Neuropelveology: An Emerging Discipline for the Management of Pelvic Neuropathies and Bladder Dysfunctions through to Spinal Cord Injury, Anti-Ageing and the Mars Mission
Abstract
1. Introduction
- The treatment of pelvic organ dysfunctions, in particular the stimulation of the genital nerves (genital nerves stimulation (GNS) therapy).
- The technique of laparoscopic implantation of neuroprothesis to the pelvic nerves (LION procedure) for the recovery of the loss of functions in people with spinal cord injuries.
- The stimulation of the pelvic autonomic nervous system for the prevention and/or treatment of general medical conditions such as osteoporosis, some cardio-vascular disease or control of sarcopenia (process of ageing).
2. Neuropelveology for the Management of Chronic Neuropathic Pelvic Pain
- (1)
- Determination of the nerve pathways involved in the relay of pain information to the brain.
- (2)
- Determination of the location of the neurological irritation/injury (troncular vs. radicular vs. spinal vs. cerebral location).
- (3)
- Determination of the type of nerve(s) lesion: irritation vs. injury (neurogenic neuropathy).
- (4)
- Neurological confirmation of the suspected diagnosis by clinical examination with in particular the transvaginal or transrectal palpation of the pelvic somatic nerves with the reproduction of the trigger pain and Tinel’s sign (eventually with selective anesthetic nerve(s) blockade).
- (5)
- Determination of a potential etiology based on patient history and diagnostic imaging.
- (6)
- Corresponding etiology-adapted therapy.
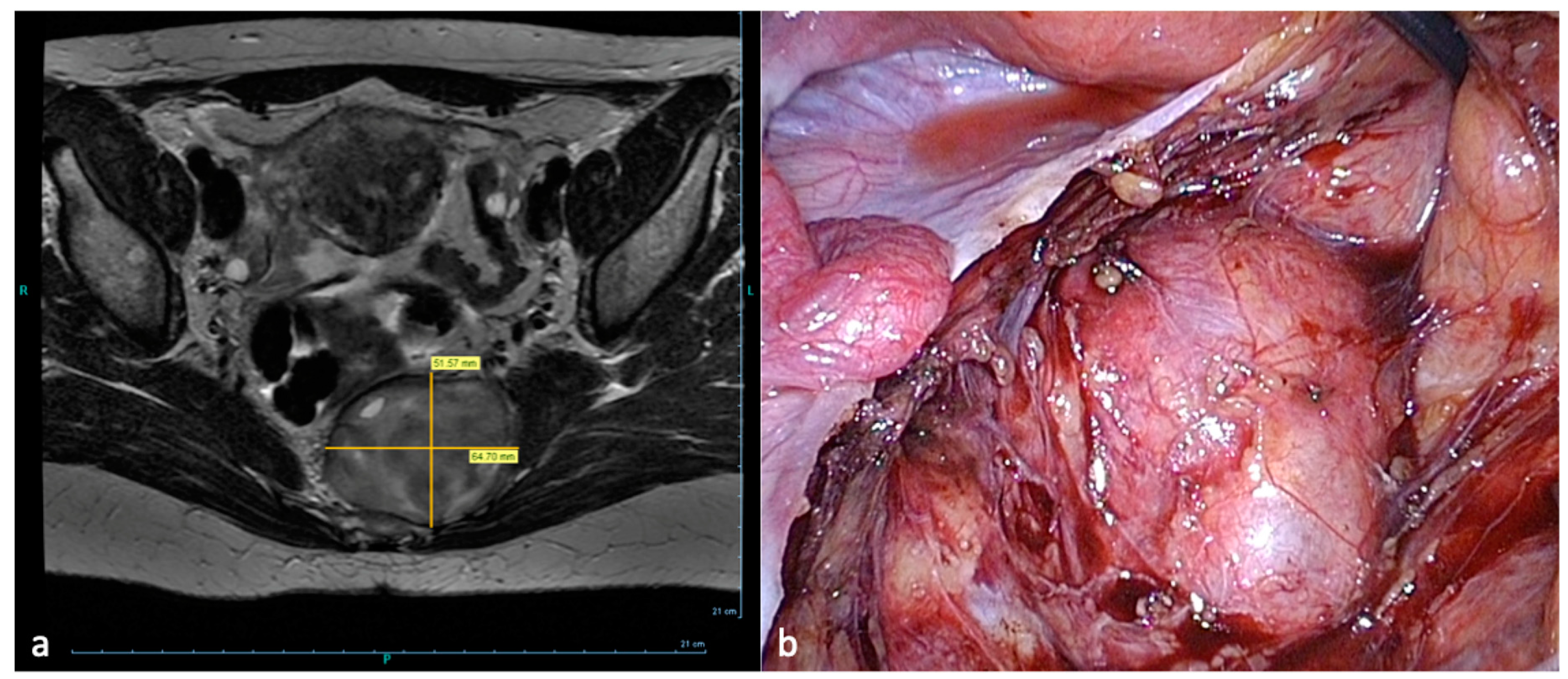
- Compression of the sacral plexus by hypertrophy or atypical insertion of the piriform muscle.
3. Genital Nerves Stimulation (GNS) Therapy
4. LION Procedures
5. Future Visions in Neuropelveology: The “in-Body-ENS”
Supplementary Materials
Funding
Conflicts of Interest
References
- Possover, M. Laparoscopic exposure and electrostimulation of the somatic and autonomous pelvic nerves: A new method for implantation of neuroprothesis in paralysed patients? J. Gynecol. Surg. Endosc. Imaging Allied Tech. 2004, 1, 87–90. [Google Scholar] [CrossRef]
- Possover, M.; Rhiem, K.; Chiantera, V. The “Laparoscopic Neuro-Navigation”—LANN: From functional cartography of the pelvic autonomous neurosystem to a new field of laparoscopic surgery. Min. Invasive Ther. Allied Technol. 2004, 13, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Possover, M.; Quakernack, J.; Chiantera, V. The “LANN-technique” to reduce the postoperative functional mobidity in laparoscopic radical pelvic surgery. J. Am. Coll. Surg. 2005, 6, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Possover, M.; Chiantera, V.; Baekerlandt, J. Anatomy of the sacral roots and the pelvic splanchnic nerves in women using the LANN technique. Surg. Lap. Endosc. Percutan. Tech. 2007, 17, 508–510. [Google Scholar] [CrossRef]
- Possover, M. The neuropelveology: A new speciality in medicine. Pelviperineology 2010, 29, 123–124. [Google Scholar]
- Possover, M.; Forman, A.; Rabischong, B.; Lemos, N.; Chiantera, V. Neuropelveology: New Groundbreaking Discipline in Medicine. J. Minim. Invasive Gynecol. 2015, 22, 1140–1141. [Google Scholar] [CrossRef]
- Possover, M.; Andersson, K.E.; Forman, A. Neuropelveology: An Emerging Discipline for the Management of Chronic Pelvic Pain. Int. Neurourol. J. 2017, 21, 243–246. [Google Scholar] [CrossRef]
- Possover, M.; Forman, A. Neuropelveological assessment of pelvic neuropathic pain. Gyn. Surg. 2014, 11, 139–144. [Google Scholar] [CrossRef]
- Possover, M. Laparoscopic therapy for endometriosis and vascular entrapment of sacral plexus. Fertil. Steril. 2011, 95, 756–758. [Google Scholar] [CrossRef]
- Possover, M.; Forman, A. Pelvic Neuralgias by Neuro-Vascular Entrapment: Anatomical Findings in a Series of 97 Consecutive Patients Treated by Laparoscopic Nerve Decompression. Pain Physician 2015, 18, E1139–E1143. [Google Scholar]
- Possover, M.; Khazali, S.; Fazel, A. The May-Thurner Syndrome as cause for Pelvic Somatic Neuropathic Pain: Diagnosis and therapeutic options. J. Gynecol. Obstet. 2021, in press. [Google Scholar]
- Possover, M. Five-Year Follow-Up after Laparoscopic Large Nerve Resection for Deep Infiltrating Sciatic Nerve Endometriosis. J. Minim. Invasive Gynecol. 2017, 24, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Possover, M.; Uehlinger, K.; Ulrich Exner, G. Laparoscopic assisted resection of an ilio-sacral chondrosarcoma: A single case report. Int. J. Surg. Case Rep. 2014, 5, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Possover, M.; Kostov, P. Laparoscopic management of sacral nerve root schwannoma with intractable vulvococcygodynia: Report of three cases and review of literature. J. Minim. Invasive Gynecol. 2013, 20, 394–397. [Google Scholar] [CrossRef]
- Possover, M. Laparoscopic management of neural pelvic pain in women secondary to pelvic surgery. Fertil. Steril. 2009, 91, 2720–2725. [Google Scholar] [CrossRef]
- Possover, M.; Lemos, N. Risks, symptoms, and management of pelvic nerve damage secondary to surgery for pelvic organ prolapse: A report of 95 cases. Int. Urogynecol. J. 2011, 22, 1485–1490. [Google Scholar] [CrossRef]
- Possover, M.; Baekelandt, J.; Chiantera, V. The Laparoscopic Implantation of Neuroprothesis (LION) Procedure to Control Intractable Abdomino-Pelvic Neuralgia. Neuromodulation 2007, 10, 18–23. [Google Scholar] [CrossRef]
- Possover, M. The laparoscopic implantation of neuroprothesis to the sacral plexus for therapy of neurogenic bladder dysfunctions after failure of percutaneous sacral nerve stimulation. Neuromodulation 2010, 13, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Possover, M. The LION procedure to the pelvic nerves for treatment of urinary and fecal disorders. Surg. Technol. Int. 2014, 24, 225–230. [Google Scholar]
- Possover, M. A new technique of laparoscopic implantation of stimulation electrode to the pudendal nerve for treatment of refractory fecal incontinence and/or overactive bladder with urinary incontinence. J. Minim. Invasive Gynecol. 2014, 21, 729. [Google Scholar] [CrossRef]
- Goldman, H.B.; Amundsen, C.L.; Mangel, J.; Grill, J.; Bennett, M.; Gustafson, K.J.; Grill, W.M. Dorsal genital nerve stimulation for the treatment of overactive bladder symptoms. Neurourol. Urodyn. 2008, 27, 499. [Google Scholar] [CrossRef]
- Farag, F.; Martens, F.M.J.; Rijkhoff, N.J.M.; Heesakkers, J.P.F.A. Dorsal genital nerve stimulation in patients with detrusor overactivity: A systematic review. Curr. Urol. Rep. 2012, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Fowler, C.; Mundy, A.; Craggs, M. Measuring the sensations of urge and bladder filling during cystometry in urge incontinence and the effects of neuromodulation. Neurourol. Urodyn. 2003, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Worsøe, J.; Fynne, L.; Laurberg, S.; Krogh, K.; Rijkhoff, N.J.M. Electrical stimulation of the dorsal clitoral nerve reduces incontinence episodes in idiopathic fecal incontinent patients: A pilot study. Colorectal Dis. 2012, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Aubin, A.; Rogez, J.M.; Douard, R.; Delmans, V. Le nerf dorsal du clitoris: De l’ anatomie a la chirurgie reconstructive du clitoris. Morphologie 2014, 98, 8. [Google Scholar] [CrossRef]
- Possover, M. The LION Procedure to the Pelvic Nerves for Recovery of Locomotion in 18 Spinal Cord Injured Peoples—A Case Series. Surg. Technol. Int. 2016, 29, 19–25. [Google Scholar]
- Possover, M. The “sacral LION procedure” for recovery of bladder/rectum/sexual functions in paraplegic patients after explantation of a previous Finetech-Brindley-Controller. J. Minim. Invasive Gynecol. 2009, 16, 98–101. [Google Scholar] [CrossRef]
- Possover, M.; Schurch, B.; Henle, K.P. New pelvic nerves stimulation strategy for recovery bladder functions and locomotion in complete paraplegics. Neurourol. Urodyn. 2010, 29, 1433–1438. [Google Scholar] [CrossRef]
- Possover, M.; Forman, A. Recovery of supraspinal control of leg movement in a chronic complete flaccid paraplegic man after continuous low-frequency pelvic nerve stimulation and FES-assisted training. Spinal Cord Ser. Cases 2017, 3, 16034. [Google Scholar] [CrossRef][Green Version]
- Possover, M. 10 years’ experience with continuous low-frequency pelvic somatic nerve stimulation for recovery of voluntary walking motion in some patients with chronic spinal cord injuries: A prospective case study of 29 patients. Arch. Phys. Med. Rehabil. 2020, in press. [Google Scholar]
- Figoni, S.F. Exercise responses and quadriplegia. Med. Sci. Sports Exerc. 1993, 25, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Scremin, A.E.; Kurta, L.; Gentili, A.; Wiseman, B.; Perell, K.; Kunkel, C.; Scremin, O.U. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch. Phys. Med. Rehabil. 1999, 80, 1531–1536. [Google Scholar] [CrossRef]
- Alfieri, V. Electrical treatment of spasticity: Reflex tonic activity in hemiplegic patients and selected specific electrostimulation. Scand. J. Rehabil. Med. 1982, 14, 177–182. [Google Scholar] [PubMed]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Levasseur, R.; Sabatier, J.P.; Potrel-Burgot, C.; Lecoq, B.; Creveuil, C.; Marcelli, C. Sympathetic nervous system as transmitter of mechanical loading in bone. Jt. Bone Spine 2003, 70, 515–519. [Google Scholar] [CrossRef]
- Rettori, R.; Planchon, M.; Porte, F.; Ghnassia, M.D. Results of epidural electrical stimulation of the spinal cord in 12 cases of arteritis of the legs. J. Mal. Vasc. 1989, 14, 267–268. [Google Scholar]
- Thawer, H.A.; Houghton, P.E. Effects of electrical stimulation on the histological properties of wounds in diabetic mice. Wound Repair Regen. 2001, 9, 107–115. [Google Scholar] [CrossRef]
- Junger, M.; Zuder, D.; Steins, A.; Hahn, M.; Klyscz, T. Treatment of venous ulcers with low frequency pulsed current (Dermapulse) effects on cutaneous microcirculation. Hautarzt 1997, 18, 879–903. [Google Scholar]
- Stromberg, B. Effects of electrical currents on wound contraction. Ann. Plast. Surg. 1988, 21, 121–123. [Google Scholar] [CrossRef]
- Mawson, A.; Siddiqui, F.; Connolly, B.; Sharp, C.; Summer, W.; Binndo, J., Jr. Effect of high voltage pulsed galvanic stimulation on sacral transcutaneous oxygen tension levels in the spinal cord injured. Paraplegia 1993, 31, 311–319. [Google Scholar] [CrossRef]
- Taylor, K.; Fish, M.R.; Mendel, F.C.; Burton, H.W. Effect of a single 30 minute treatment of high voltage pulsed current on edema formation in frog limbs. Phys. Ther. 1992, 72, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Lemos, N.; Bichuetti, D.B.; Marques, R.M.; Conde, M.S.; Oliveira, A.S.B.; Girao, M. Laparoscopic implantation of neuromodulators for treating urinary dysfunctions and improving locomotion in multiple sclerosis patients. Int. Urogynecol. J. 2015, 26, 1871–1873. [Google Scholar] [CrossRef] [PubMed]










| Visceral Pain | Somatic Pain |
|---|---|
| Pain Quality: Vague; Poorly Localized in The Entire Lower Abdomen with Radiation Into The Lower Back; Dull in Nature. | Pain Quality: Allodynia; Similar to An Electrical Shock. Very Specific Location; Precise and Clear Pain Description; Lack of Vegetative Symptoms. |
| +Vegetative Symptoms: Malaise/Oppression/Syncope, Fatigue, Irritability, Pupil Dilation, Salivation Inhibition, Tachycardia, Nausea/Vomiting, Pallor, Diaphoresis, Anxiety. | +Caudal Radiation to The Corresponding Dermatome(S) |
| +Pelvic Motor Dysfunction: Pelvic Organ Dysfunctions, Sexual Dysfunction, Locomotion Dysfunction |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Possover, M. Neuropelveology: An Emerging Discipline for the Management of Pelvic Neuropathies and Bladder Dysfunctions through to Spinal Cord Injury, Anti-Ageing and the Mars Mission. J. Clin. Med. 2020, 9, 3285. https://doi.org/10.3390/jcm9103285
Possover M. Neuropelveology: An Emerging Discipline for the Management of Pelvic Neuropathies and Bladder Dysfunctions through to Spinal Cord Injury, Anti-Ageing and the Mars Mission. Journal of Clinical Medicine. 2020; 9(10):3285. https://doi.org/10.3390/jcm9103285
Chicago/Turabian StylePossover, Marc. 2020. "Neuropelveology: An Emerging Discipline for the Management of Pelvic Neuropathies and Bladder Dysfunctions through to Spinal Cord Injury, Anti-Ageing and the Mars Mission" Journal of Clinical Medicine 9, no. 10: 3285. https://doi.org/10.3390/jcm9103285
APA StylePossover, M. (2020). Neuropelveology: An Emerging Discipline for the Management of Pelvic Neuropathies and Bladder Dysfunctions through to Spinal Cord Injury, Anti-Ageing and the Mars Mission. Journal of Clinical Medicine, 9(10), 3285. https://doi.org/10.3390/jcm9103285




