Shaving Technique and Compression Therapy for Elephantiasis Nostras Verrucosa (Lymphostatic Verrucosis) of Forefeet and Toes in End-Stage Primary Lymphedema: A 5 Year Follow-Up Study in 28 Patients and a Review of the Literature
Abstract
1. Introduction
- (1)
- elephantiasis tropica (due to filariasis);
- (2)
- elephantiasis nostras due to recurrent infections;
- (3)
- (4)
- Elephantiasis due to primary lymphedema.
2. Patients and Methods
2.1. Patients
2.2. Measurements
2.3. Gene Testing
2.4. Preoperative Treatment
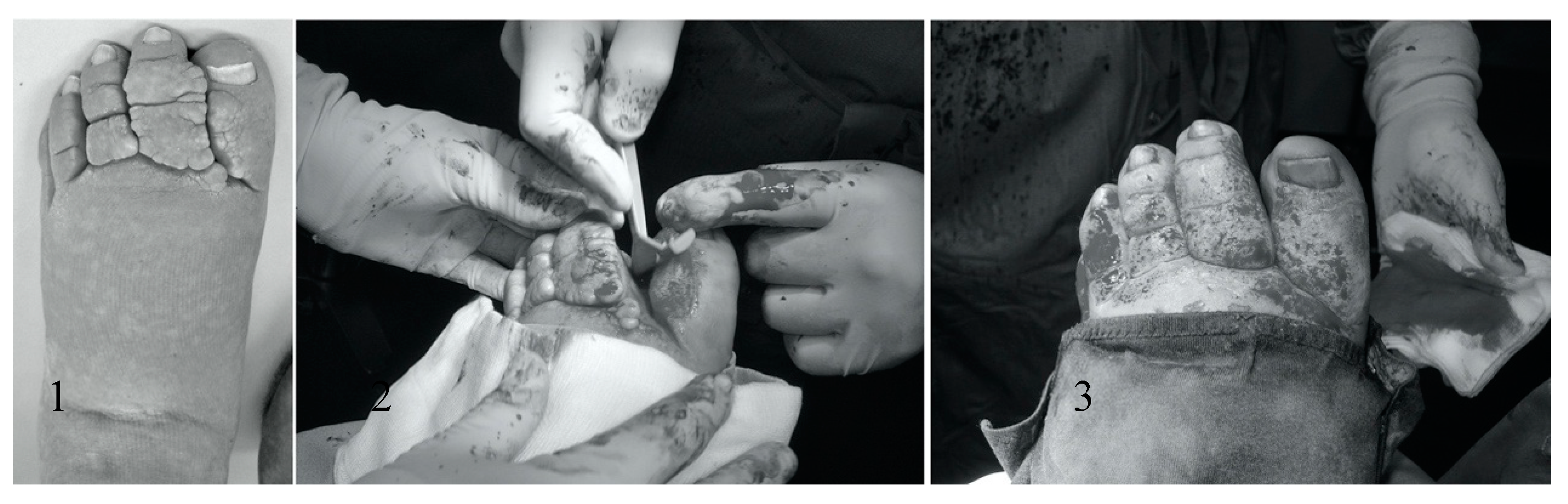
2.5. Surgical Procedure
2.6. Postoperative Treatment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fife, C.E.; Carter, M.J. Lymphedema in the morbidly obese patient: Unique challenges in a unique population. Ostomy Wound Manag. 2008, 54, 44–56. [Google Scholar]
- Sinha, P.; Tripathy, D.M.; Radhakrishnan, S.; Verghese, B.; Sunita, B.S. Tuberculosis verrucosa cutis presenting with unilateral elephantiasis nostras verrucosa of the left lower limb: A rare entity. Int. J. Mycobacteriol. 2019, 8, 202–204. [Google Scholar]
- Stemmer, R. A clinical symptom for the early and differential diagnosis of lymphedema. VASA 1976, 5, 261–262. [Google Scholar]
- International Society of Lymphology. The Diagnosis and Treatment of Peripheral Lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology 2016, 49, 170–184. [Google Scholar]
- Davis, P.L. Elephantiasis nostras verrucosa; lymphostatic verrucosis. AMA Arch. Derm. 1955, 71, 644. [Google Scholar] [CrossRef] [PubMed]
- Castellani, A. Researches on elephantiasis nostras and elephantiasis tropica with special regard to their initial stage of recurring lymphangitis (lymphangitis recurrens elephantogenica). Supplementary note. J. Trop. Med. Hyg. 1969, 72, 214–215. [Google Scholar] [PubMed]
- Wendemagegn, E.; Tirumalae, R.; Böer-Auer, A. Histopathological and immunohistochemical features of nodular podoconiosis. J. Cutan. Pathol. 2015, 42, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.M.; Zirwas, M.J.; Horst, A.V. Elephantiasis nostras verrucosa: An institutional analysis of 21 cases. J. Am. Acad. Dermatol. 2011, 64, 1104–1110. [Google Scholar] [CrossRef]
- Mendoza, N.; Li, A.; Gill, A.; Tyring, S. Filariasis: Diagnosis and treatment. Dermatol. Ther. 2009, 22, 475–490. [Google Scholar] [CrossRef]
- Korevaar, D.A.; Visser, B.J. Podoconiosis, a neglected tropical disease. Neth. J. Med. 2012, 70, 210–214. [Google Scholar]
- De Godoy, J.M.P.; Azoubel, L.M.; Godoy, M.D.F.G. Surgical treatment of elephantiasis of the feet in congenital lymphedema to facilitate the use of a compression mechanism. Int. J. Gen. Med. 2010, 3, 115–118. [Google Scholar] [CrossRef]
- Zouboulis, C.; Biczó, S.; Gollnick, H.; Reupke, H.-J.; Rinck, G.; Szabo, M.; Fekete, J.; Orfanos, C.; Zouboulis, C.C. Elephantiasis nostras verrucosa: Beneficial effect of oral etretinate therapy. Br. J. Dermatol. 1992, 127, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Marasca, C.; Mascolo, M.; Ferrillo, M.; Masarà, A.; Annunziata, M.; Iacobelli, A.; Fabbrocini, G. Acitretin may improve symptoms and exudation in patients affected by elephantiasis nostras verrucosa: Report of a case. Int. Wound J. 2018, 16, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Iwao, F.; Sato-Matsumura, K.C.; Sawamura, D.; Shimizu, H. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermat. Surg. 2004, 30, 939–941. [Google Scholar]
- Doscher, M.E.; Herman, S.; Garfein, E.S. Surgical Management of Inoperable Lymphedema: The Re-emergence of Abandoned Techniques. J. Am. Coll. Surg. 2012, 215, 278–283. [Google Scholar] [CrossRef]
- Lazzeri, D.; Larcher, L.; Huemer, G.M.; Riml, S.; Grassetti, L.; Pantaloni, M.; Li, Q.; Zhang, Y.X.; Spinelli, G.; Agostini, T. Surgical correction of rhinophyma: Comparison of two methods in a 15-year-long experience. J. Cranio Maxillofac. Surg. 2013, 41, 429–436. [Google Scholar] [CrossRef]
- Connell, F.; Brice, G.; Jeffery, S.; Keeley, V.; Mortimer, P.; Mansour, S. A new classification system for primary lymphatic dysplasias based on phenotype. Clin. Genet. 2010, 77, 438–452. [Google Scholar] [CrossRef]
- Lamprou, D.-A.A.; Voesten, H.G.J.; Damstra, R.J.; Wikkeling, O.R.M. Circumferential suction-assisted lipectomy in the treatment of primary and secondary end-stage lymphoedema of the leg. Br. J. Surg. 2016, 104, 84–89. [Google Scholar] [CrossRef]
- Damstra, R.; Van Steensel, M.A.M.; Boomsma, J.; Nelemans, P.; Veraart, J. Erysipelas as a sign of subclinical primary lymphoedema: A prospective quantitative scintigraphic study of 40 patients with unilateral erysipelas of the leg. Br. J. Dermatol. 2008, 158, 1210–1215. [Google Scholar] [CrossRef]
- Soo, J.; Bicanic, T.; Heenan, S.; Mortimer, P. Lymphatic abnormalities demonstrated by lymphoscintigraphy after lower limb cellulitis. Br. J. Dermatol. 2008, 158, 1350–1353. [Google Scholar] [CrossRef]
- Damstra, R.; Halk, A.-B.; Damstra, R.; Halk, B.; Berg, J.V.D.; Born, Y.; Butter, E.; Van Dorst, E.; Van Everdingen, J.; Feenstra, C.; et al. The Dutch lymphedema guidelines based on the International Classification of Functioning, Disability, and Health and the chronic care model. J. Vasc. Surg. 2017, 5, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Andrade, M.; Bergan, J.; Boccardo, F.; Campisi, C.; Damstra, R.; Flour, M.; Gloviczki, P.; Laredo, J.; Piller, N.; et al. Diagnosis and treatment of primary lymphedema. Consensus document of the International Union of Phlebology (IUP)-2009. Int. Angiol. 2010, 29, 454–470. [Google Scholar] [PubMed]
- Sisto, K.; Khachemoune, A. Elephantiasis nostras verrucosa: A review. Am. J. Clin. Dermatol. 2008, 9, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Arrault, M.; Vignes, S. Pediatric primary lymphoedema: A cohort of 155 children and newborns. Br. J. Dermatol. 2016, 175, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Ferrandiz, L.; Moreno-Ramirez, D.; Perez-Bernal, A.M.; Camacho, F. Elephantiasis Nostras Verrucosa Treated with Surgical Débridement. Dermatol. Surg. 2005, 31, 731. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Ahn, J.-J.; Haw, S.; Shin, M.-K.; Haw, C.-R. A Case of Elephantiasis Nostras Verrucosa. Ann. Dermatol. 2009, 21, 326–329. [Google Scholar] [CrossRef]
- Keong, N.K.; Ngi, A.T.S.; Muniandy, P.; Fei, W.V. Elephantiasis nostras verrucosa: A rare complication of lower limb lymphoedema. BMJ Case Rep. 2017, 2017, bcr-2017-221492. [Google Scholar] [CrossRef]
- Borst, G.M.; Goettler, C.E.; Kachare, S.D.; Sherman, R.A. Maggot Therapy for Elephantiasis Nostras Verrucosa Reveals New Applications and New Complications. Int. J. Low. Extrem. Wounds 2014, 13, 135–139. [Google Scholar] [CrossRef]
- Liaw, F.-Y.; Huang, C.-F.; Wu, Y.-C.; Wu, B.-Y. Elephantiasis nostras verrucose: Swelling with verrucose appearance of lower limbs. Can. Fam. Phys. Med. Fam. Can. 2012, 58, e551–e553. [Google Scholar]
- Schiavo, A.L.; Alfano, R.; Caccavale, S. Elephantiasis nostras verrucosa in a patient with obesity and chronic venous insufficiency. Int. J. Dermatol. 2013, 52, 461–462. [Google Scholar] [CrossRef]
- Hennessy, M.M.; O’Brien, G.C. Gross-dependent lower limb lymphoedema. Clin. Case Rep. 2017, 5, 150–153. [Google Scholar] [CrossRef]
- Turhan, E.; Ege, A.; Keser, S.; Bayar, A. Elephantiasis nostras verrucosa complicated with chronic tibial osteomyelitis. Arch. Orthop. Trauma Surg. 2007, 128, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, M.; Borgia, F.; Guarneri, F.; Cannavò, S.P. Elephantiasis nostras verrucosa. Int. J. Dermatol. 2000, 39, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, C.; Vaccaro, M. What is your call? Cobblestone-like skin. Elephantiasis nostras verrucosa. CMAJ 2008, 179, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.; Rodrigues, J.F.M. Elephantiasis nostras verrucosa secondary to recurrent erysipelas. BMJ Case Rep. 2017, 2017, bcr-2017-221014. [Google Scholar] [CrossRef]
- Eda, Y.; Arita, Y.; Ogasawara, N.; Hasegawa, S. Tolvaptan for the Treatment of Elephantiasis Nostras Verrucosa. Intern. Med. 2019, 58, 3347–3348. [Google Scholar] [CrossRef]
- Lee, K.R.; Bershow, A.; Crowson, A.N. Elephantiasis nostras verrucosa secondary to scleroderma. Cutis 2019, 103, E4–E6. [Google Scholar]
- Pitcher, A.A.; Pagan, C.A.; Small, K.; Otterburn, D.M. Excision of Elephantiasis Nostras Verrucosa Lesions in a Patient with Hereditary Lymphedema: Case Report and Review of the Literature. J. Foot Ankle Surg. 2015, 54, 747–750. [Google Scholar] [CrossRef]


| Diagnosis of Primary Lymphedema | ORPHAnet Code | Genetic Mutation |
|---|---|---|
| Late-onset nonhereditary lymphedema | ORPHA 90185 | Not tested: n = 13 |
| No FLT4/FOXC2: n = 5 | ||
| Results NGS: | ||
| Negative: n = 1 | ||
| FAT4: n = 1 | ||
| CCA CHrX: NM000169.2 C457G > A (Asp 153 Asn); class 3 hetrozygote: n = 1 | ||
| Lymphedema distichiasis syndrome | ORPHA 33001 | FOXC2 (n = 3) |
| Meige syndrome | ORPHA 90186 | Not tested (n = 2) |
| Generalized lymphatic dysplasia | ORPHA 2136 | No gene found (n = 1) |
| Noonan syndrome | ORPHA 648 | RIT1 gene (n = 1) |
| At Time of Admission | Follow-Up | |
|---|---|---|
| Gender (male/female) | M = 13/F = 15 | |
| Age of onset lymphedema | Δ 16.3 (0–34) | |
| Age first visit our clinic | Δ 44.7 (32–66) | |
| Duration of lymphedema at first visit in years | Δ 27.5 (6–36) | |
| Age at onset of swelling toes in years | Δ 21 (0–44) | |
| Duration of swelling toes at first visit in years | Δ 26.6 (5–50) | |
| Mean age (years) at first attack of erysipelas | Δ 20.5 (5–44) | |
| Wound healing in weeks | Δ 3.6 | |
| Wearing of toecaps | n = 28/0 | n = 28/28 |
| N erysipelas: | Δ 17.6 (self-reported and hospital records) | Δ 0.6 (0–9) (during follow-up phase) |
| ≤10 | n = 10; Δ 3.3 (1–9) | |
| >10 | n = 18; Δ 25.5 (10–50) | |
| Overweight/obesity | BMI < 28: n = 10 | BMI < 28: n = 9 |
| BMI 28–35: n = 12 | BMI 28–35: n = 13 | |
| BMI > 35: n = 6 | BMI >35: n = 6 | |
| Total mean: | Δ 30.0 (20.0–42.3) | Δ 30.7 (20.1–44.1) |
| Weight control during follow-up | Improved: n = 9 | |
| Stable: n = 14 | ||
| Worsened: n = 5 |
| Reference | Location of ENV | Diagnosis | N of Patients | Erysipelas n/year PreOP | Overweight/BMI | Type of Treatment | Compression/Toecaps | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Ferrandiz et al. [25] | Feet | EVN type 2 (infections) | 1 | Several | Unknown | Excision | Not mentioned | 1 year |
| Sinha et al. [2] | Feet | EVN type 3: cutaneous tuberculosis | 1 | NA | Normal according to picture | Medication | Not mentioned | No |
| deGodoy et al. [11] | Lower leg | EVN type 4 | 2 | NA | Unknown | Excision | Yes | No |
| Yang et al. [26] | Lower leg | EVN type 2 | 1 | Several | Unknown | Etretinate | Not mentioned | No |
| Kar Keong et al. [27] | Lower leg | EVN type 4 | 1 | 6x/2 years | Obese | Suggestion for amputation | Not mentioned | |
| Borst et al. [28] | Lower leg | EVN type 3 | 1 | Several | Unknown | Surgical debridement and maggots | Not mentioned | No |
| Liaw et al. [29] | Lower leg | EVN type 3 (incl. obesity) | 1 | NA | BMI 40.4 | Passed away | Not mentioned | No |
| Dean et al. [8] | Lower leg | EVN type 3 (mainly obesity and CVI) | 21 | Many times | BMI 30–40 (n = 2) BMI > 40 (n = 19) | Retinoids, debridement, and excision | Yes | No |
| Schiavo et al. [30] | Lower leg | EVN 3 (obesity and CVI) | 1 | NA | BMI 41 | Conservative without result | Yes | No |
| Hennessy et al. [31] | Lower leg | EVN 3 (incl. obesity) | 1 | NA | BMI 38.8 | Not mentioned | Yes | No |
| Turhan et al. [32] | 1 leg | EVN 3 (osteomyelitis/swelling) | 1 | NA | Unknown | Amputation | Not mentioned | No |
| Iwao et al. [14] | Lower leg | EVN 3 (paralysis and swelling) | 1 | NA | Unknown | Shaving | Yes/stockings | No |
| Vaccaro et al. [33] | Lower leg | EVN type 3 (obesity; internal morbidity) | 1 | NA | Severe obesity | Antibiotics and died | Not mentioned | NA |
| Guarneri et al. [34] | Lower leg | EVN 3 (comorbidly) | 1 | Several | Unknown | Conservative without clearance of skin changes | Not mentioned | No |
| Freitas et al. [35] | Lower leg | EVN 3 (comorbidly) | 1 | Several | Unknown | Antibiotics and conservative treatment | Not mentioned | No |
| Eda et al. [36] | Lower leg | EVN 3 (comorbidly) | 1 | Several | BMI 44.2 | Compression, obesity management, and tolvapan | Yes | No |
| Lee et al. [37] | Lower leg | EVN by scleroderma | 1 | Few | Unknown | Treatment of scleroderma | Not mentioned | No |
| Pitcher et al. [38] | Lower leg | EVN type 4 | 1 | Many | BMI 27 | Excision | Not mentioned | 1 year |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damstra, R.J.; Dickinson-Blok, J.L.; Voesten, H.G.J.M. Shaving Technique and Compression Therapy for Elephantiasis Nostras Verrucosa (Lymphostatic Verrucosis) of Forefeet and Toes in End-Stage Primary Lymphedema: A 5 Year Follow-Up Study in 28 Patients and a Review of the Literature. J. Clin. Med. 2020, 9, 3139. https://doi.org/10.3390/jcm9103139
Damstra RJ, Dickinson-Blok JL, Voesten HGJM. Shaving Technique and Compression Therapy for Elephantiasis Nostras Verrucosa (Lymphostatic Verrucosis) of Forefeet and Toes in End-Stage Primary Lymphedema: A 5 Year Follow-Up Study in 28 Patients and a Review of the Literature. Journal of Clinical Medicine. 2020; 9(10):3139. https://doi.org/10.3390/jcm9103139
Chicago/Turabian StyleDamstra, Robert J., Janine L. Dickinson-Blok, and Harry G.J.M. Voesten. 2020. "Shaving Technique and Compression Therapy for Elephantiasis Nostras Verrucosa (Lymphostatic Verrucosis) of Forefeet and Toes in End-Stage Primary Lymphedema: A 5 Year Follow-Up Study in 28 Patients and a Review of the Literature" Journal of Clinical Medicine 9, no. 10: 3139. https://doi.org/10.3390/jcm9103139
APA StyleDamstra, R. J., Dickinson-Blok, J. L., & Voesten, H. G. J. M. (2020). Shaving Technique and Compression Therapy for Elephantiasis Nostras Verrucosa (Lymphostatic Verrucosis) of Forefeet and Toes in End-Stage Primary Lymphedema: A 5 Year Follow-Up Study in 28 Patients and a Review of the Literature. Journal of Clinical Medicine, 9(10), 3139. https://doi.org/10.3390/jcm9103139




