The Eye as a Non-Invasive Window to the Microcirculation in Liver Cirrhosis: A Prospective Pilot Study
Abstract
1. Introduction
2. Experimental Section
2.1. Ethics
2.2. Participants
2.3. Study Visit
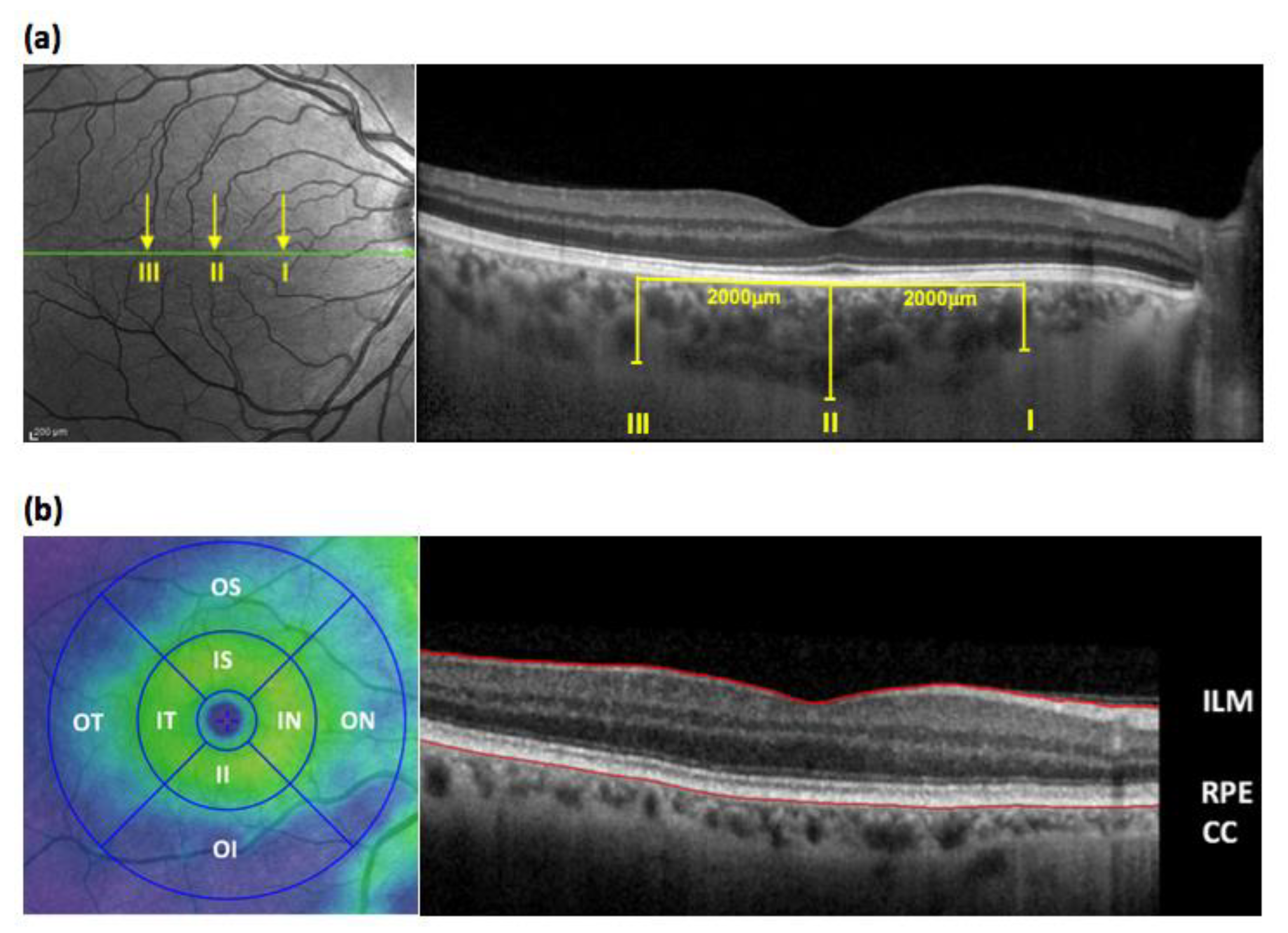
2.3.1. Optical Coherence Tomography (OCT)
2.3.2. Sample Collection and Analysis
2.4. Follow-Up
2.5. Statistics
2.5.1. Sample Size
2.5.2. Statistical analysis
3. Results
3.1. Participant Disposition
3.2. Chorioretinal Measurements in Cirrhosis
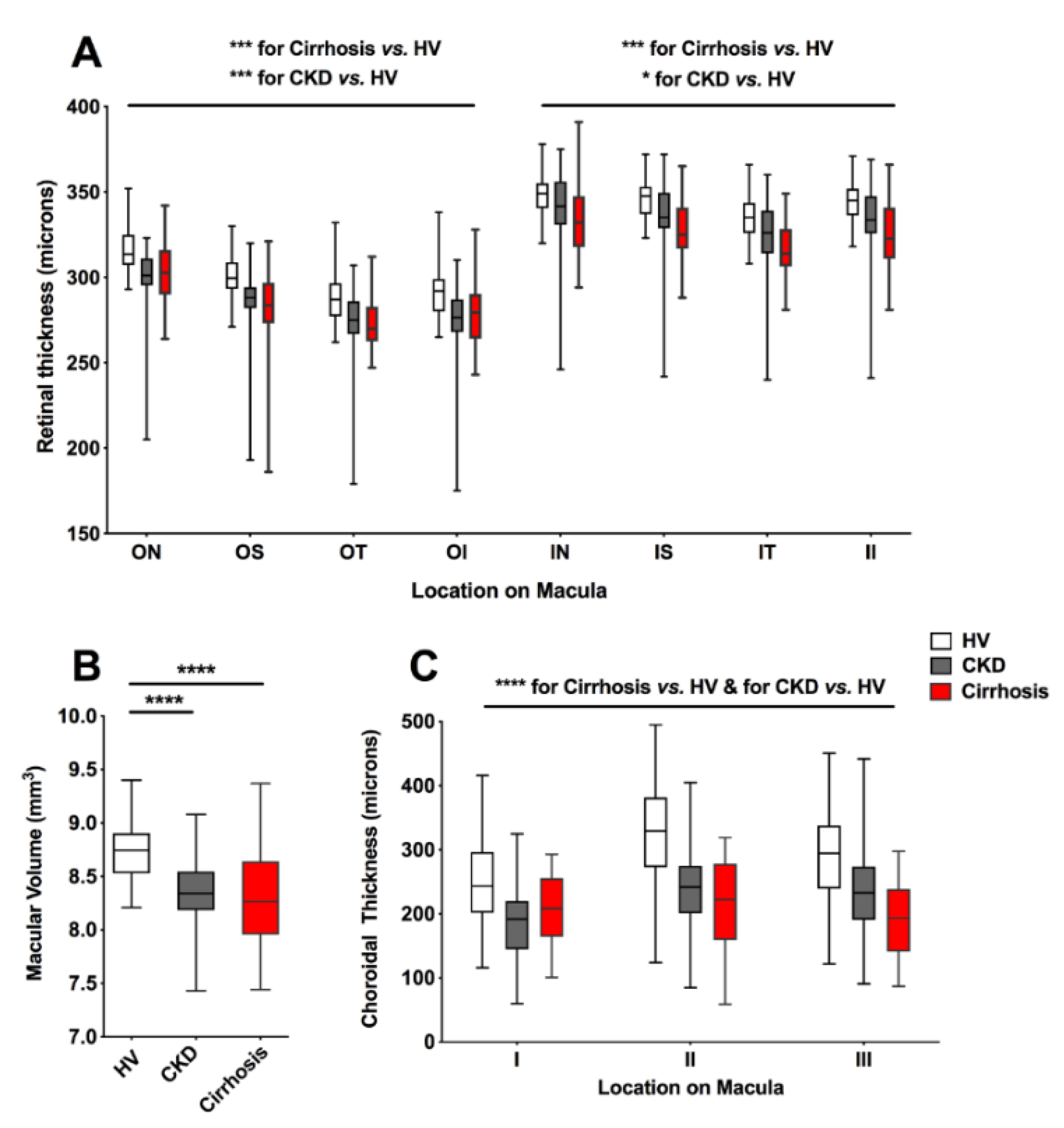
3.2.1. Chorioretinal Parameters in Cirrhosis, CKD and HV
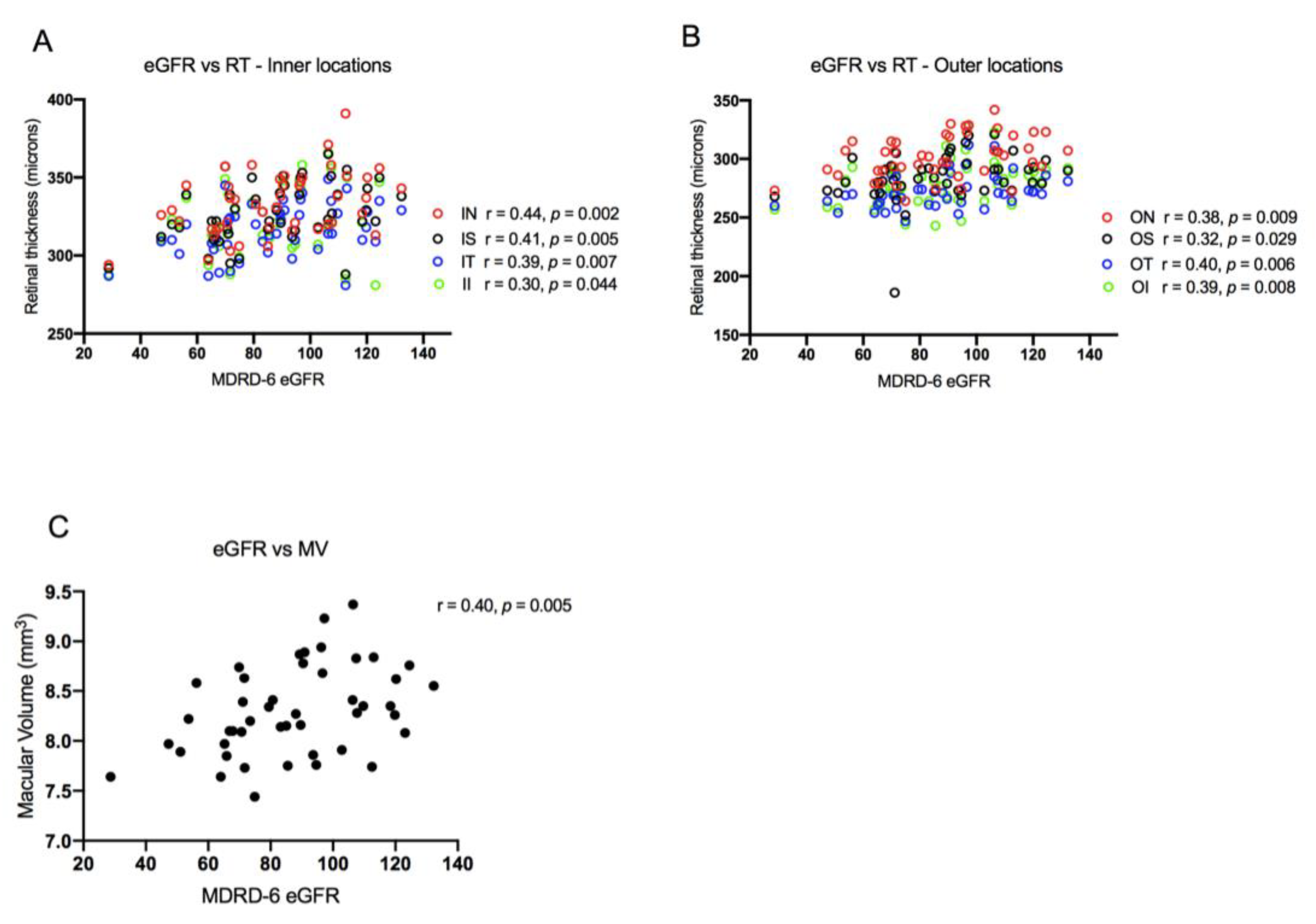
3.2.2. Correlation of Chorioretinal Parameters with Renal Function and Liver Disease Severity
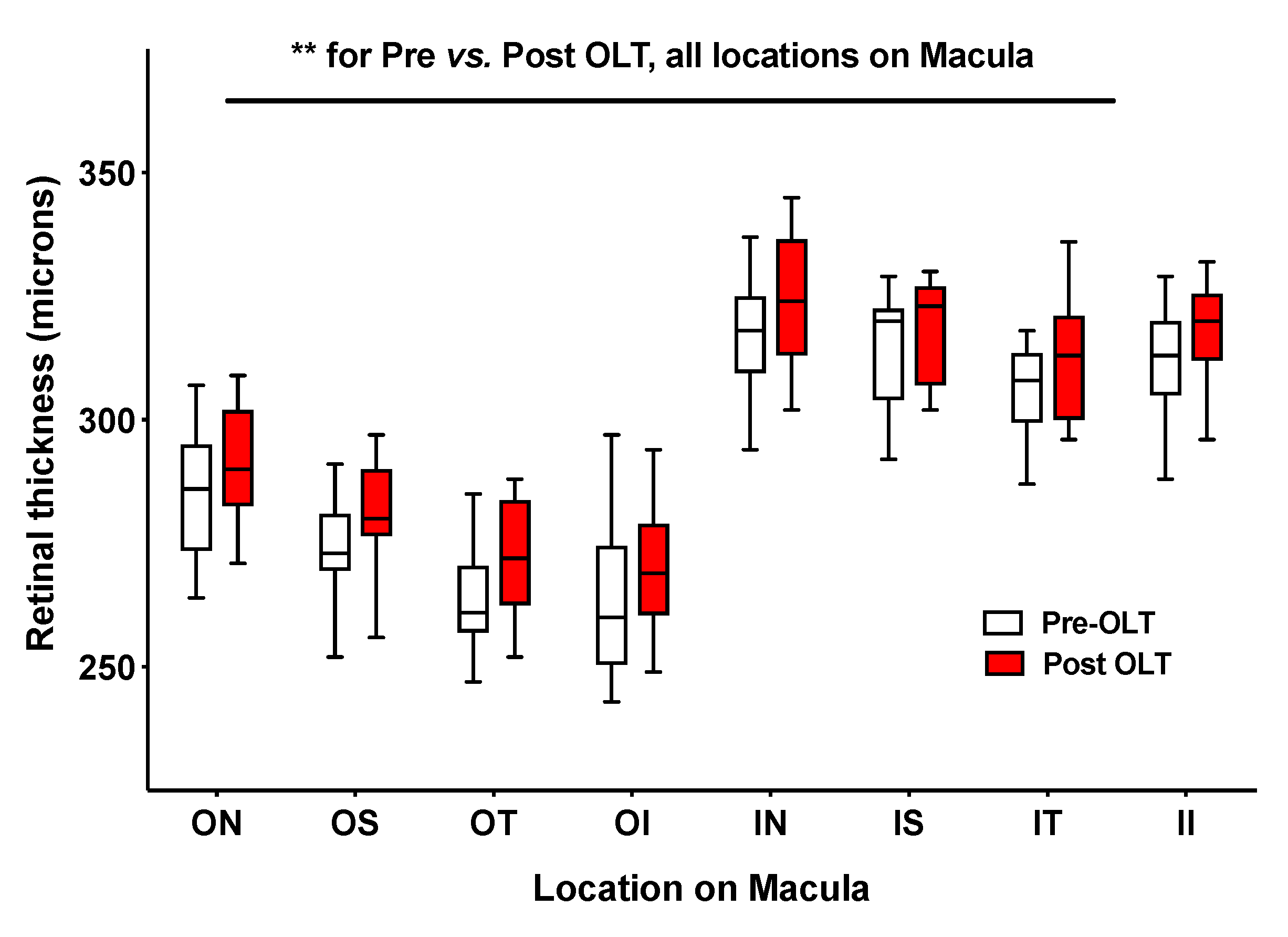
3.2.3. Alterations in Chorioretinal Parameters with Liver Transplantation
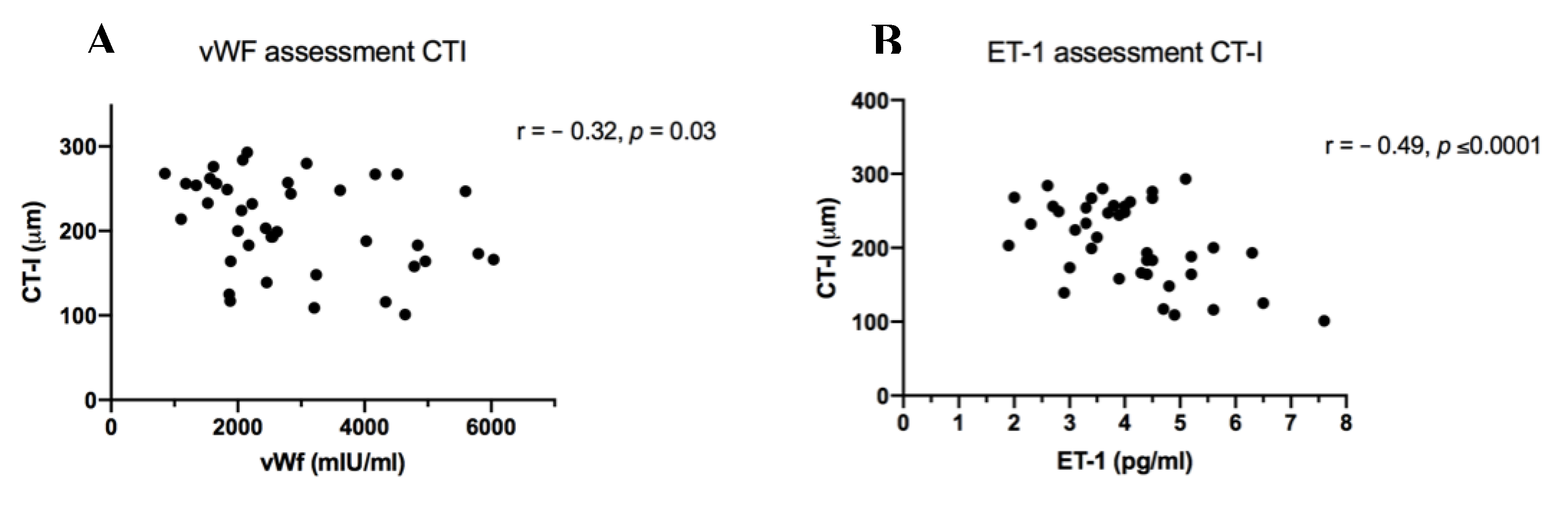
3.2.4. Chorioretinal Parameters and Markers of Inflammation and Endothelial Dysfunction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trebicka, J.; Amoros, A.; Pitarch, C.; Titos, E.; Alcaraz-Quiles, J.; Schierwagen, R.; Deulofeu, C.; Fernandez-Gomez, J.; Piano, S.; Caraceni, P.; et al. Addressing Profiles of Systemic Inflammation Across the Different Clinical Phenotypes of Acutely Decompensated Cirrhosis. Front. Immunol. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Angeli, P.; Garcia-Tsao, G.; Nadim, M.K.; Parikh, C.R. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J. Hepatol. 2019, 71, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Newby, D.E.; Hayes, P.C. Hyperdynamic circulation in liver cirrhosis: Not peripheral vasodilatation but ’splanchnic steal’. QJM Int. J. Med. 2002, 95, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.R.; Cox, E.F.; Scott, R.A.; James, M.W.; Kaye, P.; Aithal, G.P.; Francis, S.T.; Guha, I.N. Multi-organ assessment of compensated cirrhosis patients using quantitative magnetic resonance imaging. J. Hepatol. 2018, 69, 1015–1024. [Google Scholar] [CrossRef]
- McAvoy, N.C.; Semple, S.; Richards, J.M.; Robson, A.J.; Patel, D.; Jardine, A.G.; Leyland, K.; Cooper, A.S.; Newby, D.E.; Hayes, P.C. Differential visceral blood flow in the hyperdynamic circulation of patients with liver cirrhosis. Aliment. Pharmacol. Ther. 2016, 43, 947–954. [Google Scholar] [CrossRef]
- Davies, T.; Wythe, S.; O’Beirne, J.; Martin, D.; Gilbert-Kawai, E. Review article: The role of the microcirculation in liver cirrhosis. Aliment. Pharmacol. Ther. 2017, 46, 825–835. [Google Scholar] [CrossRef]
- Sakr, Y.; Dubois, M.J.; De Backer, D.; Creteur, J.; Vincent, J.L. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004, 32, 1825–1831. [Google Scholar] [CrossRef]
- D’Amico, G.; Morabito, A.; D’Amico, M.; Pasta, L.; Malizia, G.; Rebora, P.; Valsecchi, M.G. Clinical states of cirrhosis and competing risks. J. Hepatol. 2018, 68, 563–576. [Google Scholar] [CrossRef]
- Schneider, A.G.; Schelleman, A.; Goodwin, M.D.; Bailey, M.; Eastwood, G.M.; Bellomo, R. Contrast-enhanced ultrasound evaluation of the renal microcirculation response to terlipressin in hepato-renal syndrome: A preliminary report. Ren Fail. 2015, 37, 175–179. [Google Scholar] [CrossRef]
- Aykut, G.; Veenstra, G.; Scorcella, C.; Ince, C.; Boerma, C. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med. Exp. 2015, 3, 40. [Google Scholar] [CrossRef]
- Kara, A.; Akin, S.; Ince, C. Monitoring microcirculation in critical illness. Curr. Opin. Crit. Care. 2016, 22, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Hosny, H.; Tawadros Md, P.; Elayashy, M.; El-Ashmawi, H. Effect of Dexmedetomidine Infusion on Sublingual Microcirculation in Patients Undergoing On-Pump Coronary Artery Bypass Graft Surgery: A Prospective Randomized Trial. J. Cardiothorac. Vasc. Anesth. 2019, 33, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Balmforth, C.; van Bragt, J.J.; Ruijs, T.; Cameron, J.R.; Kimmitt, R.; Moorhouse, R.; Czopek, A.; Hu, M.K.; Gallacher, P.J.; Dear, J.W.; et al. Chorioretinal thinning in chronic kidney disease links to inflammation and endothelial dysfunction. JCI Insight 2016, 1, e89173. [Google Scholar] [CrossRef] [PubMed]
- Nadim, M.K.; Sung, R.S.; Davis, C.L.; Andreoni, K.A.; Biggins, S.W.; Danovitch, G.M.; Feng, S.; Friedewald, J.; Hong, J.C.; Kellum, J.A.; et al. Simultaneous liver-kidney transplantation summit: Current state and future directions. Am. J. Transpl. 2012, 12, 2901–2908. [Google Scholar] [CrossRef]
- South, K.; Lane, D.A. ADAMTS-13 and von Willebrand factor: A dynamic duo. J. Thromb. Haemost. 2018, 16, 6–18. [Google Scholar] [CrossRef]
- Dhaun, N.; Goddard, J.; Webb, D.J. The endothelin system and its antagonism in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 943–955. [Google Scholar] [CrossRef]
- Britze, J.; Frederiksen, J.L. Optical coherence tomography in multiple sclerosis. Eye 2018, 32, 884–888. [Google Scholar] [CrossRef]
- Nolan, R.C.; Narayana, K.; Galetta, S.L.; Balcer, L.J. Optical Coherence Tomography for the Neurologist. Semin. Neurol. 2015, 35, 564–577. [Google Scholar] [CrossRef]
- Farrah, T.E.; Webb, D.J.; Dhaun, N. Retinal fingerprints for precision profiling of cardiovascular risk. Nat. Rev. Cardiol. 2019, 16, 379–381. [Google Scholar] [CrossRef]
- Farrah, T.E.; Dhillon, B.; Keane, P.A.; Webb, D.J.; Dhaun, N. The eye, the kidney, and cardiovascular disease: Old concepts, better tools, and new horizons. Kidney Int. 2020, 98, 323–342. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Couper, D.J.; Cooper, L.S.; Shahar, E.; Hubbard, L.D.; Wofford, M.R.; Sharrett, A.R. Retinal microvascular abnormalities and incident stroke: The Atherosclerosis Risk in Communities Study. Lancet 2001, 358, 1134–1140. [Google Scholar] [CrossRef]
- Daien, V.; Carriere, I.; Kawasaki, R.; Cristol, J.P.; Villain, M.; Fesler, P.; Ritchie, C.W.; Delcourt, C. Retinal vascular caliber is associated with cardiovascular biomarkers of oxidative stress and inflammation: The POLA study. PLoS ONE 2013, 8, e71089. [Google Scholar] [CrossRef] [PubMed]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 2015, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Tachon, G.; Harrois, A.; Tanaka, S.; Kato, H.; Huet, O.; Pottecher, J.; Vicaut, E.; Duranteau, J. Microcirculatory alterations in traumatic hemorrhagic shock. Crit. Care Med. 2014, 42, 1433–1441. [Google Scholar] [CrossRef]
- Vellinga, N.A.; Boerma, E.C.; Koopmans, M.; Donati, A.; Dubin, A.; Shapiro, N.I.; Pearse, R.M.; Machado, F.R.; Fries, M.; Akarsu-Ayazoglu, T.; et al. International study on microcirculatory shock occurrence in acutely ill patients. Crit. Care Med. 2015, 43, 48–56. [Google Scholar] [CrossRef]
- Francoz, C.; Prie, D.; Abdelrazek, W.; Moreau, R.; Mandot, A.; Belghiti, J.; Valla, D.; Durand, F. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: Impact on the model for end-stage liver disease score. Liver Transpl. 2010, 16, 1169–1177. [Google Scholar] [CrossRef]
- Ferrara, D.; Waheed, N.K.; Duker, J.S. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin. Eye Res. 2016, 52, 130–155. [Google Scholar] [CrossRef]
- Sheikh, M.Y.; Javed, U.; Singh, J.; Choudhury, J.; Deen, O.; Dhah, K.; Peterson, M.W. Bedside sublingual video imaging of microcirculation in assessing bacterial infection in cirrhosis. Dig. Dis. Sci. 2009, 54, 2706–2711. [Google Scholar] [CrossRef]
- Gonzalez Ballerga, E.; Pozo, M.O.; Rubatto Birri, P.N.; Edul, V.S.K.; Sorda, J.A.; Daruich, J.; Dubin, A. Sublingual microcirculatory alterations in cirrhotic patients. Microcirculation 2018, 25, e12448. [Google Scholar] [CrossRef]
- Wythe, S.; Davies, T.W.; O’Beirne, J.; Martin, D.; Gilbert-Kawai, E. Observational study of the microcirculation in patients with liver cirrhosis. JGH Open 2019, 3, 518–524. [Google Scholar] [CrossRef]
| Participant Characteristics | Healthy Volunteer Cohort (n = 50) | Cirrhosis Cohort (n = 49) | CKD Cohort (n = 50) |
|---|---|---|---|
| Demographics | |||
| Age (years) | 50 ± 8 | 58 ± 9 | 53 ± 16 |
| Male sex, n (%) | 28 (56) | 29 (59) | 33 (66) |
| Clinical measurements | |||
| BMI (kg/m2) | 25.5 ± 4.3 | 27.3 ± 5.3 | 26.9 ± 5.0 |
| Systolic blood pressure (mmHg) | 129 ± 14 | 124.9 ± 17 | 134 ± 17 |
| Diastolic blood pressure (mmHg) | 81 ± 9 | 68.8 ± 9 | 78 ± 10 |
| Mean arterial pressure (mmHg) | 95 ± 16 | 87 ± 10 | 96 ± 10 |
| Serum creatinine (μmol/L) | 74 ± 11 | 70 ± 18 | 219 ± 126 |
| CKD stage | |||
| 1 (eGFR ≥ 90 + uPCR > 15) | - | 6 | 2 |
| 2 (eGFR 60–89 + uPCR > 15) | - | 5 | 8 |
| 3 (eGFR 30–59) | - | 2 | 20 |
| 4 (eGFR 15–29) | - | 0 | 13 |
| 5 (eGFR < 15) | - | 0 | 8 |
| Estimated GFR, mL/min/1.73 m2 | 98 ± 13 | 100 ± 24 | 37 ± 23 |
| Pre OLT (n = 55) | Post OLT (n = 12) | p-Value | |
|---|---|---|---|
| mean ± SD | mean ± SD | ||
| vWF (mIU/mL) | 3047 ± 1330 | 1744 ± 637 | 0.006 |
| ET-1 (pg/mL) | 3.9 ± 1.3 | 3.1 ± 0.6 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gifford, F.J.; Moroni, F.; Farrah, T.E.; Hetherington, K.; MacGillivray, T.J.; Hayes, P.C.; Dhaun, N.; Fallowfield, J.A. The Eye as a Non-Invasive Window to the Microcirculation in Liver Cirrhosis: A Prospective Pilot Study. J. Clin. Med. 2020, 9, 3332. https://doi.org/10.3390/jcm9103332
Gifford FJ, Moroni F, Farrah TE, Hetherington K, MacGillivray TJ, Hayes PC, Dhaun N, Fallowfield JA. The Eye as a Non-Invasive Window to the Microcirculation in Liver Cirrhosis: A Prospective Pilot Study. Journal of Clinical Medicine. 2020; 9(10):3332. https://doi.org/10.3390/jcm9103332
Chicago/Turabian StyleGifford, Fiona J, Francesca Moroni, Tariq E Farrah, Kirstie Hetherington, Tom J MacGillivray, Peter C Hayes, Neeraj Dhaun, and Jonathan A Fallowfield. 2020. "The Eye as a Non-Invasive Window to the Microcirculation in Liver Cirrhosis: A Prospective Pilot Study" Journal of Clinical Medicine 9, no. 10: 3332. https://doi.org/10.3390/jcm9103332
APA StyleGifford, F. J., Moroni, F., Farrah, T. E., Hetherington, K., MacGillivray, T. J., Hayes, P. C., Dhaun, N., & Fallowfield, J. A. (2020). The Eye as a Non-Invasive Window to the Microcirculation in Liver Cirrhosis: A Prospective Pilot Study. Journal of Clinical Medicine, 9(10), 3332. https://doi.org/10.3390/jcm9103332






