Defining Pre-Clinical Psoriatic Arthritis in an Integrated Dermato-Rheumatology Environment
Abstract
1. Introduction
2. From PsO to PsA in the Dermatology-Rheumatology Combined Clinic
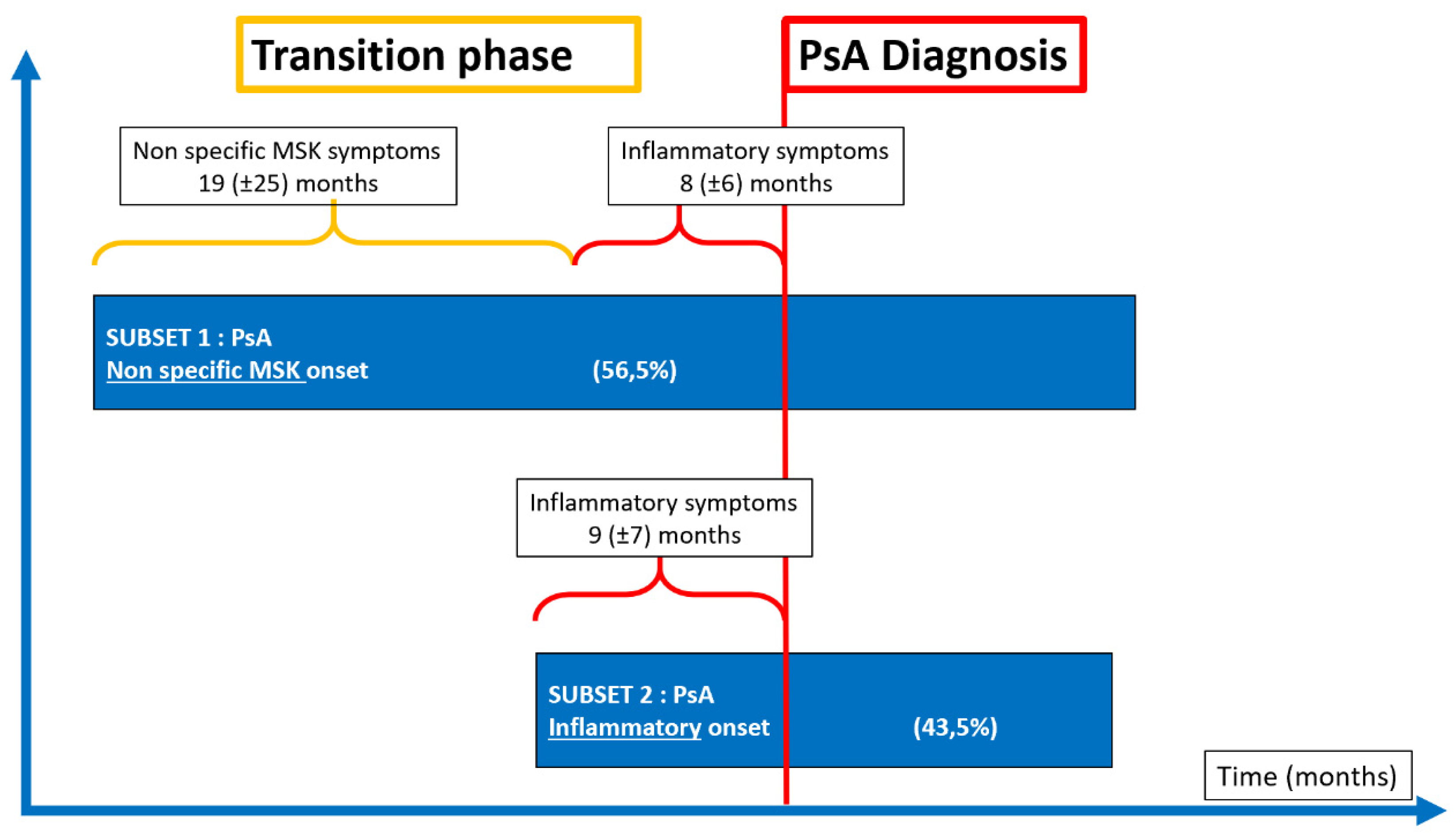
2.1. The Pre-Clinical Phases of Psoriatic Arthritis
2.2. The Need for Early Evaluation and Intervention
2.3. Recognising Psoriatic Arthritis
2.4. The Entheseal Inflammation in Early PsA
2.5. Patterns of Joint Involvement
2.6. Defining Clinical Disease Phenotypes
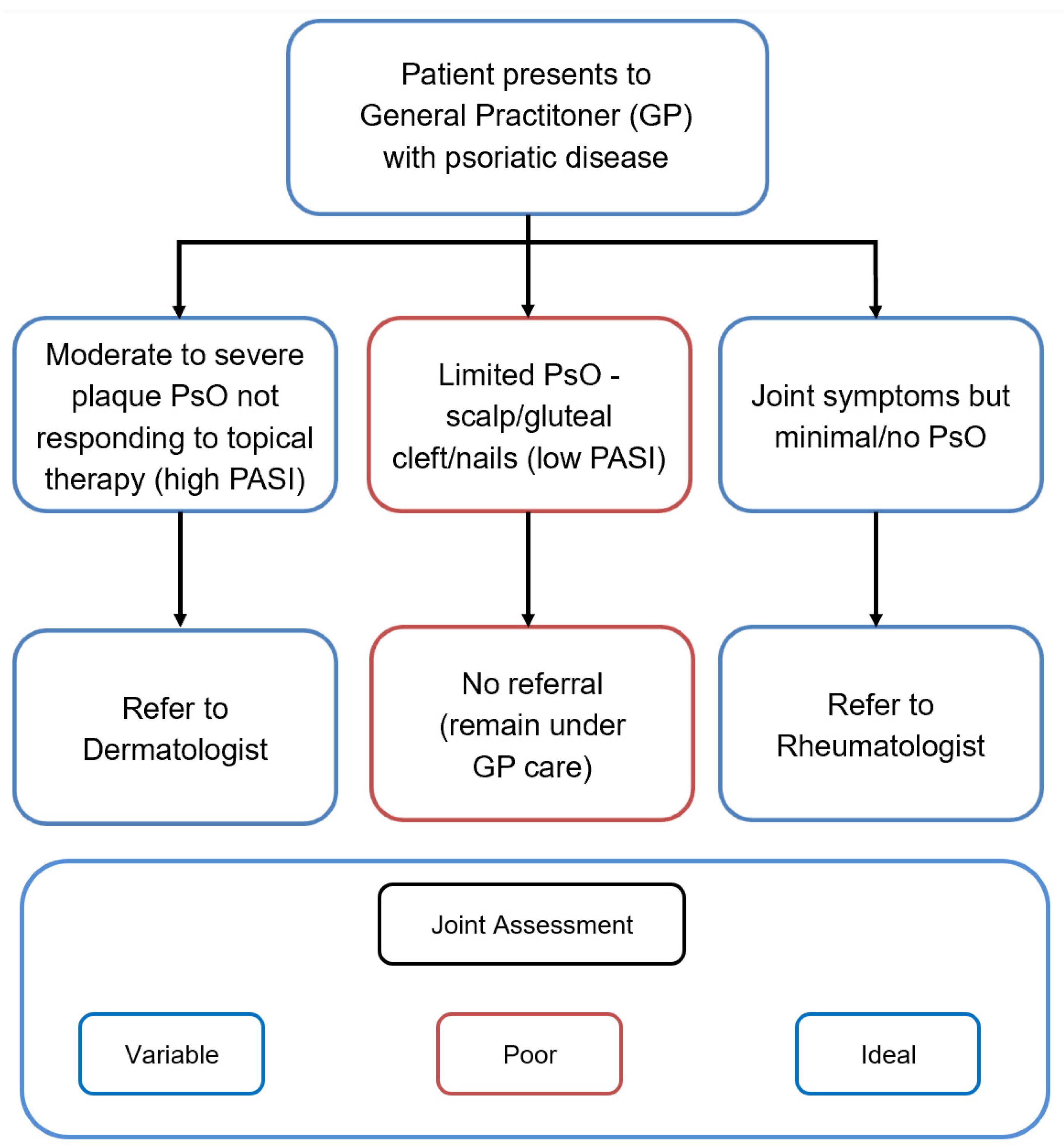
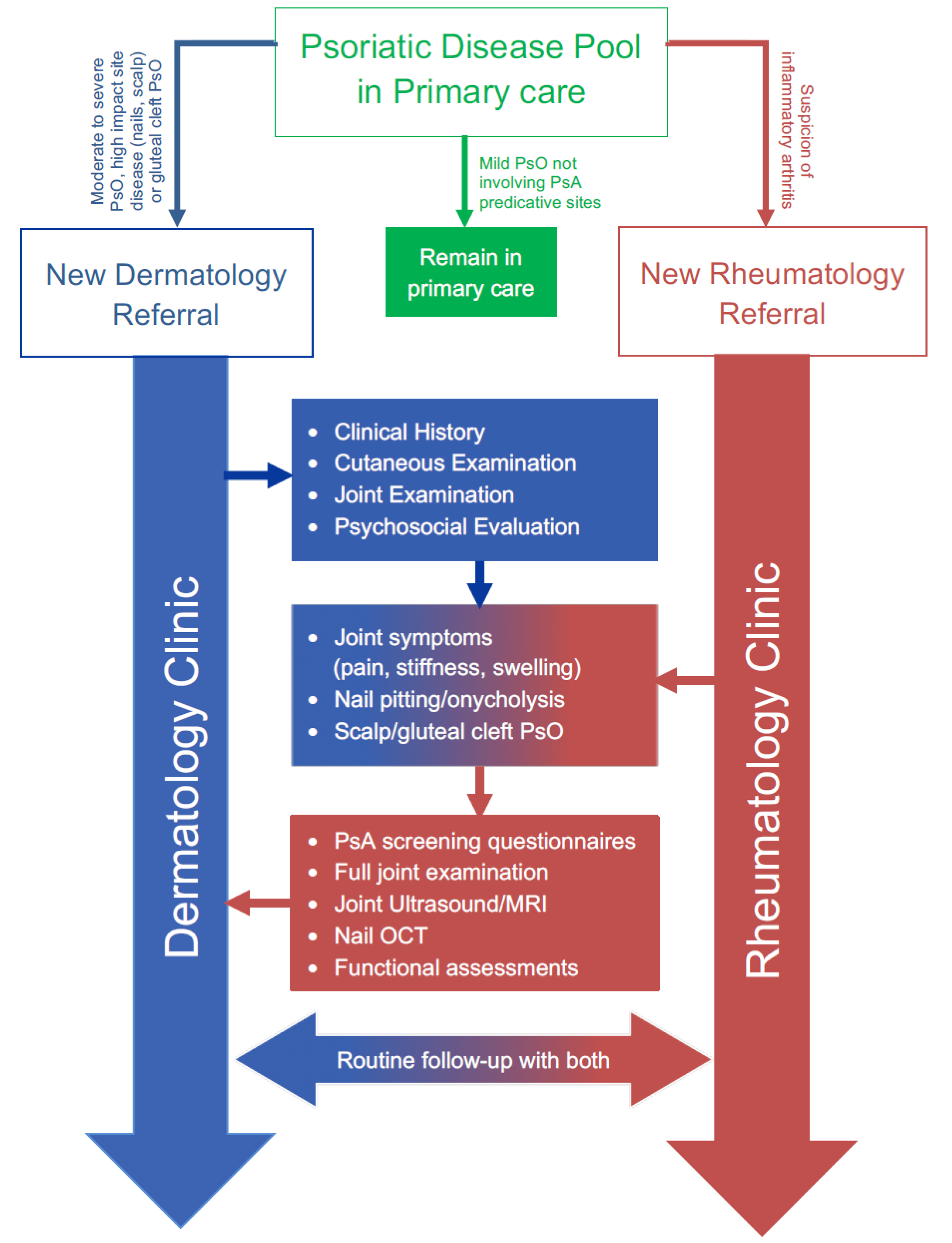
2.7. Combined Dermato-Rheumatology Assessment for Early PsA
2.8. Integrated Care for Psoriatic Disease: Current and Potential Benefits
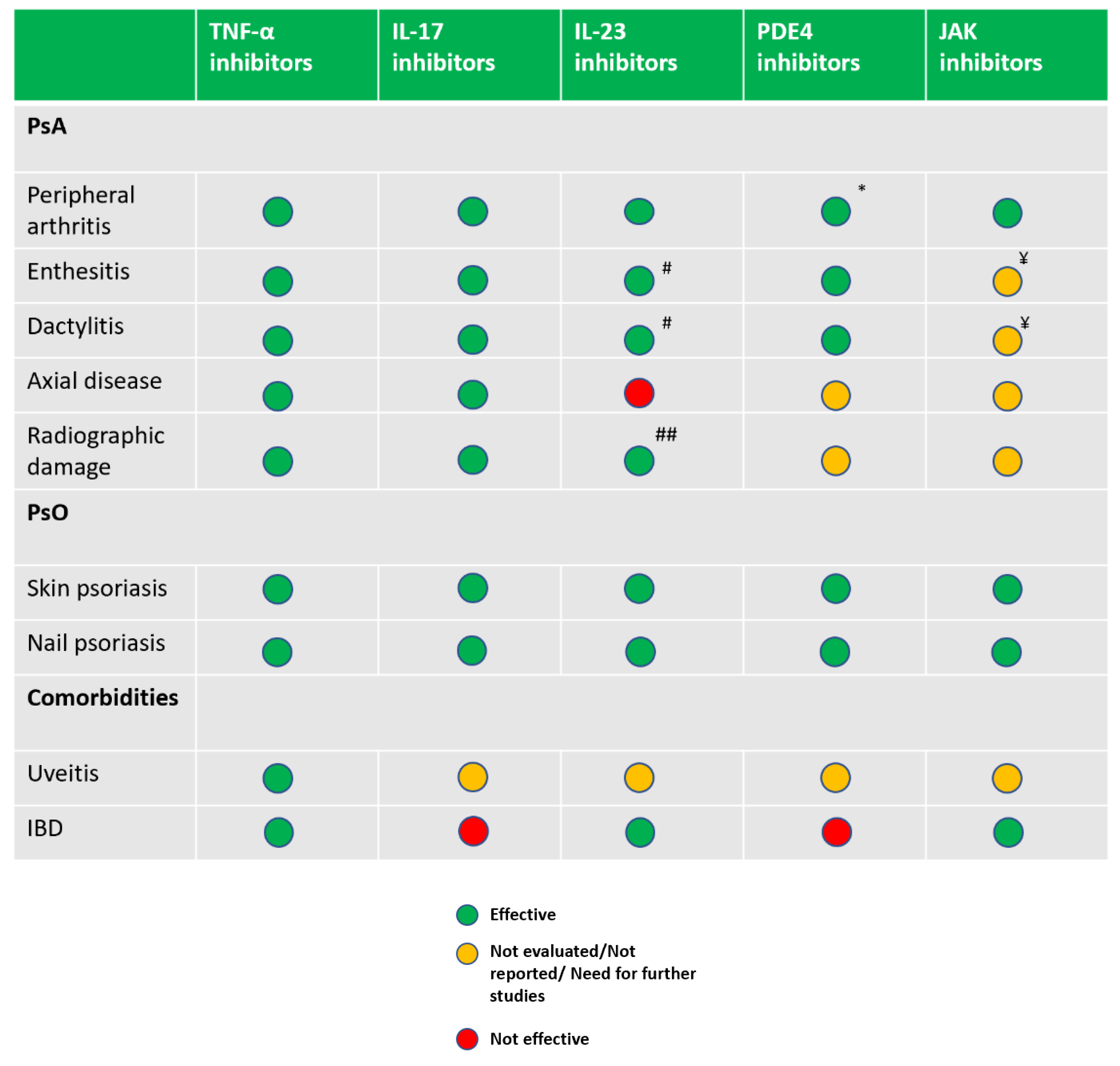
2.9. Therapeutics
3. Conclusions
Funding
Conflicts of Interest
References
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Radtke, M.A.; Reich, K.; Blome, C.; Rustenbach, S.; Augustin, M. Prevalence and clinical features of psoriatic arthritis and joint complaints in 2009 patients with psoriasis: Results of a German national survey. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 683–691. [Google Scholar] [CrossRef]
- Gisondi, P.; Girolomoni, G.; Sampogna, F.; Tabolli, S.; Abeni, D. Prevalence of psoriatic arthritis and joint complaints in a large population of Italian patients hospitalised for psoriasis. Eur. J. Dermatol. 2005, 15, 279–283. [Google Scholar]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii14–ii17. [Google Scholar] [CrossRef]
- Ogdie, A. The preclinical phase of PsA: A challenge for the epidemiologist. Ann. Rheum. Dis. 2017, 76, 1481–1483. [Google Scholar] [CrossRef]
- Ogdie, A.; Nowell, W.B.; Applegate, E.; Gavigan, K.; Venkatachalam, S.; de la Cruz, M.; Flood, E.; Schwartz, E.J.; Romero, B.; Hur, P. Patient perspectives on the pathway to psoriatic arthritis diagnosis: Results from a web-based survey of patients in the United States. BMC Rheumatol. 2020, 4, 2. [Google Scholar] [CrossRef]
- Eder, L.; Haddad, A.; Rosen, C.F.; Lee, K.-A.; Chandran, V.; Cook, R.; Gladman, D.D. The Incidence and Risk Factors for Psoriatic Arthritis in Patients with Psoriasis: A Prospective Cohort Study. Arthritis Rheumatol. (Hoboken N. J.) 2016, 68, 915–923. [Google Scholar] [CrossRef]
- Eder, L.; Polachek, A.; Rosen, C.F.; Chandran, V.; Cook, R.; Gladman, D.D. The Development of Psoriatic Arthritis in Patients with Psoriasis Is Preceded by a Period of Nonspecific Musculoskeletal Symptoms: A Prospective Cohort Study. Arthritis Rheumatol. (Hoboken N. J.) 2017, 69, 622–629. [Google Scholar] [CrossRef]
- Chang, C.A.; Gottlieb, A.B.; Lizzul, P.F. Management of psoriatic arthritis from the view of the dermatologist. Nat. Rev. Rheumatol. 2011, 7, 588–598. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Husni, M.E.; Mody, E. Psoriatic arthritis and psoriasis: Need for a multidisciplinary approach. Semin. Cutan. Med. Surg. 2005, 24, 46–51. [Google Scholar] [CrossRef]
- Gladman, D.D.; Shuckett, R.; Russell, M.L.; Thorne, J.C.; Schachter, R.K. Psoriatic arthritis (PSA)—An analysis of 220 patients. QJM Int. J. Med. 1987, 62, 127–141. [Google Scholar]
- McGonagle, D.; Gibbon, W.; Emery, P. Classification of inflammatory arthritis by enthesitis. Lancet 1998, 352, 1137–1140. [Google Scholar] [CrossRef]
- McGonagle, D.; Lories, R.J.U.; Tan, A.L.; Benjamin, M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007, 56, 2482–2491. [Google Scholar] [CrossRef]
- Zuliani, F.; Zabotti, A.; Errichetti, E.; Tinazzi, I.; Zanetti, A.; Carrara, G.; Quartuccio, L.; Sacco, S.; Giovannini, I.; Stinco, G.; et al. Ultrasonographic detection of subclinical enthesitis and synovitis: A possible stratification of psoriatic patients without clinical musculoskeletal involvement. Clin. Exp. Rheumatol. 2019, 37, 593–599. [Google Scholar] [PubMed]
- Naredo, E.; Moller, I.; de Miguel, E.; Batlle-Gualda, E.; Acebes, C.; Brito, E.; Mayordomo, L.; Moragues, C.; Uson, J.; de Agustin, J.J.; et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: A prospective case-control study. Rheumatology (Oxf. Engl.) 2011, 50, 1838–1848. [Google Scholar] [CrossRef]
- Faustini, F.; Simon, D.; Oliveira, I.; Kleyer, A.; Haschka, J.; Englbrecht, M.; Cavalcante, A.R.; Kraus, S.; Tabosa, T.P.; Figueiredo, C.; et al. Subclinical joint inflammation in patients with psoriasis without concomitant psoriatic arthritis: A cross-sectional and longitudinal analysis. Ann. Rheum. Dis. 2016, 75, 2068–2074. [Google Scholar] [CrossRef]
- Savage, L.; Goodfield, M.; Horton, L.; Watad, A.; Hensor, E.; Emery, P.; Wakefield, R.; Wittmann, M.; McGonagle, D. Regression of Peripheral Subclinical Enthesopathy in Therapy-Naive Patients Treated with Ustekinumab for Moderate-to-Severe Chronic Plaque Psoriasis: A Fifty-Two-Week, Prospective, Open-Label Feasibility Study. Arthritis Rheumatol. 2019, 71, 626–631. [Google Scholar] [CrossRef]
- Zabotti, A.; Tinazzi, I.; Aydin, S.Z.; McGonagle, D. From Psoriasis to Psoriatic Arthritis: Insights from Imaging on the Transition to Psoriatic Arthritis and Implications for Arthritis Prevention. Curr. Rheumatol. Rep. 2020, 22, 24. [Google Scholar] [CrossRef]
- Scher, J.U.; Ogdie, A.; Merola, J.F.; Ritchlin, C. Preventing psoriatic arthritis: Focusing on patients with psoriasis at increased risk of transition. Nat. Rev. Rheumatol. 2019, 15, 153–166. [Google Scholar] [CrossRef]
- Zabotti, A.; McGonagle, D.G.; Giovannini, I.; Errichetti, E.; Zuliani, F.; Zanetti, A.; Tinazzi, I.; De Lucia, O.; Batticciotto, A.; Idolazzi, L.; et al. Transition phase towards psoriatic arthritis: Clinical and ultrasonographic characterisation of psoriatic arthralgia. RMD Open 2019, 5, e001067. [Google Scholar] [CrossRef]
- Marchesoni, A.; De Marco, G.; Merashli, M.; McKenna, F.; Tinazzi, I.; Marzo-Ortega, H.; McGonagle, D.G. The problem in differentiation between psoriatic-related polyenthesitis and fibromyalgia. Rheumatology (Oxf.) 2018, 57, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, P.; Salvarani, C.; Possemato, N.; Gutierrez, M.; Grassi, W.; Gasparini, S.; Perricone, C.; Perrotta, F.M.; Grembiale, R.D.; Bruno, C.; et al. Ultrasonographic and clinical assessment of peripheral enthesitis in patients with psoriatic arthritis, psoriasis, and fibromyalgia syndrome: The ULISSE study. J. Rheumatol. 2019, 46, 904–911. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Hermann, K.-G.A.; Tan, A.L. Differentiation between osteoarthritis and psoriatic arthritis: Implications for pathogenesis and treatment in the biologic therapy era. Rheumatology (Oxf.) 2015, 54, 29–38. [Google Scholar] [CrossRef]
- Tinazzi, I.; McGonagle, D.; Biasi, D.; Confente, S.; Caimmi, C.; Girolomoni, G.; Gisondi, P. Preliminary evidence that subclinical enthesopathy may predict psoriatic arthritis in patients with psoriasis. J. Rheumatol. 2011, 38, 2691–2692. [Google Scholar] [CrossRef]
- Elnady, B.; El Shaarawy, N.K.; Dawoud, N.M.; Elkhouly, T.; Desouky, D.E.-S.; ElShafey, E.N.; El Husseiny, M.S.; Rasker, J.J. Subclinical synovitis and enthesitis in psoriasis patients and controls by ultrasonography in Saudi Arabia; incidence of psoriatic arthritis during two years. Clin. Rheumatol. 2019, 38, 1627–1635. [Google Scholar] [CrossRef]
- Wilson, F.C.; Icen, M.; Crowson, C.S.; McEvoy, M.T.; Gabriel, S.E.; Kremers, H.M. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: A population-based study. Arthritis Rheum. 2009, 61, 233–239. [Google Scholar] [CrossRef]
- Kampylafka, E.; Simon, D.; d’Oliveira, I.; Linz, C.; Lerchen, V.; Englbrecht, M.; Recn, J.; Kleyer, A.; Sticherling, M.; Schett, G.; et al. Disease Interception With interleukin-17 Inhibition in High-Risk Psoriasis Patients With Subclinical Joint Inflammation-Data From the Prospective IVEPSA Study. Available online: https://pubmed.ncbi.nlm.nih.gov/31349876/?from_term=schett+g+and+psoriasis&from_sort=date&from_pos=7 (accessed on 17 April 2020).
- Steenbergen, H.W.; van Aletaha, D.; Voorde, L.J.J.B.; de Brouwer, E.; Codreanu, C.; Combe, B.; Fonseca, J.E.; Hetland, M.L.; Humby, F.; Kvien, T.K.; et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann. Rheumatic Dis. 2017, 76, 491–496. [Google Scholar] [CrossRef]
- Ten Brinck, R.M.; van Steenbergen, H.W.; van Delft, M.A.M.; Verheul, M.K.; Toes, R.E.M.; Trouw, L.A.; van der Helm-van Mil, A.H.M. The risk of individual autoantibodies, autoantibody combinations and levels for arthritis development in clinically suspect arthralgia. Rheumatology (Oxf.) 2017, 56, 2145–2153. [Google Scholar] [CrossRef]
- Zabotti, A.; Mandl, P.; Zampogna, G.; Dejaco, C.; Iagnocco, A. One year in review 2018: Ultrasonography in rheumatoid arthritis and psoriatic arthritis. Clin. Exp. Rheumatol. 2018, 36, 519–525. [Google Scholar]
- Zabotti, A.; Finzel, S.; Baraliakos, X.; Aouad, K.; Ziade, N.; Iagnocco, A. Imaging in the preclinical phases of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 536–542. [Google Scholar]
- Ibrahim, G.H.; Buch, M.H.; Lawson, C.; Waxman, R.; Helliwell, P.S. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: The Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin. Exp. Rheumatol. 2009, 27, 469–474. [Google Scholar] [PubMed]
- Gladman, D.D.; Schentag, C.T.; Tom, B.D.M.; Chandran, V.; Brockbank, J.; Rosen, C.; Farewell, V.T. Development and initial validation of a screening questionnaire for psoriatic arthritis: The Toronto Psoriatic Arthritis Screen (ToPAS). Ann. Rheum. Dis. 2009, 68, 497–501. [Google Scholar] [CrossRef]
- Husni, M.E.; Meyer, K.H.; Cohen, D.S.; Mody, E.; Qureshi, A.A. The PASE questionnaire: Pilot-testing a psoriatic arthritis screening and evaluation tool. J. Am. Acad. Dermatol. 2007, 57, 581–587. [Google Scholar] [CrossRef]
- Tinazzi, I.; Adami, S.; Zanolin, E.M.; Caimmi, C.; Confente, S.; Girolomoni, G.; Gisondi, P.; Biasi, D.; McGonagle, D. The early psoriatic arthritis screening questionnaire: A simple and fast method for the identification of arthritis in patients with psoriasis. Rheumatology (Oxf.) 2012, 51, 2058–2063. [Google Scholar] [CrossRef]
- Villani, A.P.; Rouzaud, M.; Sevrain, M.; Barnetche, T.; Paul, C.; Richard, M.-A.; Beylot-Barry, M.; Misery, L.; Joly, P.; Le Maitre, M.; et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: Systematic review and meta-analysis. J. Am. Acad. Dermatol. 2015, 73, 242–248. [Google Scholar] [CrossRef]
- Moll, J.M.; Wright, V. Psoriatic arthritis. Semin. Arthritis Rheum. 1973, 3, 55–78. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. CASPAR Study Group Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- D’Angelo, S.; Mennillo, G.A.; Cutro, M.S.; Leccese, P.; Nigro, A.; Padula, A.; Olivieri, I. Sensitivity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. J. Rheumatol. 2009, 36, 368–370. [Google Scholar] [CrossRef]
- Buskila, D.; Langevitz, P.; Gladman, D.D.; Urowitz, S.; Smythe, H.A. Patients with rheumatoid arthritis are more tender than those with psoriatic arthritis. J. Rheumatol. 1992, 19, 1115–1119. [Google Scholar]
- Michelena, X.; Poddubnyy, D.; Marzo-Ortega, H. Axial Psoriatic Arthritis: A Distinct Clinical Entity in Search of a Definition. Rheum. Dis. Clin. N. Am. 2020, 46, 327–341. [Google Scholar] [CrossRef]
- Jacques, P.; Lambrecht, S.; Verheugen, E.; Pauwels, E.; Kollias, G.; Armaka, M.; Verhoye, M.; Van der Linden, A.; Achten, R.; Lories, R.J.; et al. Proof of concept: Enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann. Rheum. Dis. 2014, 73, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Milella, M.; Giovanna Belcastro, M.; Zollikofer, C.P.E.; Mariotti, V. The effect of age, sex, and physical activity on entheseal morphology in a contemporary Italian skeletal collection. Am. J. Phys. Anthropol. 2012, 148, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Ash, Z.R.; Tinazzi, I.; Gallego, C.C.; Kwok, C.; Wilson, C.; Goodfield, M.; Gisondi, P.; Tan, A.L.; Marzo-Ortega, H.; Emery, P.; et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann. Rheumatic Dis. 2012, 71, 553–556. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.G.; Helliwell, P.; Veale, D. Enthesitis in psoriatic disease. Dermatology 2012, 225, 100–109. [Google Scholar] [CrossRef]
- Zabotti, A.; Errichetti, E.; Zuliani, F.; Quartuccio, L.; Sacco, S.; Stinco, G.; De Vita, S. Early Psoriatic Arthritis Versus Early Seronegative Rheumatoid Arthritis: Role of Dermoscopy Combined with Ultrasonography for Differential Diagnosis. J. Rheumatol. 2018, 45, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Errichetti, E.; Zabotti, A.; Stinco, G.; Quartuccio, L.; Sacco, S.; De Marchi, G.; Piccirillo, A.; De Vita, S. Dermoscopy of nail fold and elbow in the differential diagnosis of early psoriatic arthritis sine psoriasis and early rheumatoid arthritis. J. Dermatol. 2016, 43, 1217–1220. [Google Scholar] [CrossRef]
- Kridin, K.; Vanetik, S.; Damiani, G.; Cohen, A.D. Big data highlights the association between psoriasis and fibromyalgia: A population-based study. Immunol. Res. 2020, 68, 135–140. [Google Scholar] [CrossRef]
- Marchesoni, A.; De Lucia, O.; Rotunno, L.; De Marco, G.; Manara, M. Entheseal power Doppler ultrasonography: A comparison of psoriatic arthritis and fibromyalgia. J. Rheumatol. Suppl. 2012, 89, 29–31. [Google Scholar] [CrossRef]
- Zabotti, A.; Bandinelli, F.; Batticciotto, A.; Scirè, C.A.; Iagnocco, A.; Sakellariou, G. Musculoskeletal Ultrasound Study Group of the Italian Society of Rheumatology Musculoskeletal ultrasonography for psoriatic arthritis and psoriasis patients: A systematic literature review. Rheumatology (Oxf.) 2017, 56, 1518–1532. [Google Scholar] [CrossRef]
- Raza, N.; Hameed, A.; Ali, M.K. Detection of subclinical joint involvement in psoriasis with bone scintigraphy and its response to oral methotrexate. Clin. Exp. Dermatol. 2008, 33, 70–73. [Google Scholar] [CrossRef]
- Weiner, S.M.; Jurenz, S.; Uhl, M.; Lange-Nolde, A.; Warnatz, K.; Peter, H.H.; Walker, U.A. Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis. Clin. Rheumatol. 2008, 27, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Takahashi, A.; Taniguchi, Y.; Terada, Y.; Sano, S. Detection of asymptomatic enthesitis in psoriasis patients: An onset of psoriatic arthritis? J. Dermatol. 2016, 43, 650–654. [Google Scholar] [CrossRef]
- Schett, G.; Lories, R.J.; D’Agostino, M.-A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.Z.; Ash, Z.R.; Tinazzi, I.; Castillo-Gallego, C.; Kwok, C.; Wilson, C.; Goodfield, M.; Gisondi, P.; Tan, A.L.; Marzo-Ortega, H.; et al. The link between enthesitis and arthritis in psoriatic arthritis: A switch to a vascular phenotype at insertions may play a role in arthritis development. Ann. Rheum. Dis. 2013, 72, 992–995. [Google Scholar] [CrossRef]
- Halin, C.; Fahrngruber, H.; Meingassner, J.G.; Bold, G.; Littlewood-Evans, A.; Stuetz, A.; Detmar, M. Inhibition of chronic and acute skin inflammation by treatment with a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Am. J. Pathol. 2008, 173, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Pandya, N.M.; Dhalla, N.S.; Santani, D.D. Angiogenesis—A new target for future therapy. Vascul. Pharmacol. 2006, 44, 265–274. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Boehncke, S.; Tobin, A.-M.; Kirby, B. The “psoriatic march”: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef]
- McGonagle, D.; Ash, Z.; Dickie, L.; McDermott, M.; Aydin, S.Z. The early phase of psoriatic arthritis. Ann. Rheum. Dis. 2011, 70 (Suppl. 1), i71–i76. [Google Scholar] [CrossRef]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de Wit, M.; McInnes, I.; Dougados, M.; Primdahl, J.; McGonagle, D.G.; Aletaha, D.; Balanescu, A.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar] [CrossRef]
- Singh, J.A.; Guyatt, G.; Ogdie, A.; Gladman, D.D.; Deal, C.; Deodhar, A.; Dubreuil, M.; Dunham, J.; Husni, M.E.; Kenny, S.; et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. (Hoboken N. J.) 2019, 71, 5–32. [Google Scholar] [CrossRef]
- Mease, P.J.; Gladman, D.D.; Collier, D.H.; Ritchlin, C.T.; Helliwell, P.S.; Liu, L.; Kricorian, G.; Chung, J.B. Etanercept and Methotrexate as Monotherapy or in Combination for Psoriatic Arthritis: Primary Results from a Randomized, Controlled Phase III Trial. Arthritis Rheumatol. (Hoboken N. J.) 2019, 71, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, A.; Gensler, L.S.; Sieper, J.; Clark, M.; Calderon, C.; Wang, Y.; Zhou, Y.; Leu, J.H.; Campbell, K.; Sweet, K.; et al. Three Multicenter, Randomized, Double-Blind, Placebo-Controlled Studies Evaluating the Efficacy and Safety of Ustekinumab in Axial Spondyloarthritis. Arthritis Rheumatol. (Hoboken N. J.) 2019, 71, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Østergaard, M.; Wei, J.C.-C.; Sieper, J.; Järvinen, P.; Tam, L.-S.; Salvarani, C.; Kim, T.-H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 2018, 77, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaumer, A.; Smolen, J.S.; Dougados, M.; de Wit, M.; Primdahl, J.; McInnes, I.; van der Heijde, D.; Baraliakos, X.; Falzon, L.; Gossec, L. Pharmacological treatment of psoriatic arthritis: A systematic literature research for the 2019 update of the EULAR recommendations for the management of psoriatic arthritis. Ann. Rheum. Dis. 2020, 79, 778–786. [Google Scholar]
- Mease, P.J.; Kellner, H.; Morita, A.; Kivitz, A.J.; Papp, K.A.; Aslanyan, S.; Berner, B.; Chen, S.; Eldred, A.; Behrens, F. OP0307 Efficacy and safety of risankizumab, a selective il-23p19 inhibitor, in patients with active psoriatic arthritis over 24 weeks: Results from a phase 2 trial. Ann. Rheumatic Dis. 2018, 77, 200–201. [Google Scholar]
- Kavanaugh, A.; Ritchlin, C.; Rahman, P.; Puig, L.; Gottlieb, A.B.; Li, S.; Wang, Y.; Noonan, L.; Brodmerkel, C.; Song, M.; et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: Results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann. Rheum. Dis. 2014, 73, 1000–1006. [Google Scholar]
- Mease, P.J.; Rahman, P.; Gottlieb, A.B.; Kollmeier, A.P.; Hsia, E.C.; Xu, X.L.; Sheng, S.; Agarwal, P.; Zhou, B.; Zhuang, Y.; et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1126–1136. [Google Scholar] [CrossRef]
- Wells, A.F.; Edwards, C.J.; Kivitz, A.J.; Bird, P.; Nguyen, D.; Paris, M.; Teng, L.; Aelion, J.A. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: Results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxf.) 2018, 57, 1253–1263. [Google Scholar] [CrossRef]
- Gladman, D.; Rigby, W.; Azevedo, V.F.; Behrens, F.; Blanco, R.; Kaszuba, A.; Kudlacz, E.; Wang, C.; Menon, S.; Hendrikx, T.; et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N. Engl. J. Med. 2017, 377, 1525–1536. [Google Scholar] [CrossRef]
- Gossec, L.; Smolen, J.S.; Ramiro, S.; de Wit, M.; Cutolo, M.; Dougados, M.; Emery, P.; Landewé, R.; Oliver, S.; Aletaha, D.; et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann. Rheum. Dis. 2016, 75, 499–510. [Google Scholar] [CrossRef]
| Psoriatic Arthritis | Rheumatoid Arthritis | |
|---|---|---|
| Pre-clinical disease | 70% have psoriasis | 70% have ACPA positivity |
| Duration of pre-clinical disease | 7–12 years | Up to 10 years |
| Biomarker/sensitivity | PASI score—no correlation with arthritis severity | ACPA titre—correlates with arthritis severity |
| PsA | RA | Inflammatory OA | ||
|---|---|---|---|---|
| Demographics | Male:female | 1:1 | 3:1 | 1.5:1 |
| Age at onset | 35–45 | 35–50 | 45–55 | |
| Clinical | Skin Lesions | Frequently | Uncommon | Uncommon |
| Nail Lesions | Common | Uncommon | Uncommon | |
| Dactylitis | Common | Uncommon | Uncommon | |
| Enthesitis | Common | Uncommon | Uncommon | |
| DIPJ involvement | Common | Uncommon | Common | |
| Joint erythema | Common | Uncommon | Uncommon | |
| Joint tenderness | Mild | Severe | Mild | |
| Symmetry | Less common | Common | Uncommon | |
| Back involvement | Common | Uncommon | Uncommon | |
| Sacroilitis | 50% | Rare | Uncommon | |
| Serological | Rheumatoid factor | Uncommon | Common | Uncommon |
| ACPA | Uncommon | Common | Uncommon | |
| HLA-B27 | 20–30% | Not related | Not related | |
| Imaging | Periostitis | Common | Rare | Uncommon |
| Pencil-in-cup | Common | Rare | Uncommon | |
| Ankylosis | Common | Rare | Uncommon | |
| Osteopenia | Less common | Common | Uncommon | |
| Morbidity | QoL | Reduced | Reduced | Reduced |
| Function | Reduced | Reduced | Reduced |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savage, L.; Tinazzi, I.; Zabotti, A.; Laws, P.M.; Wittmann, M.; McGonagle, D. Defining Pre-Clinical Psoriatic Arthritis in an Integrated Dermato-Rheumatology Environment. J. Clin. Med. 2020, 9, 3262. https://doi.org/10.3390/jcm9103262
Savage L, Tinazzi I, Zabotti A, Laws PM, Wittmann M, McGonagle D. Defining Pre-Clinical Psoriatic Arthritis in an Integrated Dermato-Rheumatology Environment. Journal of Clinical Medicine. 2020; 9(10):3262. https://doi.org/10.3390/jcm9103262
Chicago/Turabian StyleSavage, Laura, Ilaria Tinazzi, Alen Zabotti, Philip M. Laws, Miriam Wittmann, and Dennis McGonagle. 2020. "Defining Pre-Clinical Psoriatic Arthritis in an Integrated Dermato-Rheumatology Environment" Journal of Clinical Medicine 9, no. 10: 3262. https://doi.org/10.3390/jcm9103262
APA StyleSavage, L., Tinazzi, I., Zabotti, A., Laws, P. M., Wittmann, M., & McGonagle, D. (2020). Defining Pre-Clinical Psoriatic Arthritis in an Integrated Dermato-Rheumatology Environment. Journal of Clinical Medicine, 9(10), 3262. https://doi.org/10.3390/jcm9103262






