CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. CoQ10 Analysis by Liquid Chromatography-Tandem Mass Spectrometry
2.3. Blood–Brain Barrier Cell Culture
2.4. Coenzyme Q10 Transport Assays
2.5. Cellular CoQ10 Depletion and Mitochondrial Respiratory Chain Enzyme Activity
2.6. Cell Viability Assay
2.7. CoQ10 Partition in Serum Lipoprotein Fractions
2.8. Confocal Microscopy
2.9. Statistical Analysis
3. Results
3.1. LC-MS/MS CoQ10 Method Validation
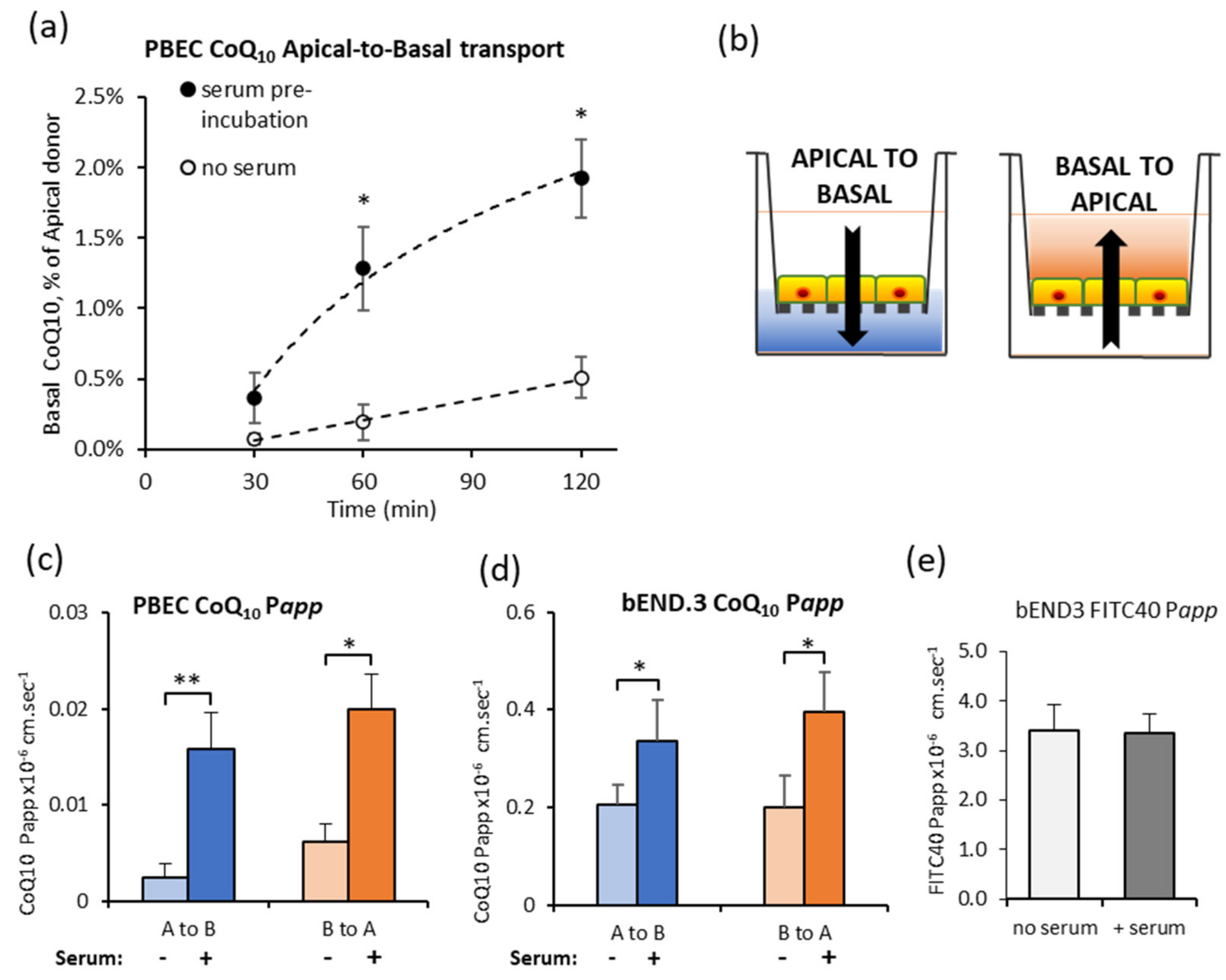
3.2. Effect of Serum Pre-Incubation on CoQ10 Transport across In Vitro BBB
3.3. CoQ10 Distribution in Serum Lipoprotein Fractions
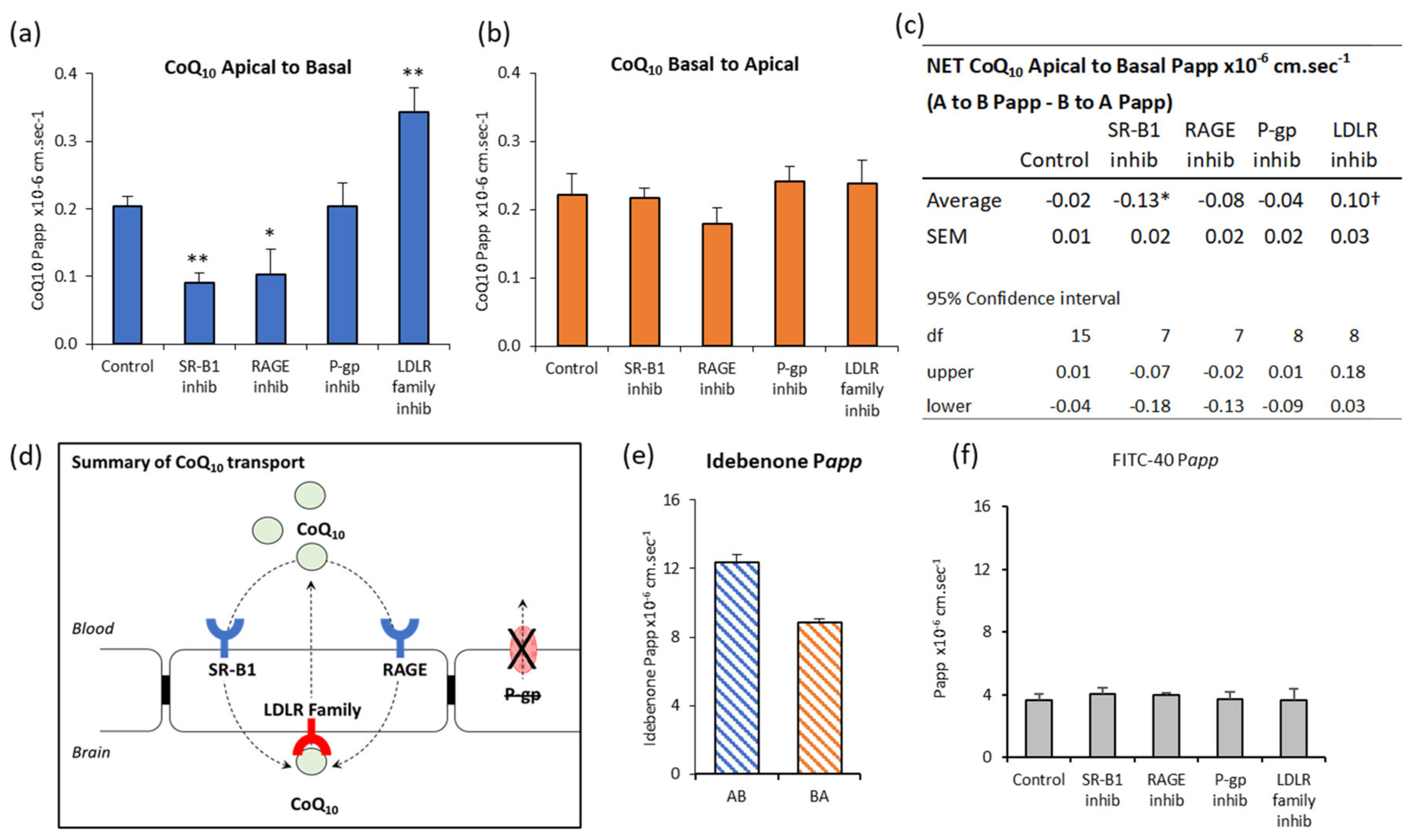
3.4. CoQ10 BBB Transport: SR-B1, LDLR, and RAGE Inhibitors
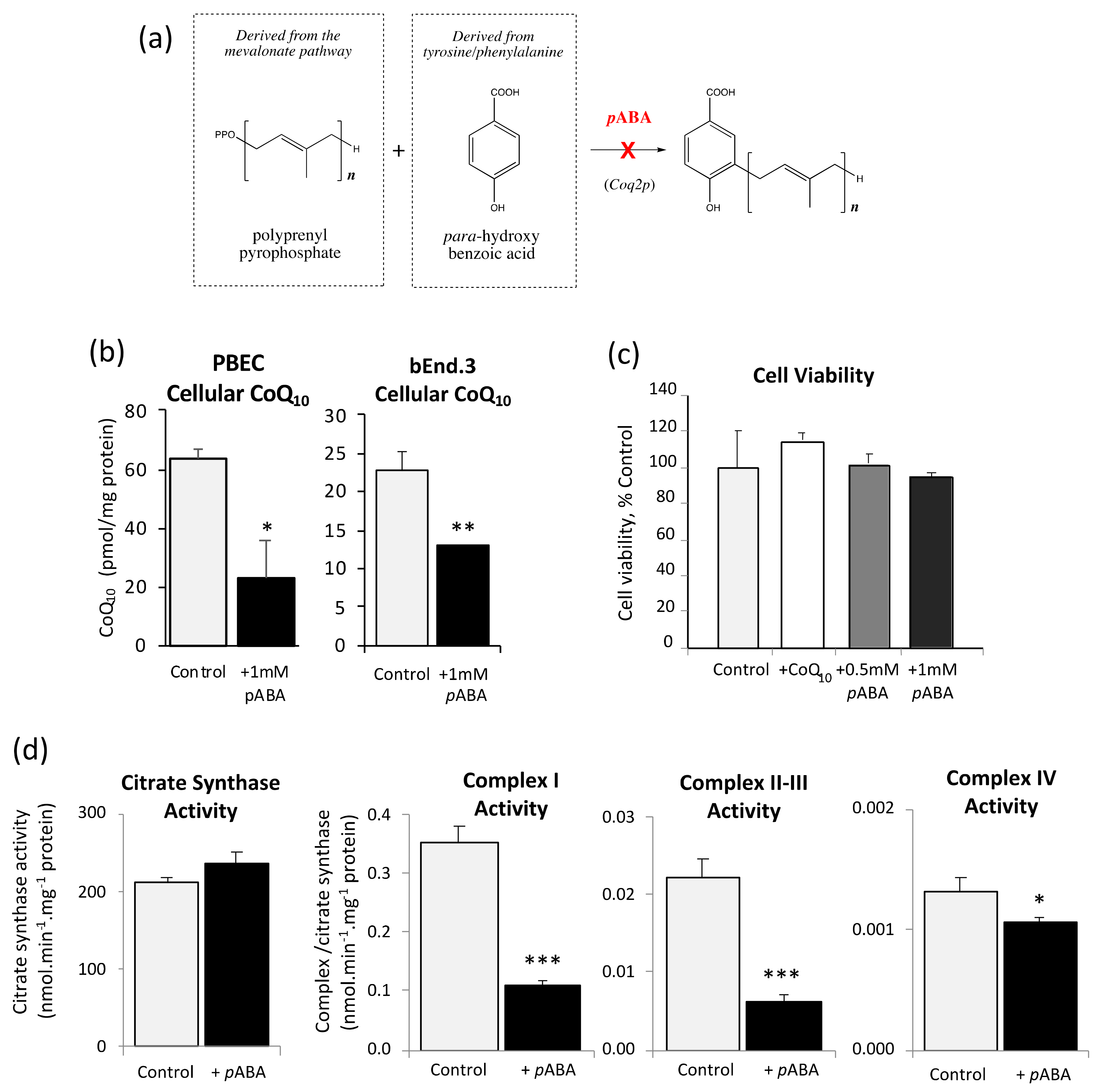
3.5. Inhibition of CoQ10 Biosynthesis
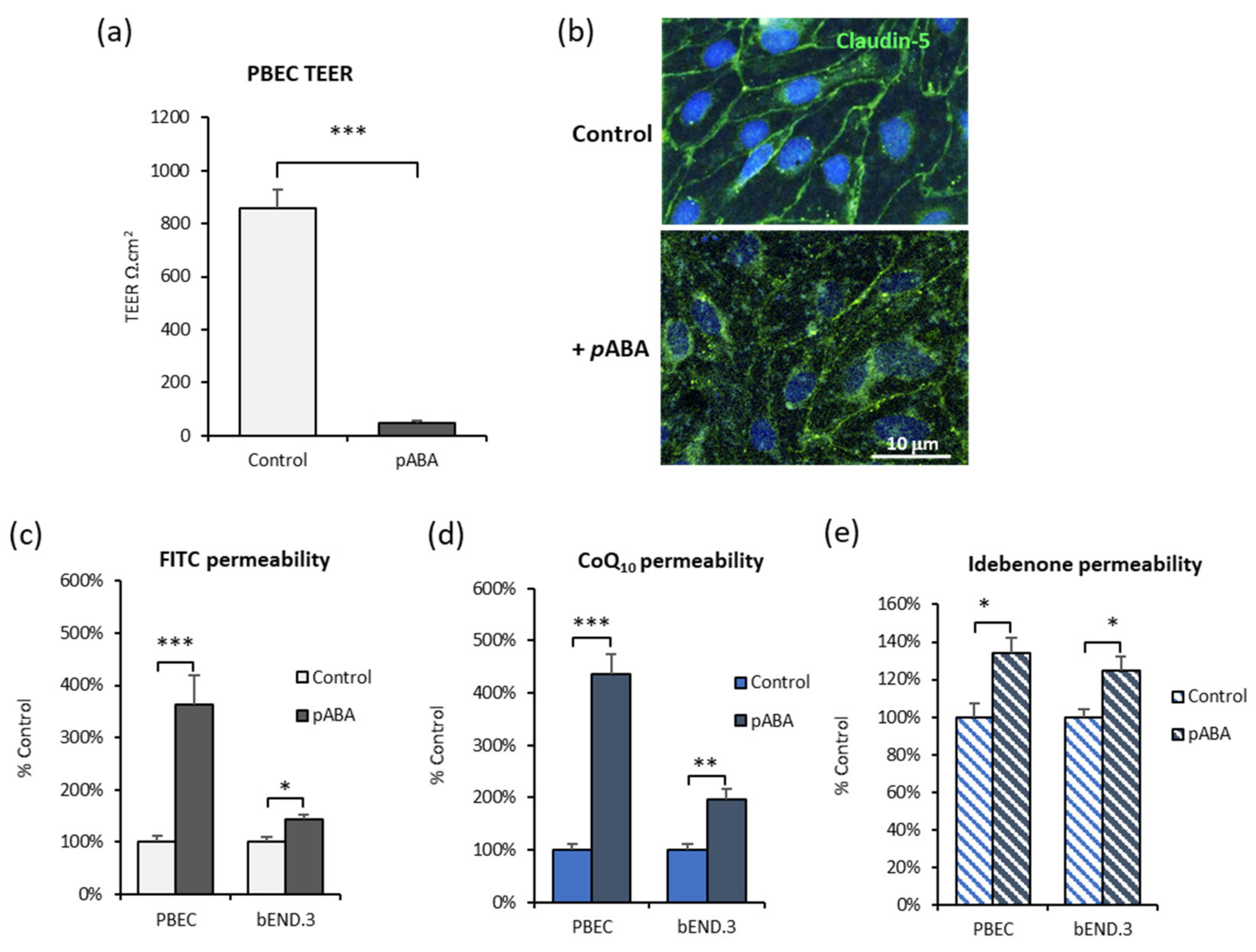
3.6. CoQ10 Defecient BBB: Effect of SR-B1, LDLR, and RAGE Inhibitors
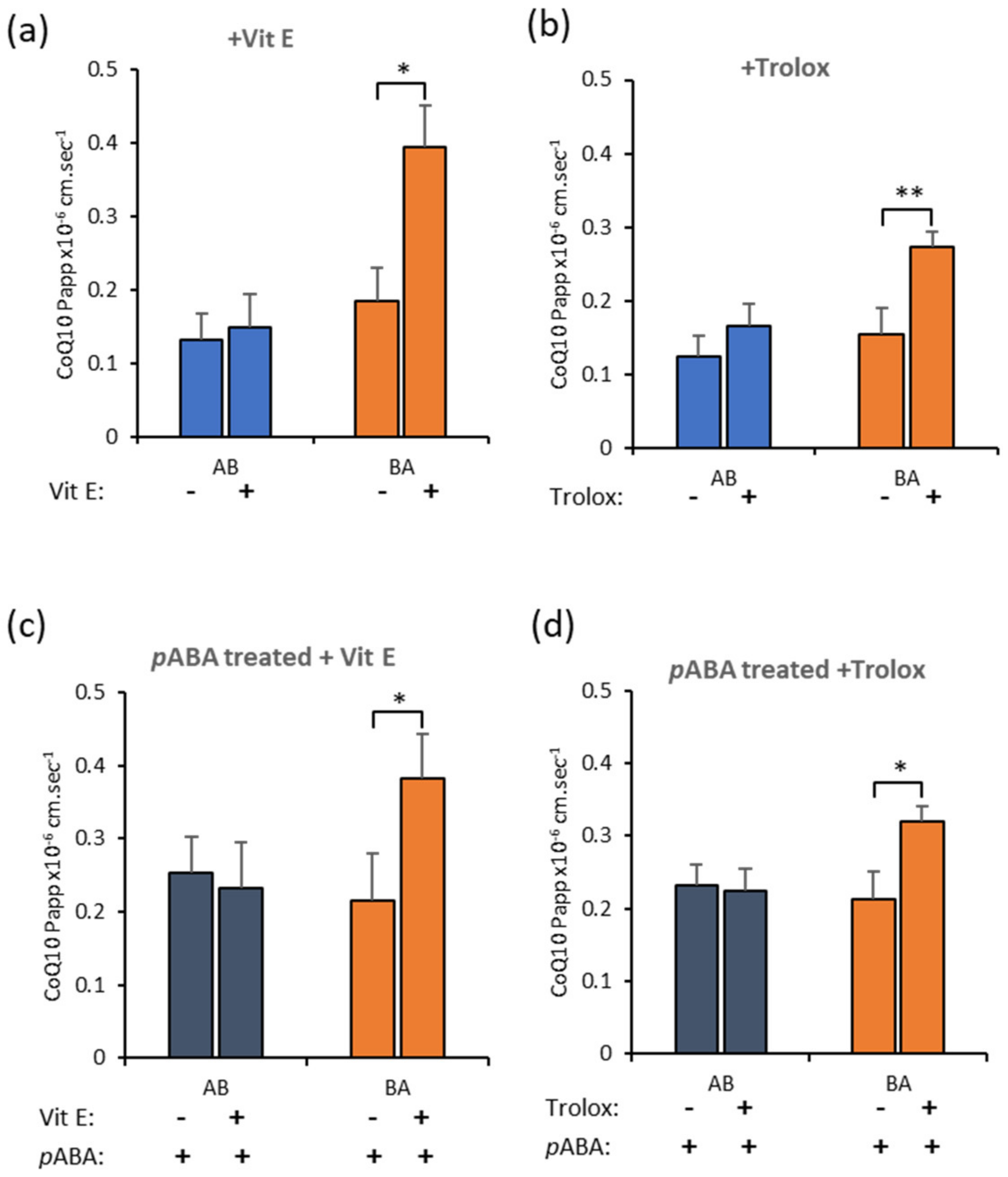
3.7. Effect of Antioxidants on CoQ10 BBB Transport
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta Mol. Basis Dis. 1995, 1271, 195–204. [Google Scholar] [CrossRef]
- Acín-Pérez, R.; Fernández-Silva, P.; Peleato, M.L.; Pérez-Martos, A.; Enriquez, J.A. Respiratory Active Mitochondrial Supercomplexes. Mol. Cell 2008, 32, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.J.; Fonseca, L.V.; Desbats, M.A.; Cerqua, C.; Zordan, R.; Trevisson, E.; Salviati, L. Coenzyme Q biosynthesis in health and disease. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Neergheen, V.; Hargreaves, I.P. Secondary Coenzyme Q10 Deficiency Causes and Consequence; Grigoryeva, S., Ed.; Coenzyme Q10 Uses, Health Effects and Role in Disease; Nova Science Publishers, Inc.: New York, NY, USA, 2018. [Google Scholar]
- Emmanuele, V.; López, L.C.; Berardo, A.; Naini, A.; Tadesse, S.; Wen, B.; D’Agostino, E.; Solomon, M.; DiMauro, S.; Quinzii, C.; et al. Heterogeneity of Coenzyme Q10 Deficiency. Arch. Neurol. 2012, 69, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Salviati, L.; Trevisson, E.; Doimo, M.; Navas, P. Primary Coenzyme Q10 Deficiency; GeneReviews®: Seattle, WA, USA, 2017. [Google Scholar]
- Salviati, L.; Sacconi, S.; Murer, L.; Zacchello, G.; Franceschini, L.; Laverda, A.M.; Basso, G.; Quinzii, C.; Angelini, C.; Hirano, M.; et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: A CoQ10-responsive condition. Neurology 2005, 65, 606–608. [Google Scholar] [CrossRef]
- Musumeci, O.; Naini, A.; Slonim, A.E.; Skavin, N.; Hadjigeorgiou, G.L.; Krawiecki, N.; Weissman, B.M.; Tsao, C.-Y.; Mendell, J.R.; Shanske, S.; et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurolofy 2001, 56, 849–855. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Tazir, M.; López, L.C.; Quinzii, C.M.; Assoum, M.; Drouot, N.; Busso, C.; Makri, S.; Ali-Pacha, L.; Benhassine, T.; et al. ADCK3, an Ancestral Kinase, Is Mutated in a Form of Recessive Ataxia Associated with Coenzyme Q10 Deficiency. Am. J. Hum. Genet. 2008, 82, 661–672. [Google Scholar] [CrossRef]
- Tomasetti, M.; Tomasetti, M.; Solenghi, M.D.; Littarru, G.P. Distribution of antioxidants among blood components and lipoproteins: Significance of lipids/CoQ10ratio as a possible marker of increased risk for atherosclerosis. BioFactors 1999, 9, 231–240. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free. Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Gosselet, F.; Candela, P.; Sevin, E.; Berezowski, V.; Cecchelli, R.; Fenart, L. Transcriptional profiles of receptors and transporters involved in brain cholesterol homeostasis at the blood–brain barrier: Use of an in vitro model. Brain Res. 2009, 1249, 34–42. [Google Scholar] [CrossRef]
- Balazs, Z.; Panzenboeck, U.; Hammer, A.; Sovic, A.; Quehenberger, O.; Malle, E.; Sattler, W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J. Neurochem. 2004, 89, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eckel, R.H. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 2014, 25, 8–14. [Google Scholar] [CrossRef]
- González-Pecchi, V.; Valdés, S.; Pons, V.; Honorato, P.; Martinez, L.O.; Lamperti, L.; Aguayo, C.; Radojkovic, C. Apolipoprotein A-I enhances proliferation of human endothelial progenitor cells and promotes angiogenesis through the cell surface ATP synthase. Microvasc. Res. 2015, 98, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bu, G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int. Rev. Cytol. 2001, 209, 79–116. [Google Scholar] [PubMed]
- Deane, R.; Wu, Z.; Zlokovic, B.V. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke 2004, 35, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, S.; Ochiai, A.; Kobayashi, M.; Sugawara, M.; Hirano, T.; Iseki, K. Interaction of Coenzyme Q10 with the Intestinal Drug Transporter P-Glycoprotein. J. Agric. Food Chem. 2008, 56, 6923–6927. [Google Scholar] [CrossRef]
- Itkonen, O.; Suomalainen, A.; Turpeinen, U. Mitochondrial Coenzyme Q10 Determination by Isotope-Dilution Liquid Chromatography–Tandem Mass Spectrometry. Clin. Chem. 2013, 59, 1260–1267. [Google Scholar] [CrossRef][Green Version]
- Duncan, A.J.; Heales, S.J.; Mills, K.; Eaton, S.; Land, J.M.; Hargreaves, I.P. Determination of Coenzyme Q10 Status in Blood Mononuclear Cells, Skeletal Muscle, and Plasma by HPLC with Di-Propoxy-Coenzyme Q10 as an Internal Standard. Clin. Chem. 2005, 51, 2380–2382. [Google Scholar] [CrossRef]
- Crane, F.L.; Lester, R.L.; Widmer, C.; Hatefi, Y.; Fechner, W.F.; Welch, E.M. Studies on the electron transport system. XVIII. Isolation of coenzyme Q (Q275) from beef heart and beef heart mitochondria. Biochim. Biophys. Acta 1959, 32, 73–79. [Google Scholar]
- Nielsen, S.S.E.; Siupka, P.; Georgian, A.; Preston, J.E.; Tóth, A.E.; Yusof, S.R.; Abbott, N.J.; Nielsen, M.S. Improved Method for the Establishment of an In Vitro Blood-Brain Barrier Model Based on Porcine Brain Endothelial Cells. J. Vis. Exp. 2017, 127, e56277. [Google Scholar] [CrossRef]
- Patabendige, A.; Skinner, R.A.; Morgan, L.; Abbott, N.J. A detailed method for preparation of a functional and flexible blood–brain barrier model using porcine brain endothelial cells. Brain Res. 2013, 1521, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Ulery, P.G.; Beers, J.; Mikhailenko, I.; E Tanzi, R.; Rebeck, G.W.; Hyman, B.T.; Strickland, D.K. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J. Biol. Chem. 2000, 275, 7410–7415. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Jayagopal, A.; Qu, Z.-C.; Parker, W.H. Ascorbic acid prevents high glucose-induced apoptosis in human brain pericytes. Biochem. Biophys. Res. Commun. 2014, 452, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.R.; Pasanisi, F.; Elliott, H.L.; Reid, J.L. Effects of verapamil and nisoldipine on human platelets: In vivo and in vitro studies. Br. J. Clin. Pharmacol. 1985, 20, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Mottier, P.; Gremaud, E.; Guy, P.A.; Turesky, R.J. Comparison of Gas Chromatography–Mass Spectrometry and Liquid Chromatography–Tandem Mass Spectrometry Methods to Quantify α-Tocopherol and α-Tocopherolquinone Levels in Human Plasma. Anal. Biochem. 2002, 301, 128–135. [Google Scholar] [CrossRef]
- Alam, S.S.; Nambudiri, A.; Rudney, H. 4-Hydroxybenzoate:Polyprenyl transferase and the prenylation of 4-aminobenzoate in mammalian tissues. Arch. Biochem. Biophys. 1975, 171, 183–190. [Google Scholar] [CrossRef]
- Duberley, K.E.C.; Abramov, A.Y.; Chalasani, A.; Heales, S.J.R.; Rahman, S.; Hargreaves, I. Human neuronal coenzyme Q10 deficiency results in global loss of mitochondrial respiratory chain activity, increased mitochondrial oxidative stress and reversal of ATP synthase activity: Implications for pathogenesis and treatment. J. Inherit. Metab. Dis. 2013, 36, 63–73. [Google Scholar] [CrossRef]
- Hargreaves, I.P.; Heales, S.J.R.; Land, J.M. Mitochondrial respiratory chain defects are not accompanied by an increase in the activities of lactate dehydrogenase or manganese superoxide dismutase in paediatric skeletal muscle biopsies. J. Inherit. Metab. Dis. 1999, 22, 925–931. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ononogbu, I.; Lewis, B. Lipoprotein fractionation by a precipitation method. a simple quantitative procedure. Clin. Chim. Acta 1976, 71, 397–402. [Google Scholar] [CrossRef]
- USA Department of Health and Human Services. Bioanalytical Method Validation, in Guidance for Industry; USA Department of Health and Human Services: Silver Spring, MD, USA, 2018. [Google Scholar]
- European Medicines Agency. Guideline on Bioanalytical Method Validation; Committee for Medicinal Products for Human Use: London, UK, 2012. [Google Scholar]
- Yubero, D.; Montero, R.; Artuch, R.; Land, J.M.; Heales, S.J.; Hargreaves, I.P. Biochemical Diagnosis of Coenzyme Q10 Deficiency. Mol. Syndromol. 2014, 5, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Leaver, N. A Practical Guide to Implementing Clinical Mass Spectrometry Systems; ILM Publications: St Albans, UK, 2011; p. 84. [Google Scholar]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–S88. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Festa, R.; Raimondo, S.; Pontecorvi, A.; Littarru, G.P. Hormonal Influence on Coenzyme Q10 Levels in Blood Plasma. Int. J. Mol. Sci. 2011, 12, 9216–9225. [Google Scholar] [CrossRef] [PubMed]
- Duberley, K.; Heales, S.; Abramov, A.; Chalasani, A.; Land, J.; Rahman, S.; Hargreaves, I. Effect of Coenzyme Q10 supplementation on mitochondrial electron transport chain activity and mitochondrial oxidative stress in Coenzyme Q10 deficient human neuronal cells. Int. J. Biochem. Cell Biol. 2014, 50, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, M.; Hoekstra, M.; Out, R.; Bos, I.S.T.; Kruijt, J.K.; Hildebrand, R.B.; Van Berkel, T.J.C. Scavenger receptor BI facilitates the metabolism of VLDL lipoproteins in vivo. J. Lipid Res. 2008, 49, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- González-Aragón, D.; Burón, M.I.; López-Lluch, G.; Hermán, M.D.; Gómez-Díaz, C.; Navas, P.; Villalba, J.M. Coenzyme Q and the regulation of intracellular steady-state levels of superoxide in HL-60 cells. BioFactors 2005, 25, 31–41. [Google Scholar] [CrossRef]
- Parikh, S.; Goldstein, A.; Karaa, A.; Koenig, M.K.; Anselm, I.; Brunel-Guitton, C.; Christodoulou, J.; Cohen, B.H.; Dimmock, D.; Enns, G.M.; et al. Patient care standards for primary mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genet. Med. 2017, 19, 1380. [Google Scholar] [CrossRef]
- Enns, G.M. Treatment of Mitochondrial Disorders: Antioxidants and beyond. J. Child Neurol. 2014, 29, 1235–1240. [Google Scholar] [CrossRef]
- Goti, D.; Hammer, A.; Galla, H.-J.; Malle, E.; Sattler, W. Uptake of Lipoprotein-Associated α-Tocopherol by Primary Porcine Brain Capillary Endothelial Cells. J. Neurochem. 2002, 74, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.T.; Yang, L.; Browne, S.; Baik, M.; Beal, M.F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. USA 1998, 95, 8892–8897. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Matson, S.; Matson, W.R.; Cormier, K.; Del Signore, S.J.; Hagerty, S.W.; Stack, E.C.; Ryu, H.; Ferrante, R.J. Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington’s disease mice. Biochim. Biophys. Acta 2006, 1762, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Sunesen, V.H.; Weber, C.; Hølmer, G.; Becker, C. Lipophilic antioxidants and polyunsaturated fatty acids in lipoprotein classes: Distribution and interaction. Eur. J. Clin. Nutr. 2001, 55, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M. Central nervous system: Cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 2009, 390, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, B.; Fenart, L.; Dehouck, M.-P.; Pierce, A.; Torpier, G.; Cecchelli, R. A New Function for the LDL Receptor: Transcytosis of LDL across the Blood–Brain Barrier. J. Cell Biol. 1997, 138, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Panzenboeck, U.; Balazs, Z.; Sovic, A.; Hrzenjak, A.; Levak-Frank, S.; Wintersperger, A.; Malle, E.; Sattler, W. ABCA1 and Scavenger Receptor Class B, Type I, Are Modulators of Reverse Sterol Transport at anin Vitro Blood-Brain Barrier Constituted of Porcine Brain Capillary Endothelial Cells. J. Biol. Chem. 2002, 277, 42781–42789. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Yamada, S.; Kumar, S.R.; Calero, M.; Bading, J.; Frangione, B.; Holtzman, D.M.; Miller, C.A.; Strickland, D.K.; Ghiso, J.; et al. Clearance of Alzheimer’s amyloid-ss (1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Investig. 2000, 106, 1489–1499. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef]
- Williams, D.L.; de La Llera-Moya, M.; Thuahnai, S.T.; Lund-Katz, S.; Connelly, M.A.; Azhar, S.; Anantharamaiah, G.M.; Phillips, M.C. Binding and Cross-linking Studies Show That Scavenger Receptor BI Interacts with Multiple Sites in Apolipoprotein A-I and Identify the Class A Amphipathic alpha-Helix as a Recognition Motif. J. Biol. Chem. 2000, 275, 18897–18904. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. Introduction to Lipids and Lipoproteins. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, H.J., Kaltsas, G., et al., Eds.; MDText.com: South Dartmouth, MA, USA, 2018; NBK305896. [Google Scholar]
- Mendivil, C.O.; Rimm, E.B.; Furtado, J.; Sacks, F.M. Apolipoprotein E in VLDL and LDL With Apolipoprotein C-III is Associated With a Lower Risk of Coronary Heart Disease. J. Am. Hear. Assoc. 2013, 2, e000130. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Wang, C.; Nyegaard, S.; Heit, B.; Fairn, G.D.; Lee, W.L. SR-BI Mediated Transcytosis of HDL in Brain Microvascular Endothelial Cells Is Independent of Caveolin, Clathrin, and PDZK1. Front. Physiol. 2017, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.M.; Sugiyama, M.G.; Fung, K.Y.; Gao, Y.; Wang, C.; Levy, A.S.; Azizi, P.; Roufaiel, M.; Zhu, S.-N.; Neculai, D.; et al. A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis. Cardiovasc. Res. 2015, 108, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Kraehling, J.R.; Chidlow, J.H.; Rajagopal, C.; Sugiyama, M.G.; Fowler, J.W.; Lee, M.Y.; Zhang, X.; Ramírez, C.M.; Park, E.J.; Tao, B.; et al. Genome-wide RNAi screen reveals ALK1 mediates LDL uptake and transcytosis in endothelial cells. Nat. Commun. 2016, 7, 13516. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G. RAGE: A single receptor fits multiple ligands. Trends Biochem. Sci. 2011, 36, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Gursky, O. Human Plasma Very Low-Density Lipoproteins Are Stabilized by Electrostatic Interactions and Destabilized by Acidic pH. J. Lipids 2011, 2011, 493720. [Google Scholar] [CrossRef]
- Wang, H.; Chen, F.; Zhong, K.L.; Tang, S.S.; Hu, M.; Long, Y.; Miao, M.X.; Liao, J.M.; Sun, H.B.; Hong, H. PPARgamma agonists regulate bidirectional transport of amyloid-beta across the blood-brain barrier and hippocampus plasticity in db/db mice. Br. J. Pharmacol. 2016, 173, 372–385. [Google Scholar] [CrossRef]
- Donahue, J.E.; Flaherty, S.L.; Johanson, C.E.; Duncan, J.A.; Silverberg, G.D.; Miller, M.C.; Tavares, R.; Yang, W.; Wu, Q.; Sabo, E.; et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006, 112, 405–415. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Du Yan, S.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar]
- Deane, R.J. Is RAGE still a therapeutic target for Alzheimer’s disease? Futur. Med. Chem. 2012, 4, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Peluso, G.; Ab Melone, M. RAGE recycles at the plasma membrane in S100B secretory vesicles and promotes Schwann cells morphological changes. J. Cell. Physiol. 2008, 217, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.Y.; McLaurin, J. Mechanisms of Amyloid-Beta Peptide Uptake by Neurons: The Role of Lipid Rafts and Lipid Raft-Associated Proteins. Int. J. Alzheimers Dis. 2010, 2011, 548380. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-J.; Hsu, F.-Y.; Chen, W.-W.; Lee, C.-H.; Lin, Y.-J.; Chen, Y.-Y.M.; Chen, C.-J.; Huang, M.-Z.; Kao, M.-C.; Chen, Y.-A.; et al. Helicobacter pylori Activates HMGB1 Expression and Recruits RAGE into Lipid Rafts to Promote Inflammation in Gastric Epithelial Cells. Front. Immunol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Dato, V.A.; Chiabrando, G.A. The Role of Low-Density Lipoprotein Receptor-Related Protein 1 in Lipid Metabolism, Glucose Homeostasis and Inflammation. Int. J. Mol. Sci. 2018, 19, 1780. [Google Scholar] [CrossRef]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M.B.; Holtzman, D.M.; Zlokovic, B.V. ApoE isoform–specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Investig. 2008, 118, 4002–4013. [Google Scholar] [CrossRef]
- Molino, Y.; David, M.; Varini, K.; Jabès, F.; Gaudin, N.; Fortoul, A.; Bakloul, K.; Masse, M.; Bernard, A.; Drobecq, L.; et al. Use of LDL receptor—targeting peptide vectors for in vitro and in vivo cargo transport across the blood-brain barrier. FASEB J. 2017, 31, 1807–1827. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Iannuzzo, G.; Parlato, A.; Cuomo, G.; Testa, C.; Coppola, M.; D’Ambrosio, G.; Oliviero, D.A.; Sarullo, S.; Vitale, G.; et al. Clinical Evidence for Q10 Coenzyme Supplementation in Heart Failure: From Energetics to Functional Improvement. J. Clin. Med. 2020, 9, 1266. [Google Scholar] [CrossRef]
- Itagaki, S.; Ochiai, A.; Kobayashi, M.; Sugawara, M.; Hirano, T.; Iseki, K. Grapefruit juice enhance the uptake of coenzyme Q10 in the human intestinal cell-line Caco-2. Food Chem. 2010, 120, 552–555. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Mitochondrial Disorders in Children; Co-enzyme Q10; National Institute for Health and Care Excellence: London, UK, 2017. [Google Scholar]
- Kashiba, M.; Terashima, M.; Sagawa, T.; Yoshimura, S.; Yamamoto, Y. Prosaposin knockdown in Caco-2 cells decreases cellular levels of coenzyme Q10 and ATP, and results in the loss of tight junction barriers. J. Clin. Biochem. Nutr. 2017, 60, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Ziosi, M.; Di Meo, I.; Kleiner, G.; Gao, X.; Barca, E.; Sanchez-Quintero, M.J.; Tadesse, S.; Jiang, H.; Qiao, C.; Rodenburg, R.J.; et al. Coenzyme Q deficiency causes impairment of the sulfide oxidation pathway. EMBO Mol. Med. 2017, 9, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.K.; Abdul Murad, N.; Yeo, A.; McKenzie, M.; Ward, M.; Chong, K.L.; Schieber, N.L.; Parton, R.G.; Lim, Y.C.; Wolvetang, E.; et al. AarF Domain Containing Kinase 3 (ADCK3) Mutant Cells Display Signs of Oxidative Stress, Defects in Mitochondrial Homeostasis and Lysosomal Accumulation. PLoS ONE 2016, 11, e0148213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quinzii, C.M.; Tadesse, S.; Naini, A.; Hirano, M. Effects of Inhibiting CoQ10 Biosynthesis with 4-nitrobenzoate in Human Fibroblasts. PLoS ONE 2012, 7, e30606. [Google Scholar] [CrossRef] [PubMed]
- Forsman, U.; Sjöberg, M.; Turunen, M.; Sindelar, P.J. 4-Nitrobenzoate inhibits coenzyme Q biosynthesis in mammalian cell cultures. Nat. Chem. Biol. 2010, 6, 515–517. [Google Scholar] [CrossRef]
- Pierrel, F. Impact of Chemical Analogs of 4-Hydroxybenzoic Acid on Coenzyme Q Biosynthesis: From Inhibition to Bypass of Coenzyme Q Deficiency. Front. Physiol. 2017, 8, 436. [Google Scholar] [CrossRef]
- Brea, G.; Haack, T.B.; Karall, D.; Ohtake, A.; Invernizzi, F.; Carrozzo, R.; Kremer, L.; Dusi, S.; Fauth, C.; Scholl-Bürgi, S.; et al. COQ4 Mutations Cause a Broad Spectrum of Mitochondrial Disorders Associated with CoQ10 Deficiency. Am. J. Hum. Genet. 2015, 96, 309–317. [Google Scholar] [CrossRef]
- Doll, D.N.; Hu, H.; Sun, J.; Lewis, S.E.; Simpkins, J.W.; Ren, X. Mitochondrial Crisis in Cerebrovascular Endothelial Cells Opens the Blood–Brain Barrier. Stroke 2015, 46, 1681–1689. [Google Scholar] [CrossRef]
- Pasquali, M.A.; Gelain, D.P.; Zeidán-Chuliá, F.; Pires, A.S.; Gasparotto, J.; Terra, S.R.; Moreira, J.C.F. Vitamin A (retinol) downregulates the receptor for advanced glycation endproducts (RAGE) by oxidant-dependent activation of p38 MAPK and NF-kB in human lung cancer A549 cells. Cell. Signal. 2013, 25, 939–954. [Google Scholar] [CrossRef]
- Bialowas-McGoey, L.A.; Lesicka, A.; Whitaker-Azmitia, P.M. Vitamin E increases S100B-mediated microglial activation in an S100B-overexpressing mouse model of pathological aging. Glia 2008, 56, 1780–1790. [Google Scholar] [CrossRef]
- A Erickson, M.; Hansen, K.; Banks, W.A. Inflammation-induced dysfunction of the low-density lipoprotein receptor-related protein-1 at the blood-brain barrier: Protection by the antioxidant N-acetylcysteine. Brain Behav. Immun. 2012, 26, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Mentor, S.; Fisher, D. Aggressive Antioxidant Reductive Stress Impairs Brain Endothelial Cell Angiogenesis and Blood Brain Barrier Function. Curr. Neurovascular Res. 2017, 14, 71–81. [Google Scholar] [CrossRef] [PubMed]

| LC-MS/MS | HPLC-UV | |
|---|---|---|
| LLOQ (nmol/L) | 0.25 | 10 |
| LLOD (nmol/L) | 0.125 | 6 |
| Linearity (nmol/L) | 500 | 200 |
| Run Time (minutes) | 7 | 25 |
| Intra-Assay Imprecision (CV%) | Inter-Assay Imprecision (CV%) | Recovery (Ave.%) | |
|---|---|---|---|
| Baseline | 3.6 | 7.2 | − |
| Low Spike (10 nmol/L) | 5.6 | 6.4 | 84 |
| High Spike (100 nmol/L) | 5.9 | − | 103 |
| EQC Plasma | − | 6.7 | − |
| Lipoprotein Fraction | Untreated Serum CoQ10 nmol/L | Supplemented Serum CoQ10 nmol/L |
|---|---|---|
| HDL | 32.7 ± 1.8 (21.7%) | 762 ± 8.1 (7.2%) |
| LDL | 117.0 ± 1.2 (77.7%) | 6718 ± 103.5 (63.8%) |
| vLDL | 0.9 ± 0.01 (0.6%) | 3060 ± 138.8 (29.0%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wainwright, L.; Hargreaves, I.P.; Georgian, A.R.; Turner, C.; Dalton, R.N.; Abbott, N.J.; Heales, S.J.R.; Preston, J.E. CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport. J. Clin. Med. 2020, 9, 3236. https://doi.org/10.3390/jcm9103236
Wainwright L, Hargreaves IP, Georgian AR, Turner C, Dalton RN, Abbott NJ, Heales SJR, Preston JE. CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport. Journal of Clinical Medicine. 2020; 9(10):3236. https://doi.org/10.3390/jcm9103236
Chicago/Turabian StyleWainwright, Luke, Iain P. Hargreaves, Ana R. Georgian, Charles Turner, R. Neil Dalton, N. Joan Abbott, Simon J. R. Heales, and Jane E. Preston. 2020. "CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport" Journal of Clinical Medicine 9, no. 10: 3236. https://doi.org/10.3390/jcm9103236
APA StyleWainwright, L., Hargreaves, I. P., Georgian, A. R., Turner, C., Dalton, R. N., Abbott, N. J., Heales, S. J. R., & Preston, J. E. (2020). CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport. Journal of Clinical Medicine, 9(10), 3236. https://doi.org/10.3390/jcm9103236







