascular leg ulcers: An Italian multicentre study. J. Wound Care 2007, 16, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Price, P. Measuring health-related quality of life in patients with chronic leg ulcers. Wounds Compend. Clin. Res. Pract. 1996, 8, 91–94. [Google Scholar]
- Husband, L.L. Venous ulceration: The pattern of pain and the paradox. Clin. Eff. Nurs. 2001, 5, 35–40. [Google Scholar] [CrossRef]
- Krasner, D. Painful venous ulcers: Themes and stories about living with the pain and suffering. J. Wound Ostomy Cont. Nurs. 1998, 25, 158–168. [Google Scholar] [CrossRef]
- Hopman, W.M.; Buchanan, M.; VanDenKerkhof, E.G.; Harrison, M.B. Pain and health-related quality of life in people with chronic leg ulcers. Chronic Dis. Inj. Can. 2013, 33, 167–174. [Google Scholar] [CrossRef]
- Frot, M.; Feine, J.S.; Bushnell, M.C. Sex differences in pain perception and anxiety. A psychophysical study with topical capsaicin. Pain 2004, 108, 230–236. [Google Scholar] [CrossRef]
- Ramírez-Maestre, C.; Esteve, R. The role of sex/gender in the experience of pain: Resilience, fear, and acceptance as central variables in the adjustment of men and women with chronic pain. J. Pain 2014, 15, 608–618. [Google Scholar] [CrossRef]
- Hyland, M.E.; Ley, A.; Thomson, B. Quality of life of leg ulcer patients: Questionnaire and preliminary findings. J. Wound Care 1994, 3, 294–298. [Google Scholar] [CrossRef]
- Closs, S.J.; Nelson, E.A.; Briggs, M. Can venous and arterial leg ulcers be differentiated by the characteristics of the pain they produce? J. Clin. Nurs. 2008, 17, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Taverner, T.; Closs, S.J.; Briggs, M. The journey to chronic pain: A grounded theory of older adults’ experiences of pain associated with leg ulceration. Pain Manag. Nurs. 2014, 15, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Chase, S.K.; Melloni, M.; Savage, A. A forever healing: The lived experience of venous ulcer disease. J. Vasc. Nurs. 1997, 15, 73–78. [Google Scholar] [CrossRef]
- Persoon, A.; Heinen, M.M.; van der Vleuten, C.J.M.; de Rooij, M.J.; van de Kerkhof, P.C.M.; van Achterberg, T. Leg ulcers: A review of their impact on daily life. J. Clin. Nurs. 2004, 13, 341–354. [Google Scholar] [CrossRef]
- Briggs, M.; Flemming, K. Living with leg ulceration: A synthesis of qualitative research. J. Adv. Nurs. 2007, 59, 319–328. [Google Scholar] [CrossRef]
- Green, J.; Jester, R. Health-related quality of life and chronic venous leg ulceration: Part 2. Br. J. Community Nurs. 2010, 15, S4–S10. [Google Scholar] [CrossRef]
- Wilson, A.B. Quality of life and leg ulceration from the patient’s perspective. Br. J. Nurs. 2004, 13, S17–S20. [Google Scholar] [CrossRef]
- Jull, A.; Walker, N.; Hackett, M.; Jones, M.; Rodgers, A.; Birchall, N.; Norton, R.; MacMahon, S. Leg ulceration and perceived health: A population based case-control study. Age Ageing 2004, 33, 236–241. [Google Scholar] [CrossRef]
- McMullen, M. The relationship between pain and leg ulcers: A critical review. Br. J. Nurs. 2004, 13, S30–S36. [Google Scholar] [CrossRef]
- Jones, J.; Barr, W.; Robinson, J.; Carlisle, C. Depression in patients with chronic venous ulceration. Br. J. Nurs. 2006, 15, S17–S23. [Google Scholar] [CrossRef]
- Wales, S. A world of pain. Nurs. Stand. 2006, 20, 24–25. [Google Scholar] [CrossRef] [PubMed]
- González-Consuegra, R.V.; Verdú, J. Quality of life in people with venous leg ulcers: An integrative review. J. Adv. Nurs. 2011, 67, 926–944. [Google Scholar] [CrossRef] [PubMed]
- Maddox, D. Effects of venous leg ulceration on patients’ quality of life. Nurs. Stand. 2012, 26, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.T.; Mościcka, P.; Jawień, A.; Cwajda-Białasik, J.; Cierzniakowska, K.; Ślusarz, R.; Hancke, E. Quality of life in patients with leg ulcers or skin lesions—A pilot study. Post. Dermatol. Alergol. 2015, 32, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Cwajda-Białasik, J.; Szewczyk, M.T.; Mościcka, P.; Jawień, A.; Ślusarz, R. Influence of ulceration etiology on the global quality of life and its specific dimensions, including the control of pain, in patients with lower limb vascular insufficiency. Post. Dermatol. Alergol. 2017, 34, 471–477. [Google Scholar] [CrossRef]
- Douglas, V. Living with a chronic leg ulcer: An insight into patients’ experiences and feelings. J. Wound Care 2001, 10, 355–360. [Google Scholar] [CrossRef]
- Heinen, M.M.; van Achterberg, T.; op Reimer, W.S.; van de Kerkhof, P.C.M.; de Laat, E. Venous leg ulcer patients: A review of the literature on lifestyle and pain-related interventions. J. Clin. Nurs. 2004, 13, 355–366. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Women | n = 485 (64.3%) |
| Mean (±SD) age (years) | 65.7 ± 12.09 |
| Age > 65 years | n = 415 (55.0%) |
| Median (range) duration of underlying disease (years) | 24 (0–70) |
| Underlying disease > 20 years | n = 392 (52.0%) |
| Median (range) duration of current ulcer (months) | 12 (1–504) |
| Duration of current ulcer > 12 months | n = 296 (39.3%) |
| Comorbidities | n = 647 (85.8%) |
| Rheumatoid arthritis | n = 118 (15.6%) |
| Arthritis | n = 234 (31.0%) |
| Diabetes mellitus | n = 160 (21.2%) |
| Atherosclerosis 1 | n = 96 (12.7%) |
| Cardiovascular disease 2 | n = 184 (24.4%) |
| Overweight/obesity (BMI ≥ 25 kg/m2) | n = 633 (84.0%) |
| Obesity (BMI ≥ 30 kg/m2) | n = 354 (46.9%) |
| Medial ulceration | n = 469 (62.2%) |
| Posterior ulceration | n = 121 (16.0%) |
| Anterior ulceration | n = 129 (17.1%) |
| Lateral ulceration | n = 216 (28.6%) |
| Circumferential ulceration | n = 24 (3.2%) |
| Posterior/circumferential ulceration | n = 141 (18.7%) |
| Ulcer locations ≥ 3 | n = 58 (7.7%) |
| Multiple ulcerations | n = 357 (47.3%) |
| Deep ulceration | n = 659 (87.4%) |
| Median (range) ulceration area at the baseline (cm2) | 8.25 (0.12–538) |
| Baseline ulceration area > 8.25 cm2 | n = 373 (49.5%) |
| Pus | n = 40 (5.3%) |
| Unpleasant smell | n = 103 (13.7%) |
| Redness | n = 472 (62.6%) |
| Swelling | n = 124 (16.4%) |
| Warmth | n = 369 (48.9%) |
| Pain | n = 646 (85.7%) |
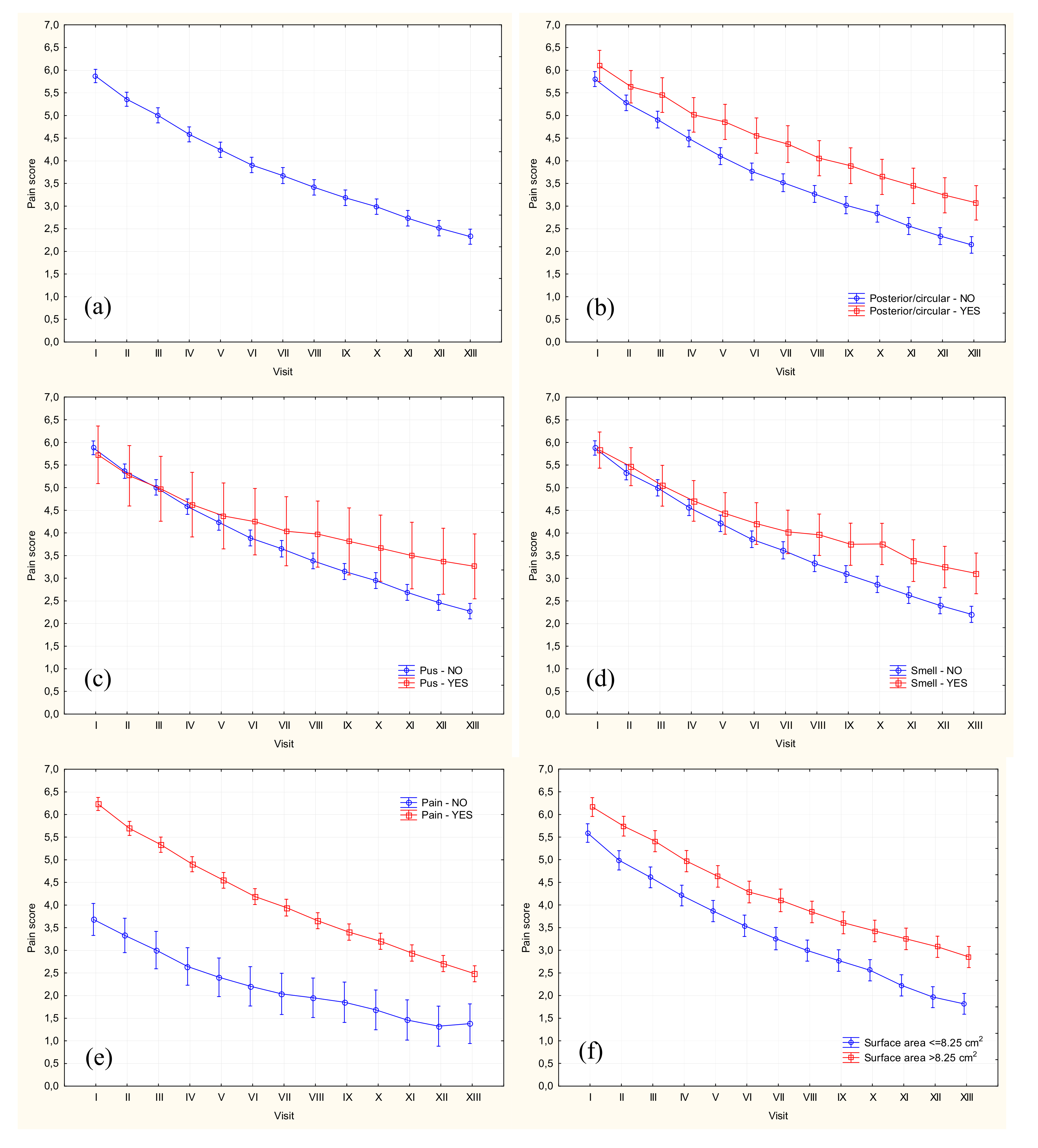
| Week | n | VAS 0 | VAS 5+ | Mean ± SD | Median | Quartiles | Range |
|---|---|---|---|---|---|---|---|
| 0 | 754 | 4 (0.53%) | 585 (77.59%) | 5.86 ± 2.05 | 6.0 | 5.0–7.5 | 0–10 |
| 1 | 752 | 22 (2.93%) | 527 (70.08%) | 5.35 ± 2.14 | 5.0 | 4.0–7.0 | 0–10 |
| 2 | 752 | 34 (4.52%) | 440 (58.51%) | 5.00 ± 2.30 | 5.0 | 3.5–6.5 | 0–10 |
| 3 | 752 | 46 (6.12%) | 391 (51.99%) | 4.58 ± 2.29 | 5.0 | 3.0-6.0 | 0–10 |
| 4 | 748 | 60 (8.02%) | 334 (44.65%) | 4.23 ± 2.34 | 4.5 | 2.5–6.0 | 0–10 |
| 5 | 748 | 80 (10.70%) | 298 (39.84%) | 3.90 ± 2.36 | 4.0 | 2.0–5.5 | 0–10 |
| 6 | 745 | 100 (13.42%) | 271 (36.38%) | 3.67 ± 2.46 | 3.5 | 2.0–5.5 | 0–10 |
| 7 | 745 | 110 (14.77%) | 238 (31.95%) | 3.41 ± 2.34 | 3.5 | 1.5–5.0 | 0–10 |
| 8 | 744 | 137 (18.41%) | 217 (29.17%) | 3.19 ± 2.39 | 3.0 | 1.0–5.0 | 0–9 |
| 9 | 744 | 154 (20.70%) | 193 (25.94%) | 2.99 ± 2.35 | 3.0 | 1.0–5.0 | 0–9 |
| 10 | 744 | 194 (26.08%) | 164 (22.04%) | 2.73 ± 2.37 | 2.5 | 0.0–4.0 | 0–10 |
| 11 | 744 | 216 (29.03%) | 164 (22.04%) | 2.51 ± 2.35 | 2.0 | 0.0–4.0 | 0–9 |
| 12 | 744 | 246 (33.06%) | 140 (18.82%) | 2.33 ± 2.31 | 2.0 | 0.0–4.0 | 0–9 |
| Predictor | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Follow-up time | 11261.4 | 12 | 938.4 | 672.18 | <0.001 |
| Age > 65 years | 23.3 | 12 | 1.9 | 1.39 | 0.163 |
| Female sex | 28.5 | 12 | 2.4 | 1.70 | 0.059 |
| Underlying disease > 20 years | 14.6 | 12 | 1.2 | 0.87 | 0.578 |
| Current ulcer > 12 months | 24.2 | 12 | 2.0 | 1.44 | 0.137 |
| Comorbidities | 27.6 | 12 | 2.3 | 1.65 | 0.071 |
| Overweight/obesity | 13.23 | 12 | 1.1 | 0.79 | 0.662 |
| Obesity | 23.4 | 12 | 2.0 | 1.40 | 0.159 |
| Medial ulceration | 27.0 | 12 | 2.3 | 1.62 | 0.079 |
| Posterior ulceration | 47.47 | 12 | 3.96 | 2.85 | 0.001 |
| Anterior ulceration | 4.64 | 12 | 0.39 | 0.28 | 0.993 |
| Lateral ulceration | 9.7 | 12 | 0.8 | 0.58 | 0.859 |
| Circumferential ulceration | 36.32 | 12 | 3.03 | 2.18 | 0.010 |
| Posterior/circumferential ulceration | 92.84 | 12 | 7.74 | 5.09 | <0.001 |
| ≥3 ulcer locations | 7.96 | 12 | 0.66 | 0.48 | 0.930 |
| Deep ulceration | 9.86 | 12 | 0.82 | 0.59 | 0.852 |
| Multiple ulcers | 18.3 | 12 | 1.5 | 1.09 | 0.361 |
| Pus | 72.85 | 12 | 6.07 | 4.37 | <0.001 |
| Unpleasant smell | 123.31 | 12 | 10.28 | 7.42 | <0.001 |
| Redness | 40.3 | 12 | 3.4 | 2.41 | 0.004 |
| Swelling | 8.82 | 12 | 0.74 | 0.53 | 0.899 |
| Warmth | 115.9 | 12 | 9.7 | 6.99 | <0.001 |
| Pain | 215.83 | 12 | 17.99 | 13.09 | <0.001 |
| Ulceration area > 8.25 cm2 | 46.2 | 12 | 3.8 | 2.76 | 0.001 |
| Predictor | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Higher pain severity | |||||
| Pus/unpleasant smell | 106.09 | 12 | 8.84 | 6.44 | <0.001 |
| Posterior/circumferential ulceration | 38.71 | 12 | 3.23 | 2.35 | 0.005 |
| Ulceration area > 8.25 cm2 | 20.42 | 12 | 1.70 | 1.24 | 0.248 |
| Lesser pain severity | |||||
| Redness | 56.08 | 12 | 4.67 | 3.41 | <0.001 |
| Warmth | 24.30 | 12 | 2.03 | 1.48 | 0.124 |
| Redness*Warmth | 133.50 | 12 | 11.13 | 8.12 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mościcka, P.; Cwajda-Białasik, J.; Jawień, A.; Sopata, M.; Szewczyk, M.T. Occurrence and Severity of Pain in Patients with Venous Leg Ulcers: A 12-Week Longitudinal Study. J. Clin. Med. 2020, 9, 3399. https://doi.org/10.3390/jcm9113399
Mościcka P, Cwajda-Białasik J, Jawień A, Sopata M, Szewczyk MT. Occurrence and Severity of Pain in Patients with Venous Leg Ulcers: A 12-Week Longitudinal Study. Journal of Clinical Medicine. 2020; 9(11):3399. https://doi.org/10.3390/jcm9113399
Chicago/Turabian StyleMościcka, Paulina, Justyna Cwajda-Białasik, Arkadiusz Jawień, Maciej Sopata, and Maria T. Szewczyk. 2020. "Occurrence and Severity of Pain in Patients with Venous Leg Ulcers: A 12-Week Longitudinal Study" Journal of Clinical Medicine 9, no. 11: 3399. https://doi.org/10.3390/jcm9113399
APA StyleMościcka, P., Cwajda-Białasik, J., Jawień, A., Sopata, M., & Szewczyk, M. T. (2020). Occurrence and Severity of Pain in Patients with Venous Leg Ulcers: A 12-Week Longitudinal Study. Journal of Clinical Medicine, 9(11), 3399. https://doi.org/10.3390/jcm9113399




