Ulcerative Colitis: Current and Emerging Treatment Strategies
Abstract
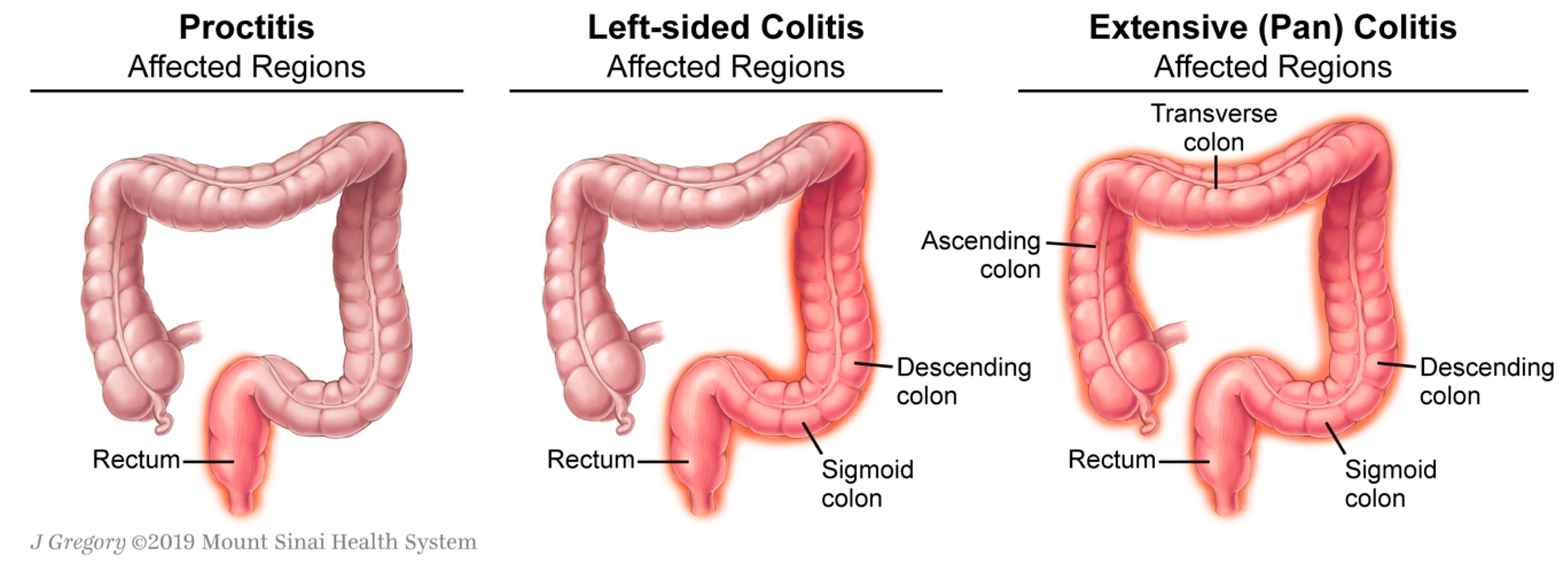
:1. Introduction
1.1. Selection of Therapy
1.2. Goals of Therapy
2. Mild-Moderate Ulcerative Colitis
3. Moderate-Severe Ulcerative Colitis
4. Acute Severe Ulcerative Colitis
5. Surgery
6. Therapies with Limited Evidence
7. Emerging Therapies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.-F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5a–36a. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.D.; Yu, A.P.; Wu, E.Q.; Xie, J.; Mulani, P.M.; Chao, J. Systematic review: The costs of ulcerative colitis in Western countries. Aliment. Pharmacol. Ther. 2010, 31, 693–707. [Google Scholar] [CrossRef]
- Roda, G.; Narula, N.; Pinotti, R.; Skamnelos, A.; Katsanos, K.H.; Ungaro, R.; Burisch, J.; Torres, J.; Colombel, J.F. Systematic review with meta-analysis: Proximal disease extension in limited ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 1481–1492. [Google Scholar] [CrossRef]
- Shah, S.C.; Colombel, J.F.; Sands, B.E.; Narula, N. Mucosal Healing Is Associated with Improved Long-term Outcomes of Patients with Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1245–1255.e8. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Ko, C.W.; Singh, S.; Feuerstein, J.D.; Falck-Ytter, C.; Falck-Ytter, Y.; Cross, R. AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 2019, 156, 748–764. [Google Scholar] [CrossRef] [Green Version]
- Truelove, S.C.; Witts, L.J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br. Med. J. 1955, 2, 1041–1048. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Chande, N.; MacDonald, J.K. Are there any differences in the efficacy and safety of different formulations of Oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? evidence from cochrane reviews. Inflamm. Bowel Dis. 2013, 19, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Bressler, B.; Marshall, J.K.; Bernstein, C.N.; Bitton, A.; Jones, J.; Leontiadis, G.I.; Panaccione, R.; Steinhart, A.H.; Tse, F.; Feagan, B. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: The Toronto consensus. Gastroenterology 2015, 148, 1035–1058.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, G.R.; Rutgeerts, P. Importance of mucosal healing in ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Manguso, F.; Zibellini, M.; Cabriada Nuno, J.L.; Goldis, A.; Tkachenko, E.; Varoli, G.; Kleczkowski, D.; Annese, V.; D’Heygere, F.; et al. Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: Results from a double-blind, randomized, parallel group study. Am. J. Gastroenterol. 2015, 110, 708–715. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Danese, S.; D’Haens, G.; Moro, L.; Jones, R.; Bagin, R.; Huang, M.; David Ballard, E.; Masure, J.; Travis, S. Induction of clinical and colonoscopic remission of mild-to-moderate ulcerative colitis with budesonide MMX 9 mg: Pooled analysis of two phase 3 studies. Aliment. Pharmacol. Ther. 2015, 41, 409–418. [Google Scholar] [CrossRef]
- Danese, S.; Siegel, C.A.; Peyrin-Biroulet, L. Review article: Integrating budesonide-MMX into treatment algorithms for mild-to-moderate ulcerative colitis. Aliment. Pharmacol. Ther. 2014, 39, 1095–1103. [Google Scholar] [CrossRef]
- In Proceedings of the Abstracts from the 2014 Advances in Inflammatory Bowel Diseases Crohn’s & Colitis Foundation’s National Clinical & Research Conference, 4–6 December 2014, Orlando, FL, USA. Inflamm. Bowel Dis. 2014, 20 (Suppl. 1), S1–S124.
- Kornbluth, A.; Sachar, D.B. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2010, 105, 501–523. quiz 524. [Google Scholar] [CrossRef]
- Naganuma, M.; Aoyama, N.; Suzuki, Y.; Nishino, H.; Kobayashi, K.; Hirai, F.; Watanabe, K.; Hibi, T. Twice-daily Budesonide 2-mg Foam Induces Complete Mucosal Healing in Patients with Distal Ulcerative Colitis. J. Crohns Colitis 2016, 10, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Bosworth, B.; Zakko, S.; Gordon, G.L.; Clemmons, D.R.; Golden, P.L.; Rolleri, R.L.; Yu, J.; Barrett, A.C.; Bortey, E.; et al. Budesonide foam induces remission in patients with mild to moderate ulcerative proctitis and ulcerative proctosigmoiditis. Gastroenterology 2015, 148, 740–750.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanauer, S.B.; Robinson, M.; Pruitt, R.; Lazenby, A.J.; Persson, T.; Nilsson, L.G.; Walton-Bowen, K.; Haskell, L.P.; Levine, J.G. Budesonide enema for the treatment of active, distal ulcerative colitis and proctitis: A dose-ranging study. U.S. Budesonide enema study group. Gastroenterology 1998, 115, 525–532. [Google Scholar] [CrossRef]
- Gross, V.; Bar-Meir, S.; Lavy, A.; Mickisch, O.; Tulassay, Z.; Pronai, L.; Kupcinskas, L.; Kiudelis, G.; Pokrotnieks, J.; Kovacs, A.; et al. Budesonide foam versus budesonide enema in active ulcerative proctitis and proctosigmoiditis. Aliment. Pharmacol. Ther. 2006, 23, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.J.; Fockens, P.; Meijer, J.W.; van der Heide, H.; Wiltink, E.H.; Tytgat, G.N. Beclomethasone dipropionate (3 mg) versus 5-aminosalicylic acid (2 g) versus the combination of both (3 mg/2 g) as retention enemas in active ulcerative proctitis. Eur. J. Gastroenterol. Hepatol. 1996, 8, 549–553. [Google Scholar] [CrossRef]
- Jarnerot, G.; Hertervig, E.; Friis-Liby, I.; Blomquist, L.; Karlen, P.; Granno, C.; Vilien, M.; Strom, M.; Danielsson, A.; Verbaan, H.; et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: A randomized, placebo-controlled study. Gastroenterology 2005, 128, 1805–1811. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [Green Version]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95. quiz e14–5. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Stidham, R.W.; Lee, T.C.; Higgins, P.D.; Deshpande, A.R.; Sussman, D.A.; Singal, A.G.; Elmunzer, B.J.; Saini, S.D.; Vijan, S.; Waljee, A.K. Systematic review with network meta-analysis: The efficacy of anti-tumour necrosis factor-alpha agents for the treatment of ulcerative colitis. Aliment. Pharmacol. Ther. 2014, 39, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Gibson, P.R.; van Langenberg, D.R. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told? World J. Gastroenterol. 2017, 23, 6385–6402. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, R.; Ghosh, S.; Middleton, S.; Marquez, J.R.; Scott, B.B.; Flint, L.; van Hoogstraten, H.J.; Chen, A.C.; Zheng, H.; Danese, S.; et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014, 146, 392–400.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Proudfoot, J.A.; Dulai, P.S.; Jairath, V.; Fumery, M.; Xu, R.; Feagan, B.G.; Sandborn, W.J. No Benefit of Concomitant 5-Aminosalicylates in Patients with Ulcerative Colitis Escalated to Biologic Therapy: Pooled Analysis of Individual Participant Data From Clinical Trials. Am. J. Gastroenterol. 2018, 113, 1197–1205. [Google Scholar] [CrossRef]
- Soler, D.; Chapman, T.; Yang, L.L.; Wyant, T.; Egan, R.; Fedyk, E.R. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J. Pharmacol. Exp. Ther. 2009, 330, 864–875. [Google Scholar] [CrossRef] [Green Version]
- Bickston, S.J.; Behm, B.W.; Tsoulis, D.J.; Cheng, J.; MacDonald, J.K.; Khanna, R.; Feagan, B.G. Vedolizumab for induction and maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2014, Cd007571. [Google Scholar] [CrossRef]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Danese, S.; Colombel, J.F.; Toruner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Hanauer, S.; Panaccione, R.; Danese, S.; Cheifetz, A.; Reinisch, W.; Higgins, P.D.R.; Woodworth, D.A.; Zhang, H.; Friedman, G.S.; Lawendy, N.; et al. Tofacitinib Induction Therapy Reduces Symptoms within 3 Days for Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- U.S. FOOD & DRUG Administration. Xeljanz, Xeljanz XR (tofacitinib): Drug Safety Communication—Due to an Increased Risk of Blood Clots and Death with Higher Dose. 2019. Available online: https://www.fda.gov/safety/medwatch-safety-alerts-human-medical-products/xeljanz-xeljanz-xr-tofacitinib-drug-safety-communication-due-increased-risk-blood-clots-and-death (accessed on 20 November 2019).
- Lynch, R.W.; Lowe, D.; Protheroe, A.; Driscoll, R.; Rhodes, J.M.; Arnott, I.D. Outcomes of rescue therapy in acute severe ulcerative colitis: Data from the United Kingdom inflammatory bowel disease audit. Aliment. Pharmacol. Ther. 2013, 38, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Walsh, C.M.; Steinhart, A.H.; Griffiths, A.M. Response to corticosteroids in severe ulcerative colitis: A systematic review of the literature and a meta-regression. Clin. Gastroenterol. Hepatol. 2007, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, S.C.; Flood, L.M.; Kilander, A.F.; Lofberg, R.; Persson, T.B.; Sjodahl, R.I. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 1998, 10, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.; Satsangi, J.; Lemann, M. Predicting the need for colectomy in severe ulcerative colitis: A critical appraisal of clinical parameters and currently available biomarkers. Gut 2011, 60, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, M.; Magnuson, A.; Bjork, J.; Benoni, C.; Almer, S.; Friis-Liby, I.; Hertervig, E.; Olsson, M.; Karlen, P.; Eriksson, A.; et al. Infliximab as rescue therapy in hospitalised patients with steroid-refractory acute ulcerative colitis: A long-term follow-up of 211 Swedish patients. Aliment. Pharmacol. Ther. 2013, 38, 377–387. [Google Scholar] [CrossRef]
- Choy, M.C.; Seah, D.; Faleck, D.M.; Shah, S.C.; Chao, C.Y.; An, Y.K.; Radford-Smith, G.; Bessissow, T.; Dubinsky, M.C.; Ford, A.C.; et al. Systematic Review and Meta-analysis: Optimal Salvage Therapy in Acute Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 1169–1186. [Google Scholar] [CrossRef]
- Shah, S.C.; Naymagon, S.; Panchal, H.J.; Sands, B.E.; Cohen, B.L.; Dubinsky, M.C. Accelerated Infliximab Dosing Increases 30-Day Colectomy in Hospitalized Ulcerative Colitis Patients: A Propensity Score Analysis. Inflamm. Bowel Dis. 2018, 24, 651–659. [Google Scholar] [CrossRef]
- Shah, S.C.; Naymagon, S.; Cohen, B.L.; Sands, B.E.; Dubinsky, M.C. There is Significant Practice Pattern Variability in the Management of the Hospitalized Ulcerative Colitis Patient at a Tertiary Care and IBD Referral Center. J. Clin. Gastroenterol. 2018, 52, 333–338. [Google Scholar] [CrossRef]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef]
- Cohen, R.D.; Stein, R.; Hanauer, S.B. Intravenous cyclosporin in ulcerative colitis: A five-year experience. Am. J. Gastroenterol. 1999, 94, 1587–1592. [Google Scholar] [CrossRef]
- Narula, N.; Marshall, J.K.; Colombel, J.F.; Leontiadis, G.I.; Williams, J.G.; Muqtadir, Z.; Reinisch, W. Systematic Review and Meta-Analysis: Infliximab or Cyclosporine as Rescue Therapy in Patients with Severe Ulcerative Colitis Refractory to Steroids. Am. J. Gastroenterol. 2016, 111, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Durai, D.; Hawthorne, A.B. Review article: How and when to use ciclosporin in ulcerative colitis. Aliment. Pharmacol. Ther. 2005, 22, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Pellet, G.; Stefanescu, C.; Carbonnel, F.; Peyrin-Biroulet, L.; Roblin, X.; Allimant, C.; Nachury, M.; Nancey, S.; Filippi, J.; Altwegg, R.; et al. Efficacy and Safety of Induction Therapy with Calcineurin Inhibitors in Combination with Vedolizumab in Patients with Refractory Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 494–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laharie, D.; Bourreille, A.; Branche, J.; Allez, M.; Bouhnik, Y.; Filippi, J.; Zerbib, F.; Savoye, G.; Nachury, M.; Moreau, J.; et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: A parallel, open-label randomised controlled trial. Lancet 2012, 380, 1909–1915. [Google Scholar] [CrossRef] [Green Version]
- Ordas, I.; Domenech, E.; Manosa, M.; Garcia-Sanchez, V.; Iglesias-Flores, E.; Penalva, M.; Canas-Ventura, A.; Merino, O.; Fernandez-Banares, F.; Gomollon, F.; et al. Long-Term Efficacy and Safety of Cyclosporine in a Cohort of Steroid-Refractory Acute Severe Ulcerative Colitis Patients from the ENEIDA Registry (1989–2013): A Nationwide Multicenter Study. Am. J. Gastroenterol. 2017, 112, 1709–1718. [Google Scholar] [CrossRef]
- Seagrove, A.C.; Alam, M.F.; Alrubaiy, L.; Cheung, W.Y.; Clement, C.; Cohen, D.; Grey, M.; Hilton, M.; Hutchings, H.; Morgan, J.; et al. Randomised controlled trial. Comparison Of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: Trial design and protocol (CONSTRUCT). BMJ Open 2014, 4, e005091. [Google Scholar] [CrossRef] [Green Version]
- Laharie, D.; Bourreille, A.; Branche, J.; Allez, M.; Bouhnik, Y.; Filippi, J.; Zerbib, F.; Savoye, G.; Vuitton, L.; Moreau, J.; et al. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut 2018, 67, 237–243. [Google Scholar] [CrossRef]
- Saito, K.; Katsuno, T.; Nakagawa, T.; Saito, M.; Sazuka, S.; Sato, T.; Matsumura, T.; Arai, M.; Miyauchi, H.; Matsubara, H. Predictive factors of response to intravenous ciclosporin in severe ulcerative colitis: The development of a novel prediction formula. Aliment. Pharmacol. Ther. 2012, 36, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Navas-Lopez, V.M.; Blasco, A.J.; Serrano, M.J.; Giron Fernandez-Crehuet, F.; Argos Rodriguez, M.D.; Sierra Salinas, C. Oral tacrolimus for pediatric steroid-resistant ulcerative colitis. J. Crohns Colitis 2014, 8, 64. [Google Scholar] [CrossRef]
- Ziring, D.A.; Wu, S.S.; Mow, W.S.; Martin, M.G.; Mehra, M.; Ament, M.E. Oral tacrolimus for steroid-dependent and steroid-resistant ulcerative colitis in children. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 306. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, X.; Chen, Z.; He, A.; Zheng, Z.; Liu, G. Efficacy of adjuvant curcumin therapy in ulcerative colitis: A meta-analysis of randomized controlled trials. J. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Soo, W.; Bryant, R.V.; Jairath, V.; Hart, A.L.; Andrews, J.M. Systematic review with meta-analysis: Faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Lowenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef] [PubMed]
- Berinstein, J.A.; Steiner, C.A.; Regal, R.E.; Allen, J.I.; Kinnucan, J.A.R.; Stidham, R.W.; Waljee, A.K.; Bishu, S.; Aldrich, L.B.; Higgins, P.D.R. Efficacy of Induction Therapy with High-Intensity Tofacitinib in 4 Patients with Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 988–990.e1. [Google Scholar] [CrossRef]
- Bekheit, M.; Baddour, N.; Katri, K.; Taher, Y.; El Tobgy, K.; Mousa, E. Hyperbaric oxygen therapy stimulates colonic stem cells and induces mucosal healing in patients with refractory ulcerative colitis: A prospective case series. BMJ Open Gastroenterol. 2016, 3, e000082. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ferrante, M.; Bhandari, B.R.; Berliba, E.; Feagan, B.G.; Hibi, T.; Tuttle, J.L.; Klekotka, P.; Friedrich, S.; Durante, M.; et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients with Ulcerative Colitis. Gastroenterology 2019. [Google Scholar] [CrossRef] [Green Version]
| Trade Name | Formulation | Dose/Frequency Induction | Dose/Frequency Maintenance |
|---|---|---|---|
| Asacol | Oral | 2.4–4.8 g daily in three divided doses | 1.6 to 2.4 g daily in one-three divided doses |
| Delzicol | Oral | 2.4–4.8 g daily in three divided doses | 1.6 g to 2.4 g in one-three divided doses |
| Lialda | Oral | 2.4–4.8 g once daily | 2.4–3.6 g once daily |
| Pentasa | Oral | 2–4 g daily in two to four divided doses | 1.5–4 g daily in four divided doses |
| Apriso | Oral | 1.5–4.5 g once daily | 1.5–3 g daily |
| Colazal | Oral | 2.25 g three times daily | 1.5–3 g twice daily |
| Canasa | Suppository | 1 g (1 suppository) once-twice daily | 1 g (1 suppository) daily |
| Rowasa | Enema | 4 g (one 60 mL unit) daily once-twice daily | 2 to 4 g (30 to 60 mL unit) daily |
| Therapeutic Class | Mechanism of Action | Formulation |
|---|---|---|
| Anti-TNF agents | Monoclonal antibodies directed against TNF-alpha, an inflammatory cytokine | |
| • Infliximab | Intravenous | |
| • Adalimumab | Subcutaneous | |
| • Golimumab | Subcutaneous | |
| Anti-integrin agents | Monoclonal antibody directed against α4β7 cell surface glycoprotein on B and T lymphocytes | |
| • Vedolizumab | Intravenous | |
| Anti-interleukin agents | Monoclonal antibody directed against p40 subunit of IL-12 and IL-23 | |
| • Ustekinumab | Intravenous (1st dose), subcutaneous | |
| Janus-kinase inhibitors | Small molecule janus kinase 1 and 3 inhibitor | |
| • Tofacitinib | Oral |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayal, M.; Shah, S. Ulcerative Colitis: Current and Emerging Treatment Strategies. J. Clin. Med. 2020, 9, 94. https://doi.org/10.3390/jcm9010094
Kayal M, Shah S. Ulcerative Colitis: Current and Emerging Treatment Strategies. Journal of Clinical Medicine. 2020; 9(1):94. https://doi.org/10.3390/jcm9010094
Chicago/Turabian StyleKayal, Maia, and Shailja Shah. 2020. "Ulcerative Colitis: Current and Emerging Treatment Strategies" Journal of Clinical Medicine 9, no. 1: 94. https://doi.org/10.3390/jcm9010094
APA StyleKayal, M., & Shah, S. (2020). Ulcerative Colitis: Current and Emerging Treatment Strategies. Journal of Clinical Medicine, 9(1), 94. https://doi.org/10.3390/jcm9010094





