Musculoskeletal Ultrasound in Monitoring Clinical Response to Treatment in Acute Symptomatic Psoriatic Dactylitis: Results from a Multicentre Prospective Observational Study
Abstract
1. Introduction
2. Experimental Section
2.1. Clinical Assesment
2.2. Ultrasound Assesment
2.3. Statistical Analysis
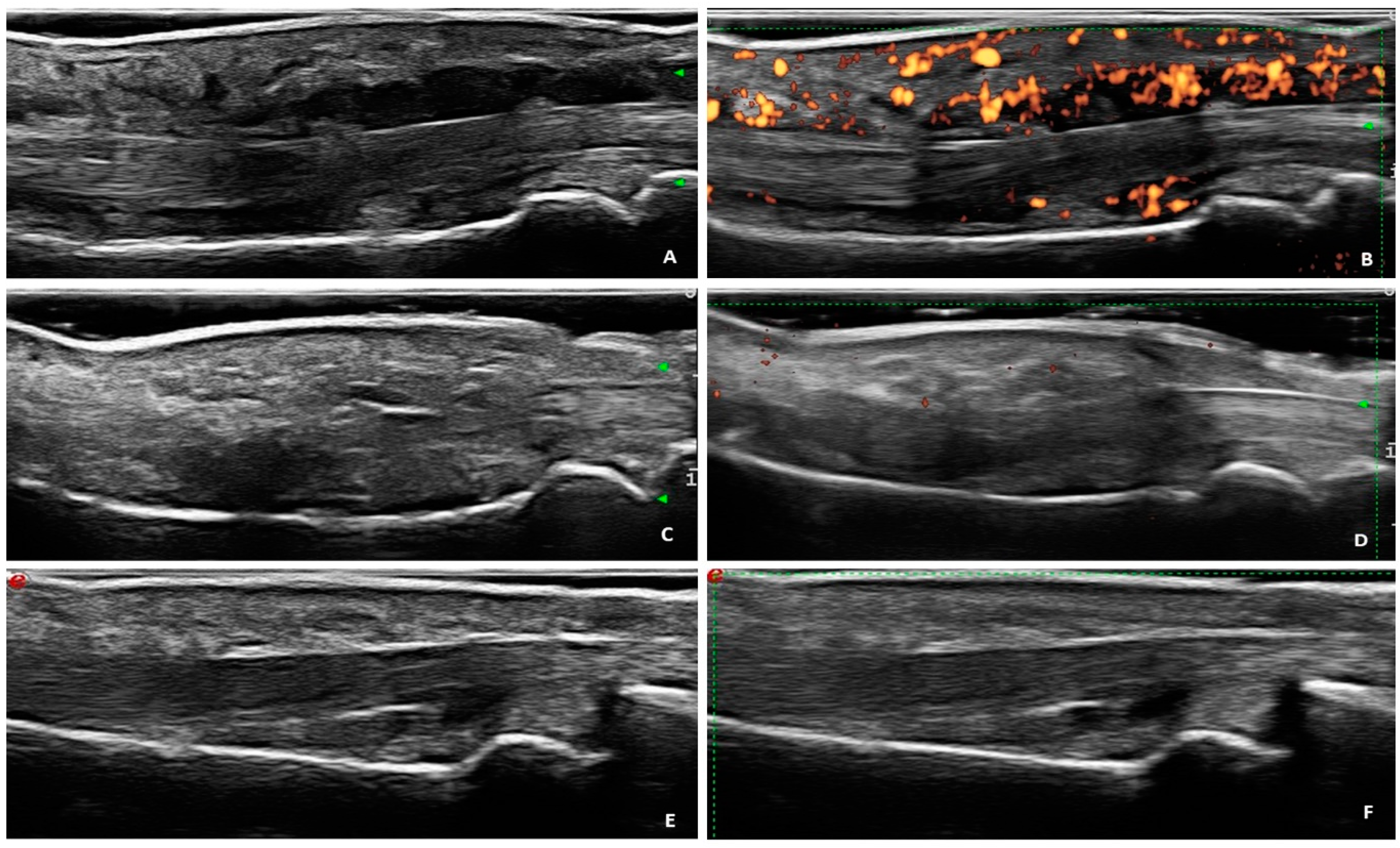
3. Results
3.1. Patients’ Characteristics and Clinical Findings
3.2. Ultrasound Findings
3.3. Correlation between Ultrasound and Clinical Parameters
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Tillett, W.; Costa, L.; Jadon, D.; Wallis, D.; Cavill, C.; McHugh, J.; Korendowych, E.; McHugh, N. The ClASsification for Psoriatic ARthritis (CASPAR) criteria—A retrospective feasibility, sensitivity, and specificity study. J. Rheumatol. 2012, 39, 154–156. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ziouzina, O.; Thavaneswaran, A.; Chandran, V. Dactylitis in psoriatic arthritis: Prevalence and response to therapy in the biologic era. J. Rheumatol. 2013, 40, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Kaeley, G.S.; Eder, L.; Aydin, S.Z.; Gutierrez, M.; Bakewell, C. Dactylitis: A hallmark of psoriatic arthritis. Semin. Arthritis Rheum. 2018, 48, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, I.; Scarano, E.; Padula, A.; Giasi, V. Dactylitis involving most of the fingers. Clin. Exp. Rheumatol. 2003, 21, 406. [Google Scholar] [PubMed]
- Helliwell, P.S.; Firth, J.; Ibrahim, G.H.; Melsom, R.D.; Shah, I.; Turner, D.E. Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J. Rheumatol. 2005, 32, 1745–1750. [Google Scholar] [PubMed]
- Healy, P.J.; Helliwell, P.S. Measuring dactylitis in clinical trials: Which is the best instrument to use? J. Rheumatol. 2007, 34, 1302–1306. [Google Scholar] [PubMed]
- Coates, L.C.; Kavanaugh, A.; Mease, P.J.; Soriano, E.R.; Acosta-Felquer, M.L.; Armstrong, A.W.; Bautista-Molano, W.; Boehncke, W.-H.; Campbell, W.; Cauli, A.; et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016, 68, 1060–1071. [Google Scholar] [CrossRef]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de Wit, M.; McInnes, I.; Dougados, M.; Primdahl, J.; McGonagle, D.G.; Aletaha, D.; Balanescu, A.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar] [CrossRef]
- McGonagle, D.; Tan, A.L.; Watad, A.; Helliwell, P. Pathophysiology, assessment and treatment of psoriatic dactylitis. Nat. Rev. Rheumatol. 2019, 15, 113–122. [Google Scholar] [CrossRef]
- Olivieri, I.; Barozzi, L.; Pierro, A.; De Matteis, M.; Padula, A.; Pavlica, P. Toe dactylitis in patients with spondyloarthropathy: Assessment by magnetic resonance imaging. J. Rheumatol. 1997, 24, 926–930. [Google Scholar] [PubMed]
- Healy, P.J.; Groves, C.; Chandramohan, M.; Helliwell, P.S. MRI changes in psoriatic dactylitis—Extent of pathology, relationship to tenderness and correlation with clinical indices. Rheumatology 2008, 47, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, I.; Scarano, E.; Padula, A.; D’Angelo, S.; Salvarani, C.; Cantini, F.; Niccoli, L.; Barozzi, L. Fast spin echo-T2-weighted sequences with fat saturation in toe dactylitis of spondyloarthritis. Clin. Rheumatol. 2008, 27, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.; Fukuba, E.; Halliday, N.A.; Tanner, S.F.; Emery, P.; McGonagle, D. High-resolution MRI assessment of dactylitis in psoriatic arthritis shows flexor tendon pulley and sheath-related enthesitis. Ann. Rheum. Dis. 2015, 74, 185–189. [Google Scholar] [CrossRef]
- Tinazzi, I.; McGonagle, D.; Aydin, S.Z.; Chessa, D.; Marchetta, A.; Macchioni, P. “Deep Koebner” phenomenon of the flexor tendon-associated accessory pulleys as a novel factor in tenosynovitis and dactylitis in psoriatic arthritis. Ann. Rheum. Dis. 2018, 77, 922–925. [Google Scholar] [CrossRef]
- Tinazzi, I.; McGonagle, D.; Zabotti, A.; Chessa, D.; Marchetta, A.; Macchioni, P. Comprehensive evaluation of finger flexor tendon entheseal soft tissue and bone changes by ultrasound can differentiate psoriatic arthritis and rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 785–790. [Google Scholar]
- Tinazzi, I.; McGonagle, D.; Macchioni, P.; Aydin, S.Z. Power Doppler enhancement of accessory pulleys confirming disease localization in psoriatic dactylitis. Rheumatology 2019, 59, 2030–2034. [Google Scholar] [CrossRef]
- Girolimetto, N.; Costa, L.; Mancarella, L.; Addimanda, O.; Bottiglieri, P.; Santelli, F.; Meliconi, R.; Peluso, R.; Del Puente, A.; Macchioni, P.; et al. Symptomatic psoriatic dactylitis is associated with ultrasound determined extra-synovial inflammatory features and shorter disease duration. Clin. Rheumatol. 2019, 38, 903–911. [Google Scholar] [CrossRef]
- Girolimetto, N.; Macchioni, P.; Tinazzi, I.; Costa, L.; McGonagle, D.; Peluso, R.; Del Puente, A.; Addimanda, O.; Marchetta, A.; Possemato, N.; et al. Ultrasonographic evidence of predominance of acute extracapsular and chronic intrasynovial patterns in 100 cases of psoriatic hand dactylitis. J. Rheumatol. 2020, 47, 227–233. [Google Scholar] [CrossRef]
- Girolimetto, N.; Macchioni, P.; Tinazzi, I.; Costa, L.; Peluso, R.; Tasso, M.; Bascherini, V.; Addimanda, O.; Marchetta, A.; Possemato, N.; et al. Predominant ultrasonographic extracapsular changes in symptomatic psoriatic dactylitis: Results from a multicenter cross-sectional study comparing symptomatic and asymptomatic hand dactylitis. Clin. Rheumatol. 2020, 39, 1157–1165. [Google Scholar] [CrossRef]
- Girolimetto, N.; Macchioni, P.; Tinazzi, I.; Costa, L.; Peluso, R.; Tasso, M.; Bottiglieri, P.; Marchetta, A.; Possemato, N.; Salvarani, C.; et al. Association between Leeds Dactylitis Index and ultrasonographic features: A multicentre study on psoriatic hand dactylitis. Clin. Exp. Rheumatol. 2020. [Google Scholar]
- Schoels, M.M.; Aletaha, D.; Alasti, F.; Smolen, J.S. Disease activity in psoriatic arthritis (PsA): Defining remission and treatment success using the DAPSA score. Ann. Rheum. Dis. 2016, 75, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, R.; Caso, F. Spondyloarthritis: Which composite measures to use in psoriatic arthritis? Nat. Rev. Rheumatol. 2018, 14, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Marchesoni, A.; Olivieri, I.; Salvarani, C.; Pipitone, N.; D’Angelo, S.; Mathieu, A.; Cauli, A.; Punzi, L.; Ramonda, R.; Scarpa, R.; et al. Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian Society of Rheumatology. Clin. Exp. Rheumatol. 2017, 35, 991–1010. [Google Scholar]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.B.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]
- Girolimetto, N.; Macchioni, P.; Citriniti, G.; Tinazzi, I.; Bascherini, V.; Martinis, F.; Marchetta, A.; Possemato, N.; Tasso, M.; Peluso, R.; et al. Effectiveness of steroid injection for hand psoriatic dactylitis: Results from a multicentre prospective observational study. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef]
- Möller, I.; Janta, I.; Backhaus, M.; Ohrndorf, S.; Bong, D.A.; Martinoli, C.; Filippucci, E.; Sconfienza, L.M.; Terslev, L.; Damjanov, N.; et al. The 2017 EULAR standardised procedures for ultrasound imaging in rheumatology. Ann. Rheum. Dis. 2017, 76, 1974–1979. [Google Scholar] [CrossRef]
- Naredo, E.; D’Agostino, M.A.; Wakefield, R.J.; Möller, I.; Balint, P.V.; Filippucci, E.; Iagnocco, A.; Karim, Z.; Terslev, L.; Bong, D.A.; et al. Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1328–1334. [Google Scholar] [CrossRef]
- Zabotti, A.; Sakellariou, G.; Tinazzi, I.; Idolazzi, L.; Batticciotto, A.; Canzoni, M.; Carrara, G.; De Lucia, O.; Figus, F.; Girolimetto, N.; et al. Novel and reliable DACTylitis glObal Sonographic (DACTOS) score in psoriatic arthritis. Ann. Rheum. Dis. 2020, 79, 1037–1043. [Google Scholar] [CrossRef]
- Tinazzi, I.; Idolazzi, L.; Zabotti, A.; Arancio, L.; Batticiotto, A.; Caimmi, C.; De Lucia, O.; Fassio, A.; Girolimetto, N.; Macchioni, P.; et al. Ultrasonographic detection, definition and quantification of soft tissue oedema in psoriatic dactylitis. Med. Ultrason. 2019, 21, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Bruyn, G.A.; Iagnocco, A.; Naredo, E.; Balint, P.V.; Gutierrez, M.; Hammer, H.B.; Collado, P.; Filippou, G.; Schmidt, W.A.; Jousse-Joulin, S.; et al. OMERACT definitions for ultrasonographic pathologies and elementary lesions of rheumatic disorders 15 years on. J. Rheumatol. 2019, 46, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Terslev, L.; Naredo, E.; Aegerter, P.; Wakefield, R.J.; Backhaus, M.; Balint, P.; Bruyn, G.A.W.; Iagnocco, A.; Jousse-Joulin, S.; Schmidt, W.A.; et al. Scoring ultrasound synovitis in rheumatoid arthritis: A EULAR-OMERACT ultrasound taskforce-Part 2: Reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open 2017, 3, e000427. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.-A.; Terslev, L.; Aegerter, P.; Backhaus, M.; Balint, P.; Bruyn, G.A.; Filippucci, E.; Grassi, W.; Iagnocco, A.; Jousse-Joulin, S.; et al. Scoring ultrasound synovitis in rheumatoid arthritis: A EULAR-OMERACT ultrasound taskforce-Part 1: Definition and development of a standardised, consensus-based scoring system. RMD Open 2017, 3, e000428. [Google Scholar] [CrossRef] [PubMed]
- Husted, J.A.; Cook, R.J.; Farewell, V.T.; Gladman, D.D. Methods for assessing responsiveness: A critical review and recommendations. J. Clin. Epidemiol. 2000, 53, 459–468. [Google Scholar] [CrossRef]
| Patients (56) | |
| Female n (%) | 23 (41%) |
| Mean age, years (mean ± SD) | 49.2 + 13.8 |
| Dactylitic fingers (83) | |
| Dactylitis duration, weeks (mean ± SD) | 23.3±22.3 |
| PsA disease duration at dactylitic episode, months (mean ± SD) | 49.5 ± 44.4 |
| Psoriasis duration at dactylitic episode, months (mean ± SD) | 69.3 ± 52.8 |
| ESR, mm/hr (mean ± SD) | 18.9 ± 13.3 |
| CRP, mg/dl (mean ± SD) | 1.4 ± 1.8 |
| TJC 68 joints (mean ± SD) | 7.5 ± 4.2 |
| SJC 66 joints (mean ± SD) | 2.8 ± 2.9 |
| Nail involvement (n (%)) | 59 (71%) |
| Presence of at least one enthesitis (n (%)) | 43 (52%) |
| Enthesitis count (mean ± SD) | 1.1 ± 1.3 |
| Therapy at baseline | |
| No therapy (n (%)) | 27 (32%) |
| csDMARDs (n (%)) | 53 (64%) |
| Oral steroids (n (%)) | 3 (4%) |
| Variable | Grade 0 | Grade 1 | Grade 2 | Grade 3 | p-Value |
|---|---|---|---|---|---|
| GS flexor tenosynovitis | |||||
| T0 | 6 (7.1%) | 26 (31.0%) | 45 (53.6%) | 7 (8.3%) | |
| T1 | 24 (28.6%) | 41 (48.8%) | 16 (19.0%) | 3 (3.6%) | T0 vs. T1; p < 0.001 |
| T3 | 41 (48.8%) | 29 (34.5%) | 13 (15.5%) | 1 (1.2%) | T0 vs. T3; p < 0.001 |
| PD flexor tenosynovitis | |||||
| T0 | 16 (19.0%) | 9 (10.7%) | 51 (60.7%) | 8 (9.5%) | |
| T1 | 45 (53.6%) | 19 (22.6%) | 16 (19.0%) | 4 (4.8%) | T0 vs. T1; p < 0.001 |
| T3 | 56 (66.7%) | 10 (11.9%) | 17 (20.2%) | 1 (1.2%) | T0 vs. T3; p < 0.001 |
| GS soft tissue oedema | |||||
| T0 | 4 (4.8%) | 32 (38.1%) | 38 (45.2%) | 10 (11.9%) | |
| T1 | 25 (29.8%) | 39 (46.4%) | 17 (20.2%) | 3 (3.6%) | T0 vs. T1; p < 0.001 |
| T3 | 35 (41.7%) | 29 (34.5%) | 16 (19.0%) | 4 (4.8%) | T0 vs. T3; p < 0.001 |
| PD soft tissue oedema | |||||
| T0 | 8 (9.5%) | 25 (29.8%) | 42 (50.0%) | 9 (10.7%) | |
| T1 | 27 (32.1%) | 29 (34.5%) | 26 (31.0%) | 2 (2.4%) | T0 vs. T1; p < 0.001 |
| T3 | 33 (39.3%) | 27 (32.1%) | 22 (26.2%) | 2 (2.4%) | T0 vs. T3; p < 0.001 |
| MCP GSperitendon extensor inflammation | |||||
| T0 | 69 (94.5%) | 4 (5.5%) | |||
| T1 | 69 (94.5%) | 4 (5.5%) | T0 vs. T1; p = ns | ||
| T3 | 70 (95.8%) | 3 (4.2%) | T0 vs. T3; p = ns | ||
| MCP PDperitendon extensor inflammation | |||||
| T0 | 81 (96.4%) | 2 (2.4%) | 1 (1.2%) | ||
| T1 | 81 (96.4%) | 3 (3.6%) | T0 vs. T1; p = ns | ||
| T3 | 82 (97.2%) | 2 (2.4%) | T0 vs. T3; p = ns | ||
| PIP GSperitendon extensor inflammation | |||||
| T0 | 71 (97.3%) | 2 (2.7%) | |||
| T1 | 71 (97.2%) | 2 (2.7%) | T0 vs. T1; p = ns | ||
| T3 | 72 (98.6%) | 1 (1.4%) | T0 vs. T3; p = ns | ||
| PIP PDperitendon extensor inflammation | |||||
| T0 | 71 (97.3%) | 2 (2.7%) | |||
| T1 | 72 (98.6%) | 1 (1.4%) | T0 vs. T1; p = ns | ||
| T3 | 72 (98.6%) | 1 (1.4%) | T0 vs. T3; p = ns | ||
| MCP synovitis (combined score) | |||||
| T0 | 70 (89.7%) | 4 (5.1%) | 1 (1.3%) | 3 (3.8%) | |
| T1 | 72 (92.3%) | 2 (2.6%) | 1 (1.3%) | 3 (3.8%) | T0 vs. T1; p = ns |
| T3 | 70 (89.7%) | 5 (6.4%) | 1 (1.3%) | 2 (2.6%) | T0 vs. T3; p = ns |
| PIP synovitis (combined score) | |||||
| T0 | 59 (75.6%) | 3 (3.8%) | 6 (7.7%) | 10 (12.8%) | |
| T1 | 59 (75.6%) | 5 (6.4%) | 3 (3.8%) | 11 (14.1%) | T0 vs. T1; p = ns |
| T3 | 60 (76.9%) | 1 (1.3%) | 9 (11.5%) | 8 (10.3%) | T0 vs. T3; p = ns |
| DIP synovitis (combined score) | |||||
| T0 | 71 (91.0%) | 1 (1.3%) | 6 (7.7%) | 0 | |
| T1 | 71 (91.0%) | 0 | 7 (9.0%) | 0 | T0 vs. T3; p = ns |
| T3 | 73 (93.6%) | 0 | 5 (6.4%) | 0 | T0 vs. T1; p = ns |
| Variable | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| GS flexor tenosynovitis | ||||
| MTR at T1 (46 fingers) | 15 (32.6%) | 26 (56.5%) | 3 (6.5%) | 2 (4.3%) |
| Non MTR at T1 (37 fingers) | 9 (24.3%) | 14 (37.8%) | 13 (35.1%) | 1 (2.7%) |
| p = 0.013 | ||||
| MTR at T3 (54 fingers) | 31 (57.4%) | 20 (37.0%) | 2 (3.7%) | 1 (1.9%) |
| Non MTR at T3 (29 fingers) | 10 (34.5%) | 8 (27.6%) | 11 (37.9%) | 0 |
| p = 0.001 | ||||
| PD flexor tenosynovitis | ||||
| MTR at T1 (46 fingers) | 31 (67.4%) | 9 (19.6%) | 5 (10.9%) | 1 (2.2%) |
| Non MTR at T1 (37 fingers) | 14 (37.8%) | 9 (23.3%) | 11 (29.7%) | 3 (8.1%) |
| p = 0.032 | ||||
| MTR at T3 (54 fingers) | 45 (83.5%) | 4 (7.4%) | 4 (7.4%) | 1 (1.9%) |
| Non MTR at T3 (29 fingers) | 11 (37.9%) | 5 (17.2%) | 13 (44.8%) | 0 |
| p < 0.001 | ||||
| GS soft tissue oedema | ||||
| MTR at T1 (46 fingers) | 20 (43.5%) | 20 (43.5%) | 6 (13.6%) | 0 |
| Non MTR at T1 (37 fingers) | 5 (13.5%) | 18 (48.6%) | 11 (29.7%) | 3 (8.1%) |
| p = 0.005 | ||||
| MTR at T3 (54 fingers) | 30 (55.6%) | 19 (35.2%) | 4 (7.4%) | 1 (1.9%) |
| Non MTR at T3 (29 fingers) | 5 (17.2%) | 9 (31.0%) | 12 (41.4%) | 3 (10.3%) |
| p < 0.001 | ||||
| PD soft tissue oedema | ||||
| MTR at T1 (46 fingers) | 18 (39.1%) | 15 (32.6%) | 13 (28.3%) | 0 |
| Non MTR at T1 (37 fingers) | 9 (24.3%) | 13 (35.1%) | 13 (35.1%) | 2 (5.4%) |
| p = 0.239 | ||||
| MTR at T3 (54 fingers) | 27 (50.0%) | 15 (27.8%) | 12 (22.2%) | 0 |
| Non MTR at T3 (29 fingers) | 4 (20.7%)6 | 11 (37.9%) | 10 (34.5%) | 2 (6.9%) |
| p = 0.023 | ||||
| MCP synovitis (combined score) | ||||
| MTR at T1 (46 fingers) | 44 (95.7%) | 2 (4.3%) | 0 | 0 |
| Non MTR at T1 (37 fingers) | 32 (86.5%) | 1 (2.7%) | 1 (2.7%) | 3 (8.1%) |
| p = 0.150 | ||||
| MTR at T3 (54 fingers) | 54 (100%) | 0 | 0 | 0 |
| Non MTR at T3 (29 fingers) | 21 (72.4%) | 5 (17.2%) | 1 (3.4%) | 2 (6.9%) |
| p = 0.001 | ||||
| PIP synovitis (combined score) | ||||
| MTR at T1 (46 fingers) | 37 (80.4%) | 5 (10.9%) | 2 (4.3%) | 2 (4.3%) |
| Non MTR at T1 (37 fingers) | 26 (70.3%) | 1 (2.7%) | 1 (2.7%) | 9 (24.3%) |
| p = 0.037 | ||||
| MTR at T3 (54 fingers) | 43 (79.6%) | 1 (1.9%) | 5 (9.3%) | 5 (9.3%) |
| Non MTR at T3 (29 fingers) | 21 (72.4%) | 1 (3.4%) | 4 (13.8%) | 3 (10.3%) |
| p = 0.871 | ||||
| DIP synovitis (combined score) | ||||
| MTR at T1 (46 fingers) | 42 (93.5%) | 0 | 3 (6.5%) | 0 |
| Non MTR at T1 (37 fingers) | 33 (89.2%) | 0 | 4 (10.8%) | 0 |
| p = 0.485 | ||||
| MTR at T3 (54 fingers) | 51 (94.4%) | 0 | 3 (5.6%) | 0 |
| Non MTR at T3 (29 fingers) | 27 (93.1%) | 0 | 2 (6.9%) | 0 |
| p = 0.807 | ||||
| VAS-p Delta T0 vs. T1 | VAS-FI Delta T0 vs. T1 | LDI-b Delta T0 vs. T1 | |
|---|---|---|---|
| GS FT Delta T0 vs. T1 | 0.537 (<0.001) | 0.570 (<0.001) | 0.467(<0.001) |
| PD FT Delta T0 vs. T1 | 0.594 (<0.001) | 0.579 (<0.001) | 0.545 (<0.001) |
| GS STO Delta T0 vs. T1 | 0.420 (<0.001) | 0.500 (<0.001) | 0.386 (<0.001) |
| PD STO Delta T0 vs. T1 | 0.400 (<0.001) | 0.410 (<0.001) | 0.293 (0.009) |
| VAS-p Delta T0 vs. T3 | VAS-FI Delta T0 vs. T3 | LDI-b Delta T0 vs. T3 | |
| GS FT Delta T0 vs. T3 | 0.590 (<0.001) | 0.618 (<0.001) | 0.548 (<0.001) |
| PD FT Delta T0 vs. T3 | 0.525 (<0.001) | 0.507 (<0.001) | 0.482 (<0.001) |
| GS STO Delta T0 vs. T3 | 0.526 (<0.001) | 0.514 (<0.001) | 0.462 (<0.001) |
| PD STO Delta T0 vs. T3 | 0.396 (0.001) | 0.383 (0.001) | 0.258 (0.023) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolimetto, N.; Macchioni, P.; Possemato, N.; Tinazzi, I.; Bascherini, V.; Citriniti, G.; McConnell, R.; Marchetta, A.; Peluso, R.; Sabbatino, V.; et al. Musculoskeletal Ultrasound in Monitoring Clinical Response to Treatment in Acute Symptomatic Psoriatic Dactylitis: Results from a Multicentre Prospective Observational Study. J. Clin. Med. 2020, 9, 3127. https://doi.org/10.3390/jcm9103127
Girolimetto N, Macchioni P, Possemato N, Tinazzi I, Bascherini V, Citriniti G, McConnell R, Marchetta A, Peluso R, Sabbatino V, et al. Musculoskeletal Ultrasound in Monitoring Clinical Response to Treatment in Acute Symptomatic Psoriatic Dactylitis: Results from a Multicentre Prospective Observational Study. Journal of Clinical Medicine. 2020; 9(10):3127. https://doi.org/10.3390/jcm9103127
Chicago/Turabian StyleGirolimetto, Nicolò, Pierluigi Macchioni, Niccolò Possemato, Ilaria Tinazzi, Vittoria Bascherini, Giorgia Citriniti, Rebecca McConnell, Antonio Marchetta, Rosario Peluso, Vincenzo Sabbatino, and et al. 2020. "Musculoskeletal Ultrasound in Monitoring Clinical Response to Treatment in Acute Symptomatic Psoriatic Dactylitis: Results from a Multicentre Prospective Observational Study" Journal of Clinical Medicine 9, no. 10: 3127. https://doi.org/10.3390/jcm9103127
APA StyleGirolimetto, N., Macchioni, P., Possemato, N., Tinazzi, I., Bascherini, V., Citriniti, G., McConnell, R., Marchetta, A., Peluso, R., Sabbatino, V., Salvarani, C., Scarpa, R., Costa, L., & Caso, F. (2020). Musculoskeletal Ultrasound in Monitoring Clinical Response to Treatment in Acute Symptomatic Psoriatic Dactylitis: Results from a Multicentre Prospective Observational Study. Journal of Clinical Medicine, 9(10), 3127. https://doi.org/10.3390/jcm9103127






