Diagnostic Power of Galectin-3 in Rheumatic Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Sampling
2.3. Laboratory Assessments
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calabresi, E.; Petrelli, F.; Bonifacio, A.F.; Puxeddu, I.; Alunno, A. One year in review 2018: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 175–184. [Google Scholar] [PubMed]
- Stern, E.P.; Denton, C.P. The pathogenesis of systemic sclerosis. Rheum Dis Clin. North. Am. 2015, 41, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, D.; Elefante, E.; Calabresi, E.; Signorini, V.; Bortoluzzi, A.; Tani, C. One year in review 2019: Systemic lupus erythematosus. Clin. Exp. Rheumatol. 2019, 37, 715–722. [Google Scholar] [PubMed]
- Saroha, A.; Biswas, S.; Chatterjee, B.P.; Das, H.R. Altered glycosylation and expression of plasma alpha-1-acid glycoprotein and haptoglobin in rheumatoid arthritis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1839–1843. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Ohshima, S.; Kuchen, S.; Seemayer, C.A.; Kyburz, D.; Hirt, A.; Klinzing, S.; Michel, B.A.; Gay, R.E.; Liu, F.T.; Gay, S.; et al. Galectin-3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2788–2795. [Google Scholar] [CrossRef]
- Henderson, N.C.; Seith, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef]
- Chen, H.Y.; Liu, F.T.; Yang, R.Y. Roles of galectin-3 in immune responses. Arch. Immunol. Ther. Exp. 2005, 53, 497–504. [Google Scholar]
- Li, L.C.; Li, J.; Gao, J. Functions of galectin-3 and its role in fibrotic diseases. J. Pharm. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef]
- Dhirapong, A.; Lleo, A.; Leung, P.; Gershwin, M.E.; Liu, F.T. The immunological potential of galectin-1 and -3. Autoimmun. Rev. 2009, 8, 360–363. [Google Scholar] [CrossRef]
- Nishi, Y.; Sano, H.; Kawashima, T.; Okada, T.; Kuroda, T.; Kikkawa, K.; Kawashima, S.; Tanabe, M.; Goto, T.; Matsuzawa, Y.; et al. Role of galectin-3 in human pulmonary fibrosis. Allergol. Int. 2007, 56, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. Classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- De Oliveria, F.L.; Gatto, M.; Bassi, N.; Luisetto, R.; Ghirardello, A.; Punzi, L.; Doria, A. Galectin-3 in autoimmunity and autoimmune diseases. Exp. Biol. Med. 2015, 240, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A. Practical experience using galectin-3 in heart failure. Clin. Chem. Lab. Med. 2014, 52, 1425–1431. [Google Scholar] [CrossRef]
- De Boer, R.A.; Yu, L.; Van Veldhuisen, D.J. Galectin-3 in cardiac remodeling and heart failure. Curr. Heart Fail. Rep. 2010, 7, 1–8. [Google Scholar] [CrossRef]
- Hrynchyshyn, N.; Jourdain, P.; Desnos, M.; Diebold, B.; Funck, F. Galectin-3: A new biomarker for the diagnosis, analysis and prognosis of acute and chronic heart failure. Arch. Cardiovasc. Dis. 2013, 106, 541–546. [Google Scholar] [CrossRef]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef]
- Ezzat, M.H.; El-Gammasy, T.M.; Shaheen, K.Y.; Osman, A.O. Elevated production of Gal-3 is correlated with juvenile idiopathic arthritis disease activity, severity, and progression. Int. J. Rheum. Dis. 2011, 14, 345–352. [Google Scholar] [CrossRef]
- Koca, S.S.; Akbas, F.; Ozgen, M.; Yolbas, S.; Ilhan, N.; Gundogdu, B.; Isik, A. Serum Gal-3 level in systemic sclerosis. Clin. Rheumatol. 2014, 33, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.-Y.; Wang, J.; Gao, X.-L.; Hu, Y.-B. Serum galectin-3 concentrations in patients with ankylosing spondylitis. J. Clin. Lab. Anal. 2019, 33, e22914. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, S.W.; Song, J.K.; Park, J.J.; Bae, Y.D.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Serum Gal-3 and Gal-3 binding protein levels in Behçet’s disease and their association with disease activity. Clin. Exp. Rheumatol. 2007, 25, S41–S45. [Google Scholar] [PubMed]
- Lukyanov, P.; Furtak, V.; Ochieng, J. Galectin-3 interacts with membrane lipids and penetrates the lipid bilayer. Biochem. Biophys. Res. Commun. 2005, 338, 1031–1036. [Google Scholar] [CrossRef]
| Age (Years) | Disease Duration | Galectin-3 (ng/mL) | ESR (mm/h) | CRP (mg/L) | PLT (109/L) | HGB (g/dL) | |
|---|---|---|---|---|---|---|---|
| RA n = 82 | 58.5 20–85 | 9 years 2 months–35 years | 18.75 3.8–50.3 p = 0.000 * | 47.5 6–120 p = 0.000 * p = 0.002 # | 6.4 0.3–151.9 p < 0.001 * | 289 24–839 p = 0.020 * | 11.65 7.9–15.2 p < 0.000 * |
| SSc n = 49 | 52 19–77 | 6.5 years 3 months–30 years | 19.4 2.5–64.7 p = 0.000 * | 23 2–100 p = 0.000 * | 2.3 0.2–306.1 p = 0.002 * | 279 193–398 p = 0.008 * | 12.35 8.5–14.8 p < 0.001 * |
| SLE n = 18 | 38 23–70 | 9.7 years 1 year–21 years | 19.2 7.5–117.6 p < 0.001 * | 30 10–100 p = 0.000 * | 3.85 0.2–56.5 p < 0.001 * | 228 97–588 p = 0.822 | 12.3 8.8–13.6 p < 0.001 * |
| Control group n = 30 | 25 21–54 | - | 9.45 7.3–15 | 5.5 4–8 | 0.65 0.3–3.2 | 235 166–386 | 13.7 11.3–15.5 |
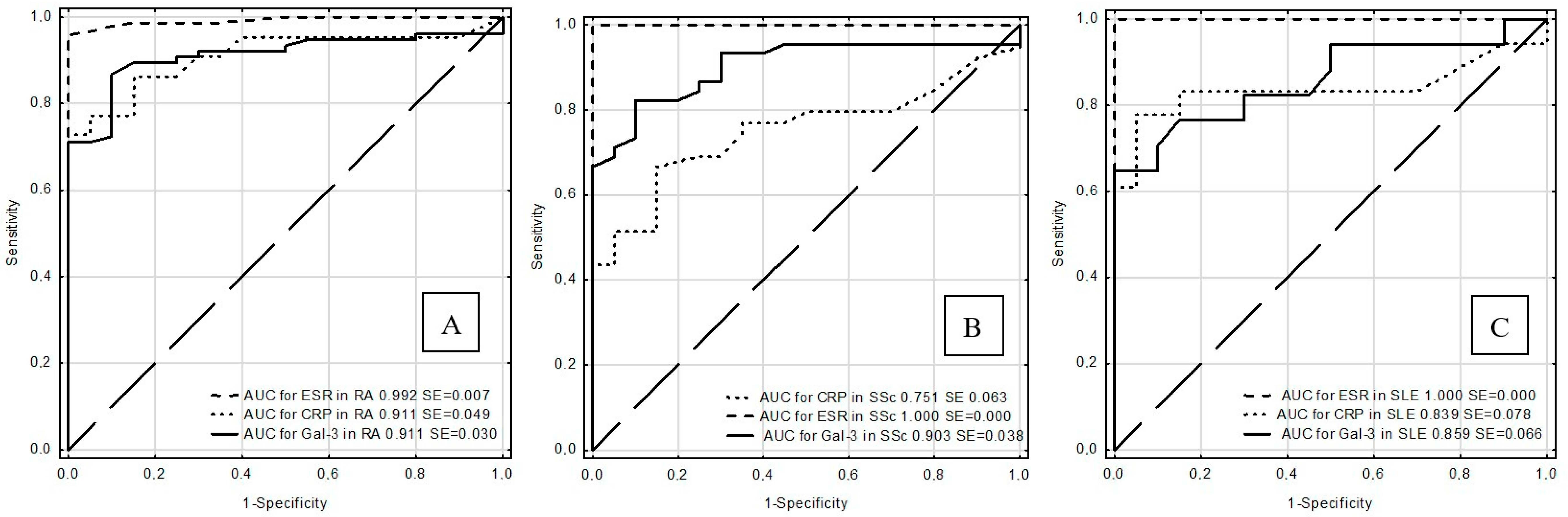
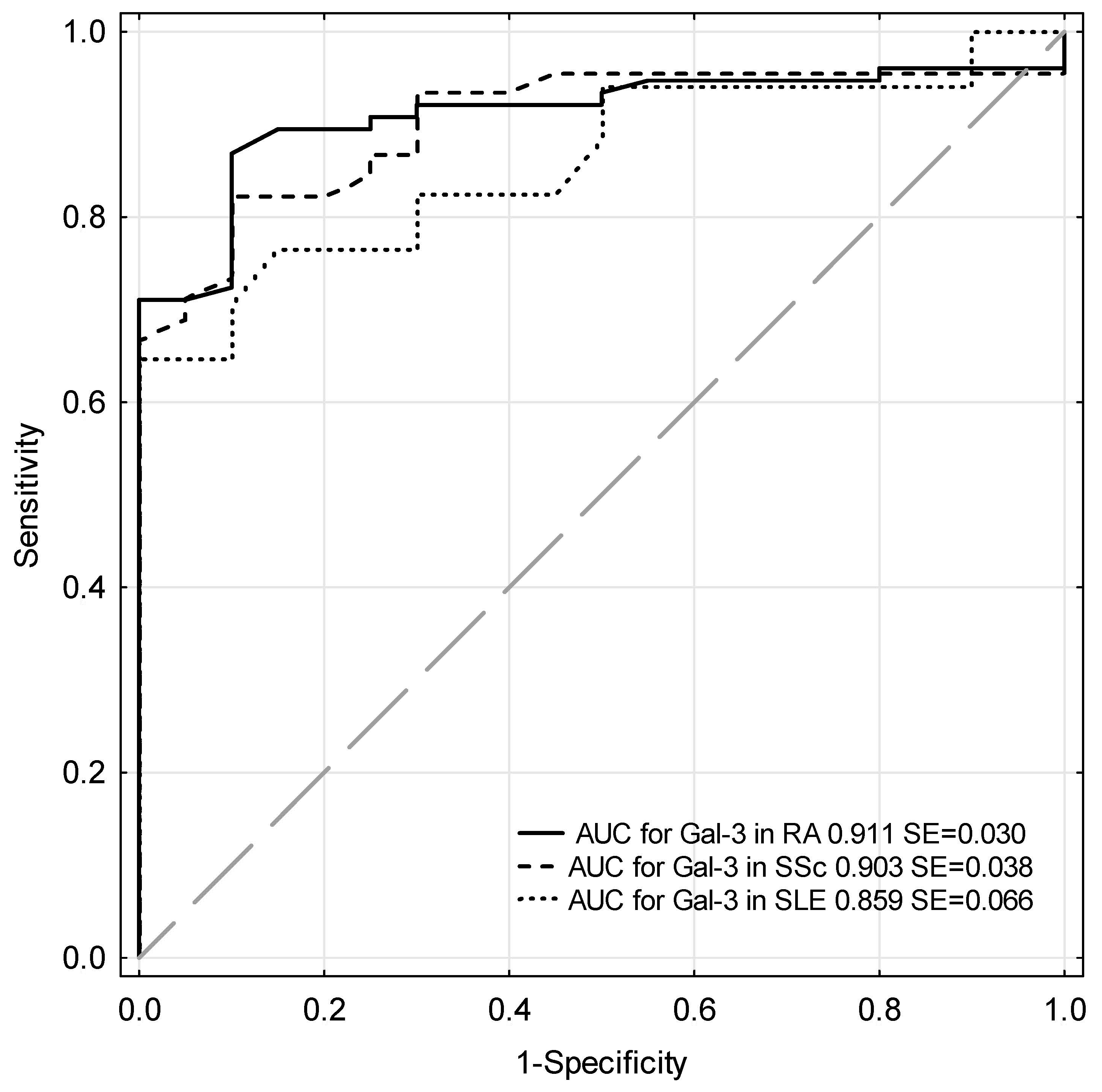
| Rheumatic Disease | Test | Cut-off (ng/mL) | Sensitivity (%) | Specificity (%) | ACC (%) | PPV (%) | NPV (%) | AUC ± SE |
|---|---|---|---|---|---|---|---|---|
| RA | Gal-3 | 15 | 71.1 | 95 | 76 | 98.2 | 46.3 | 0.911 ± 0.030 |
| ESR | 8 | 98.6 | 85 | 95.7 | 95.9 | 94.4 | 0.992 ± 0.007 | |
| CRP | 3.2 | 72.7 | 95 | 83.3 | 94.1 | 76 | 0.911 ± 0.049 | |
| SSc | Gal-3 | 15 | 68.9 | 95 | 76.9 | 96.9 | 57.6 | 0.903 ± 0.038 |
| ESR | 8 | 100 | 85 | 88.9 | 70 | 100 | 1.000 ± 0.000 | |
| CRP | 3.2 | 43.6 | 95 | 61 | 94.4 | 46.3 | 0.751 ± 0.063 | |
| SLE | Gal-3 | 15 | 64.7 | 95 | 81.1 | 91.7 | 76 | 0.859 ± 0.066 |
| ESR | 8 | 100 | 85 | 92.3 | 86.4 | 100 | 1.000 ± 0.000 | |
| CRP | 3.2 | 61.1 | 95 | 78.9 | 91.7 | 73.1 | 0.839 ± 0.078 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszewska, E.; Cylwik, B.; Gińdzieńska-Sieśkiewicz, E.; Kowal-Bielecka, O.; Mroczko, B.; Chrostek, L. Diagnostic Power of Galectin-3 in Rheumatic Diseases. J. Clin. Med. 2020, 9, 3312. https://doi.org/10.3390/jcm9103312
Gruszewska E, Cylwik B, Gińdzieńska-Sieśkiewicz E, Kowal-Bielecka O, Mroczko B, Chrostek L. Diagnostic Power of Galectin-3 in Rheumatic Diseases. Journal of Clinical Medicine. 2020; 9(10):3312. https://doi.org/10.3390/jcm9103312
Chicago/Turabian StyleGruszewska, Ewa, Bogdan Cylwik, Ewa Gińdzieńska-Sieśkiewicz, Otylia Kowal-Bielecka, Barbara Mroczko, and Lech Chrostek. 2020. "Diagnostic Power of Galectin-3 in Rheumatic Diseases" Journal of Clinical Medicine 9, no. 10: 3312. https://doi.org/10.3390/jcm9103312
APA StyleGruszewska, E., Cylwik, B., Gińdzieńska-Sieśkiewicz, E., Kowal-Bielecka, O., Mroczko, B., & Chrostek, L. (2020). Diagnostic Power of Galectin-3 in Rheumatic Diseases. Journal of Clinical Medicine, 9(10), 3312. https://doi.org/10.3390/jcm9103312






