Wedge-Shaped Implants for Minimally Invasive Treatment of Narrow Ridges: A Multicenter Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
- Bone height in the programmed implant site ≥10 mm and bone width comprised between 3.5 and 5 mm (measured 1 mm below the most cranial point of the alveolar crest);
- Healed bone crest (almost three months elapsed after extraction or tooth loss);
- Patient age >18 years;
- Patients able to examine and understand the study protocol.
- General contraindications to implant surgery;
- Immunosuppressed or immunocompromised;
- Irradiated in head and neck area within the past five years;
- Treated or under treatment with intravenous antiresorptives;
- Uncontrolled diabetes (glycated hemoglobin ≥7.5%);
- Pregnant or lactating;
- Poor oral hygiene or motivation (full mouth plaque score >30% and full mouth bleeding score >20%);
- Untreated periodontitis;
- Alcohol or drugs abuse;
- Psychiatric problems or unrealistic expectations;
- Participating in other studies, if the present protocol could not be properly followed.
2.2. Surgical and Prosthetic Procedures
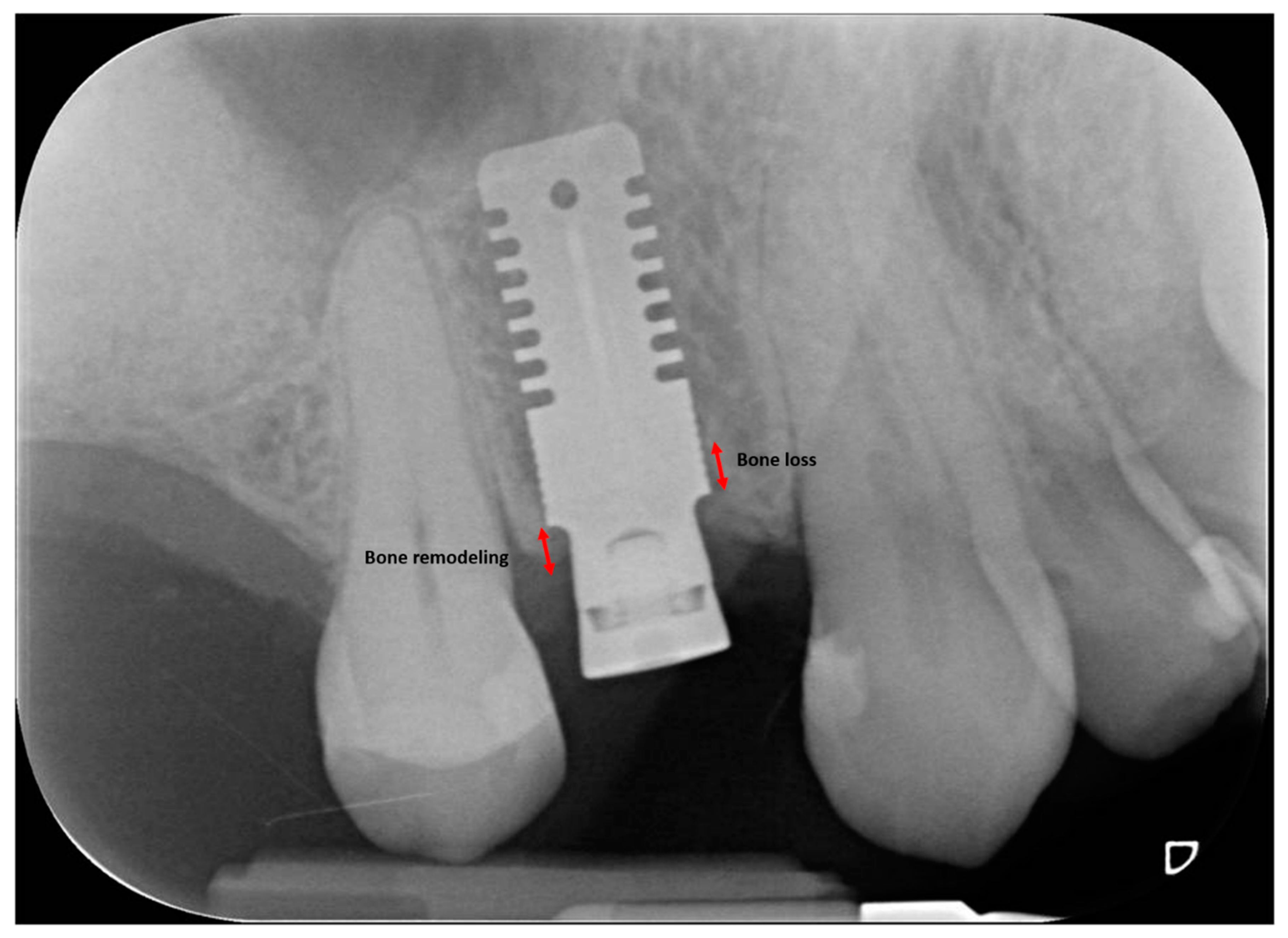
2.3. Radiographic Measurements
2.4. Patient-Centered Outcomes
- Intra-operative discomfort perceived by the patient (VRSdiscomfort): recorded immediately after surgery on a 5-point visual rating scale (VRS) ranging from “0—no discomfort” to “4—very severe discomfort”;
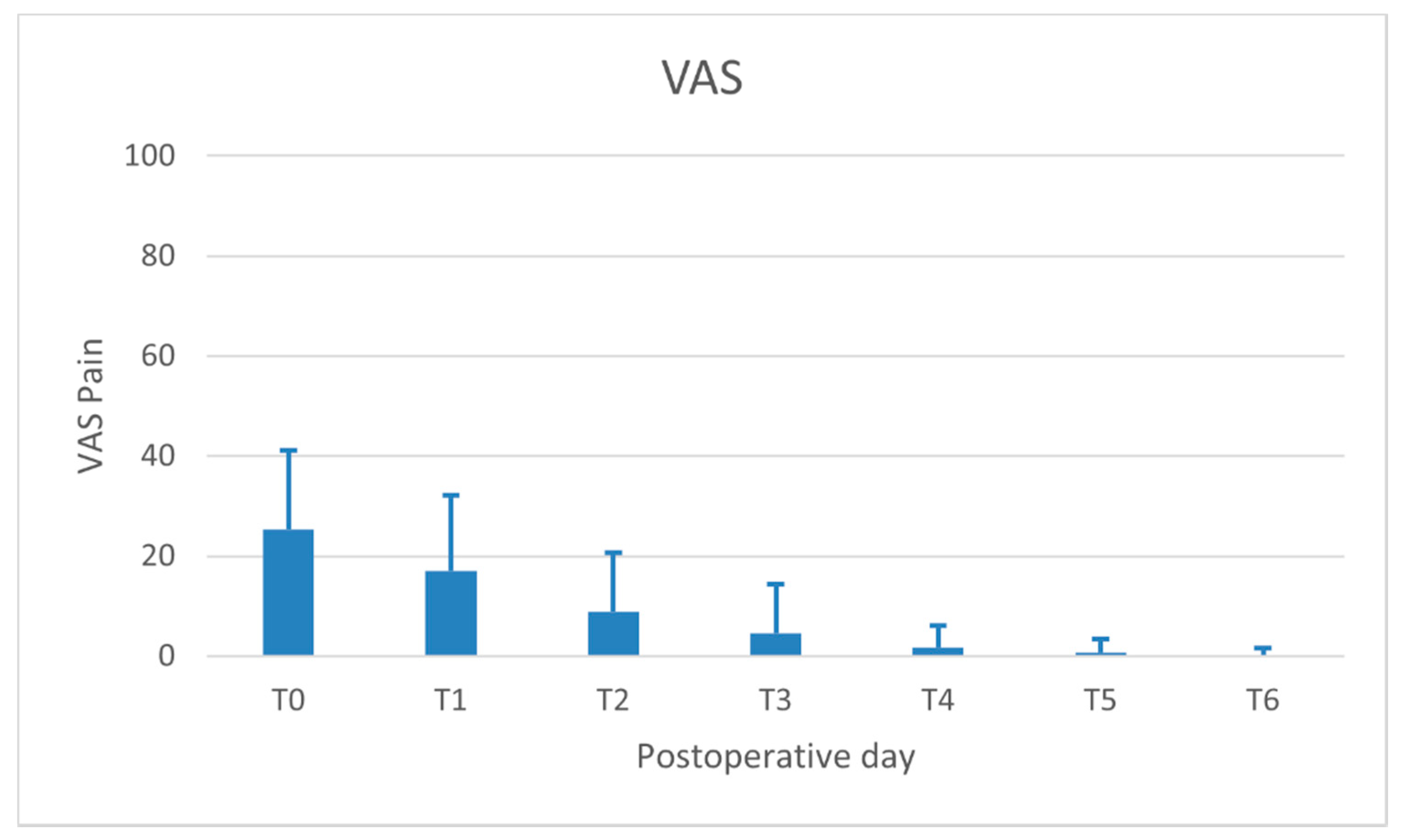
- Pain perceived by the patient (VASpain): recorded daily (evening) for 7 days following surgery on a 100-mm visual analogue scale (VAS) (ranging from “no pain” to “intolerable pain”). The patient was asked to place a line perpendicular to the VAS line at the point representing pain intensity;
- Use of painkillers (i.e., the number of paracetamol 500 mg tablets) assumed by the patient up to the 6th postoperative day.
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Duration of the Surgical Procedure
3.3. Clinical Outcomes
3.4. Patient−Centered Outcomes
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Ortega-Oller, I.; Suárez, F.; Galindo-Moreno, P.; Torrecillas-Martínez, L.; Monje, A.; Catena, A.; Wang, H.L. The influence of implant diameter on its survival: A meta-analysis based on prospective clinical trials. J. Periodontol. 2014, 85, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Schiegnitz, E.; Al-Nawas, B. Narrow-diameter implants: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.K.; Cawood, J.I.; Stoelinga, P.J.; Lowe, D. What is the quality of the evidence base for pre-implant surgery of the atrophic jaw? Int. J. Oral Maxillofac. Surg. 2008, 37, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Rahman, L.; Buser, R.; Janner, S.F.M.; Belser, U.C.; Buser, D. Effectiveness of contour augmentation with guided bone regeneration: 10-year results. J. Dent. Res. 2018, 97, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Stacchi, C.; Visintini, E.; Di Iorio, D.; Putignano, A.; Breschi, L.; Di Lenarda, R. Clinical and histologic evaluation of fresh frozen human bone grafts for horizontal reconstruction of maxillary alveolar ridges. Int. J. Periodontics Restor. Dent. 2011, 31, 535–544. [Google Scholar]
- Starch-Jensen, T.; Becktor, J.P. Maxillary alveolar ridge expansion with split-crest technique compared with lateral ridge augmentation with autogenous bone block graft: A systematic review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef]
- Chappuis, V.; Cavusoglu, Y.; Buser, D.; von Arx, T. Lateral ridge augmentation using autogenous block grafts and guided bone regeneration: A 10-year prospective case series study. Clin. Implant Dent. Relat. Res. 2017, 19, 85–96. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Sanz-Martín, I.; Figuero, E.; Sanz, M. Effectiveness of lateral bone augmentation on the alveolar crest dimension: A systematic review and meta-analysis. J. Dent. Res. 2015, 94, 128S–142S. [Google Scholar] [CrossRef]
- Stacchi, C.; Spinato, S.; Lombardi, T.; Bernardello, F.; Bertoldi, C.; Zaffe, D.; Nevins, M. Minimally invasive management of implant-supported rehabilitation in the posterior maxilla, Part II. Surgical techniques and decision tree. Int. J. Periodontics Restor. Dent. 2020, 40, e95–e102. [Google Scholar] [CrossRef]
- Linkow, L.I. The blade vent—A new dimension in endosseous implantology. Dent. Concepts 1968, 11, 3–12. [Google Scholar] [PubMed]
- Fritz, M.E. Overview of clinical trials on endosseous implants. Ann. Periodontol. 1997, 2, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Noack, N.; Willer, J.; Hoffmann, J. Long-term results after placement of dental implants: Longitudinal study of 1,964 implants over 16 years. Int. J. Oral Maxillofac. Implant. 1999, 14, 748–755. [Google Scholar]
- Diotallevi, P.; Dal Carlo, L.; Pasqualini, M.E.; Mazziotti, S.; Nardone, M.; Moglioni, E. Radiological evaluation of long term complications of oral rehabilitations of thin ridges with titanium blade implants. J. Osseointegr. 2014, 6, 11–14. [Google Scholar]
- Dal Carlo, L.; Pasqualini, M.; Shulman, M.; Rossi, F.; Comola, G.; Manenti, P.; Candotto, V.; Lauritano, D.; Zampetti, P. Endosseous distal extension (EDE) blade implant technique useful to provide stable pillars in the ipotrophic lower posterior sector: 22 years statistical survey. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838092. [Google Scholar] [CrossRef]
- Food and Drug Administration, H.H.S. Dental devices; reclassification of blade-form endosseous dental implant. Final order. Fed. Regist. 2014, 79, 34623–34625. [Google Scholar]
- Koike, M.; Greer, P.; Owen, K.; Lilly, G.; Murr, L.E.; Gaytan, S.M.; Martinez, E.; Okabe, T. Evaluation of titanium alloys fabricated using rapid prototyping technologies-electron beam melting and laser beam melting. Materials (Basel) 2011, 4, 1776–1792. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Linkevicius, T.; Puisys, A.; Linkevicius, R.; Alkimavicius, J.; Gineviciute, E.; Linkeviciene, L. The influence of submerged healing abutment or subcrestal implant placement on soft tissue thickness and crestal bone stability. A 2-year randomized clinical trial. Clin. Implant Dent. Relat. Res. 2020, 22, 497–506. [Google Scholar] [CrossRef]
- Gomez-Roman, G.; Launer, S. Peri-implant bone changes in immediate and non-immediate root-analog stepped implants-a matched comparative prospective study up to 10 years. Int. J. Implant Dent. 2016, 2, 15. [Google Scholar] [CrossRef]
- Spray, J.R.; Black, C.G.; Morris, H.F.; Ochi, S. The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Ann. Periodontol. 2000, 5, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Darby, I.B.; Reynolds, E.C. A prospective clinical study of non-submerged immediate implants: Clinical outcomes and esthetic results. Clin. Oral Implants Res. 2007, 18, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Nohra, J.; Dagher, M.; Matni, G.; Mokbel, N.; Jobaili, E.; Naaman, N. Effect of primary stability and soft- and hard-tissue thickness on marginal bone loss: A prospective pilot study. Implant Dent. 2018, 27, 542–546. [Google Scholar] [CrossRef]
- Pramstraller, M.; Farina, R.; Franceschetti, G.; Pramstraller, C.; Trombelli, L. Ridge dimensions of the edentulous posterior maxilla: A retrospective analysis of a cohort of 127 patients using computerized tomography data. Clin. Oral Implant. Res. 2011, 22, 54–61. [Google Scholar] [CrossRef]
- Katsoulis, J.; Enkling, N.; Takeichi, T.; Urban, I.A.; Mericske-Stern, R.; Avrampou, M. Relative bone width of the edentulous maxillary ridge. Clinical implications of digital assessment in presurgical implant planning. Clin. Implant Dent. Relat. Res. 2012, 14, e213–e223. [Google Scholar] [CrossRef]
- Braut, V.; Bornstein, M.M.; Kuchler, U.; Buser, D. Bone dimensions in the posterior mandible: A retrospective radiographic study using cone beam computed tomography. Part 2—Analysis of edentulous sites. Int. J. Periodontics Restor. Dent. 2014, 34, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Mardas, N.; Chadha, V. Clinical outcomes of implants following lateral bone augmentation: Systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J. Clin. Periodontol. 2008, 35, 173–202. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, U.; von Arx, T. Horizontal ridge augmentation in conjunction with or prior to implant placement in the anterior maxilla: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Brunski, J.B. Biomechanical factors affecting the bone-dental implant interface. Clin. Mater. 1992, 10, 153–201. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Brunski, J.B.; Moccia, A.F., Jr.; Pollock, S.R.; Korostoff, E.; Trachtenberg, D.I. The influence of functional use of endosseous dental implants on the tissue implant interface: I. Histological aspects. J. Dent. Res. 1979, 58, 1953–1969. [Google Scholar] [CrossRef] [PubMed]
- Lum, L.B.; Beirne, O.R.; Curtis, D.A. Histological evaluation of HA-coated vs. uncoated titanium blade implants in delayed and immediately loaded applications. Int. J. Oral Maxillofac. Implant. 1991, 6, 456–462. [Google Scholar]
- Rashad, A.; Sadr-Eshkevari, P.; Weuster, M.; Schmitz, I.; Prochnow, N.; Maurer, P. Material attrition and bone micromorphology after conventional and ultrasonic implant site preparation. Clin. Oral Implant. Res. 2013, 24, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Berton, F.; Turco, G.; Franco, M.; Navarra, C.O.; Andolsek, F.; Maglione, M.; Di Lenarda, R. Micromorphometric analysis of bone blocks harvested with eight different ultrasonic and sonic devices for osseous surgery. J. Craniomaxillofac. Surg. 2016, 44, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Bassi, F.; Troiano, G.; Rapani, A.; Lombardi, T.; Jokstad, A.; Sennerby, L.; Schierano, G. Piezoelectric bone surgery for implant site preparation compared with conventional drilling techniques: A systematic review, meta-analysis and trial sequential analysis. Int. J. Oral Implantol. 2020, 13, 141–158. [Google Scholar]
- Li, J.; Yin, X.; Huang, L.; Mouraret, S.; Brunski, J.B.; Cordova, L.; Salmon, B.; Helms, J.A. Relationships among bone quality, implant osseointegration, and Wnt signaling. J. Dent. Res. 2017, 96, 822–831. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Östman, P.O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontology 2000 2017, 73, 41–50. [Google Scholar] [CrossRef]
- Spinato, S.; Stacchi, C.; Lombardi, T.; Bernardello, F.; Messina, M.; Zaffe, D. Biological width establishment around dental implants is influenced by abutment height irrespective of vertical mucosal thickness: A cluster randomized controlled trial. Clin. Oral Implant. Res. 2019, 30, 649–659. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; León-Cano, A.; Monje, A.; Ortega-Oller, I.; O’Valle, F.; Catena, A. Abutment height influences the effect of platform switching on peri-implant marginal bone loss. Clin. Oral Implant. Res. 2016, 27, 167–173. [Google Scholar] [CrossRef]
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors influencing early marginal bone loss around dental implants positioned subcrestally: A multicenter prospective clinical study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef]
- Spinato, S.; Stacchi, C.; Lombardi, T.; Bernardello, F.; Messina, M.; Dovigo, S.; Zaffe, D. Influence of abutment height and vertical mucosal thickness on early marginal bone loss around implants. A randomized clinical trial with an 18-month post-loading clinical and radiographic evaluation. Int. J. Oral Implantol. 2020, 13, 279–290. [Google Scholar]
- Aloy-Prósper, A.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M.; Camacho-Alonso, F.; Peñarrocha-Diago, M. Peri-implant hard and soft tissue stability in implants placed simultaneously versus delayed with intraoral block bone grafts in horizontal defects: A retrospective case series study. Int. J. Oral Maxillofac. Implant. 2016, 31, 133–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meloni, S.M.; Jovanovic, S.A.; Urban, I.; Canullo, L.; Pisano, M.; Tallarico, M. Horizontal ridge augmentation using GBR with a native collagen membrane and 1:1 ratio of particulated xenograft and autologous bone: A 1-year prospective clinical study. Clin. Implant Dent. Relat. Res. 2017, 19, 38–45. [Google Scholar] [CrossRef]
- Bruschi, G.B.; Capparé, P.; Bravi, F.; Grande, N.; Gherlone, E.; Gastaldi, G.; Crespi, R. Radiographic evaluation of crestal bone level in split-crest and immediate implant placement: Minimum 5-year follow-up. Int. J. Oral Maxillofac. Implant. 2017, 32, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Strietzel, F.P.; Nowak, M.; Küchler, I.; Friedmann, A. Peri-implant alveolar bone loss with respect to bone quality after use of the osteotome technique: Results of a retrospective study. Clin. Oral Implant. Res. 2002, 13, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; van Staden, R.; Loo, Y.C.; Johnson, N.; Ivanovski, S.; Meredith, N. Influence of bone and dental implant parameters on stress distribution in the mandible: A finite element study. Int. J. Oral Maxillofac. Implant. 2009, 24, 866–876. [Google Scholar]
- Kim, S.; Lee, V.J.; Lee, S.; Moon, H.S.; Chung, M.K. Assessment of pain and anxiety following surgical placement of dental implants. Int. J. Oral Maxillofac. Implant. 2013, 28, 531–535. [Google Scholar] [CrossRef]
- Hashem, A.A.; Claffey, N.M.; O’Connell, B. Pain and anxiety following the placement of dental implants. Int. J. Oral Maxillofac. Implant. 2006, 21, 943–950. [Google Scholar]
- Pereira, G.M.; Cota, L.O.; Lima, R.P.; Costa, F.O. Effect of preemptive analgesia with ibuprofen in the control of postoperative pain in dental implant surgeries: A randomized, triple-blind controlled clinical trial. J. Clin. Exp. Dent. 2020, 12, e71–e78. [Google Scholar] [CrossRef]
- Scarano, A.; Carinci, F.; Lorusso, F.; Festa, F.; Bevilacqua, L.; Santos de Oliveira, P.; Maglione, M. Ultrasonic vs drill implant site preparation: Post-operative pain measurement through vas, swelling and crestal bone remodeling: A randomized clinical study. Materials (Basel) 2018, 11, 2516. [Google Scholar] [CrossRef]
- Pistilli, R.; Felice, P.; Piattelli, M.; Nisii, A.; Barausse, C.; Esposito, M. Blocks of autogenous bone versus xenografts for the rehabilitation of atrophic jaws with dental implants: Preliminary data from a pilot randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 153–171. [Google Scholar] [PubMed]
- Payer, M.; Tan, W.C.; Han, J.; Ivanovski, S.; Mattheos, N.; Pjetursson, B.E.; Zhuang, L.; Fokas, G.; Wong, M.C.M.; Acham, S.; et al. on behalf of the International Team for Implantology (ITI) Antibiotic Study Group. The effect of systemic antibiotics on clinical and patient-reported outcome measures of oral implant therapy with simultaneous guided bone regeneration. Clin. Oral Implant. Res. 2020, 31, 442–451. [Google Scholar] [CrossRef] [PubMed]


| Patients/Implants | 44/59 |
|---|---|
| Age (mean ± SD) | 59.5 ± 12.0 |
| Gender | 15 male/29 female |
| Smoking | 31 no smoker/13 smoker |
| History of Periodontitis | 25 no/19 yes |
| Implant Location | 14 maxilla/45 mandible |
| Implant Length | 9 mm (19)/11 mm (40) |
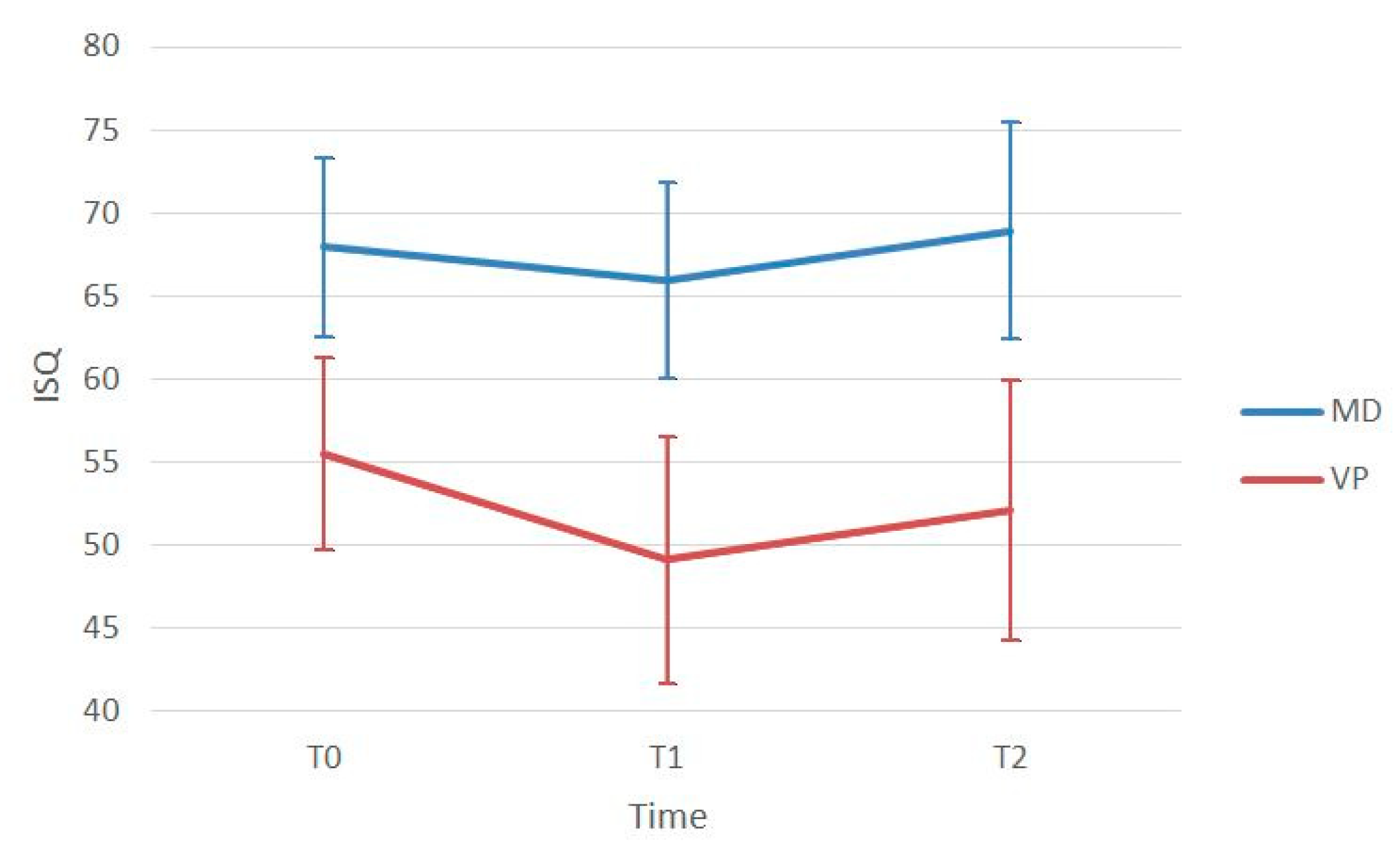
| ISQ T0 | ISQ T1 | T1-T0 (p-Value) | ISQ T2 | T2-T0 (p-Value) | T2-T1 (p-Value) | |
|---|---|---|---|---|---|---|
| Mesio-Distal | 67.96 ± 5.41 | 65.93 ± 5.71 | 0.007 * | 68.93 ± 6.51 | 0.727 | <0.001 * |
| Bucco-Lingual | 55.48 ± 5.79 | 49.12 ± 7.47 | <0.001 * | 52.10 ± 7.79 | 0.004 * | <0.001 * |
| p-value | <0.001 * | <0.001 * | < 0.001 * |
| Mesial | p-Value | Distal | p-Value | Mean | p-Value | |
|---|---|---|---|---|---|---|
| MBL T1-T0 | 0.38 ± 0.46 | <0.001 * | 0.40 ± 0.58 | <0.001 * | 0.38 ± 0.48 | <0.001 * |
| MBL T2-T0 | 0.43 ± 0.57 | <0.001 * | 0.60 ± 0.74 | <0.001 * | 0.60 ± 0.52 | <0.001 * |
| MBL T2-T1 | 0.09 ± 0.22 | <0.001 * | 0.20 ± 0.36 | <0.001 * | 0.20 ± 0.19 | <0.001 * |
| Variable | MBL (T1−T0) Coeff. [95% CI] | MBL (T2−T0) Coeff. [95% CI]–p-Value | MBL (T2−T1) Coeff. [95% CI]–p-Value |
|---|---|---|---|
| Gender | 0.058 [−0.19/0.31] | −0.065 [−0.34/0.21] | −0.110 [−0.21/−0.11] |
| Age | −0.006 [−0.02/0.05] | −0.004 [−0.02/0.08] | 0.002 [−0.003/0.006] |
| Smoking | 0.018 [−0.19/0.23] | 0.032 [−0.18/0.26] | 0.017 [−0.07/0.101] |
| History of Periodontitis | −0.110 [−0.38/0.14] | −0.190 [−0.48/0.09] | −0.042 [−0.15/0.06] |
| Implant Length | −0.013 [−0.14/0.11] | −0.062 [−0.20/0.08] | −0.047 [−0.09/0.002] |
| Implant Location | 0.283 [−0.02/0.57] | 0.322 [−0.009/0.636] * | 0.003 [−0.121/0.113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vercellotti, T.; Troiano, G.; Oreglia, F.; Lombardi, T.; Gregorig, G.; Morella, E.; Rapani, A.; Stacchi, C. Wedge-Shaped Implants for Minimally Invasive Treatment of Narrow Ridges: A Multicenter Prospective Cohort Study. J. Clin. Med. 2020, 9, 3301. https://doi.org/10.3390/jcm9103301
Vercellotti T, Troiano G, Oreglia F, Lombardi T, Gregorig G, Morella E, Rapani A, Stacchi C. Wedge-Shaped Implants for Minimally Invasive Treatment of Narrow Ridges: A Multicenter Prospective Cohort Study. Journal of Clinical Medicine. 2020; 9(10):3301. https://doi.org/10.3390/jcm9103301
Chicago/Turabian StyleVercellotti, Tomaso, Giuseppe Troiano, Francesco Oreglia, Teresa Lombardi, Gianluca Gregorig, Emanuele Morella, Antonio Rapani, and Claudio Stacchi. 2020. "Wedge-Shaped Implants for Minimally Invasive Treatment of Narrow Ridges: A Multicenter Prospective Cohort Study" Journal of Clinical Medicine 9, no. 10: 3301. https://doi.org/10.3390/jcm9103301
APA StyleVercellotti, T., Troiano, G., Oreglia, F., Lombardi, T., Gregorig, G., Morella, E., Rapani, A., & Stacchi, C. (2020). Wedge-Shaped Implants for Minimally Invasive Treatment of Narrow Ridges: A Multicenter Prospective Cohort Study. Journal of Clinical Medicine, 9(10), 3301. https://doi.org/10.3390/jcm9103301









