Kinetics of Tear Fluid Proteins after Endothelial Keratoplasty and Predictive Factors for Recovery from Corneal Haze
Abstract
1. Introduction
2. Experimental Section
2.1. Human Subjects, Patient Characteristics, and Clinical Specimens
2.2. Tear Protein Extraction
2.3. Multiplex Analysis of Cytokines and Chemokines in Tear Fluid
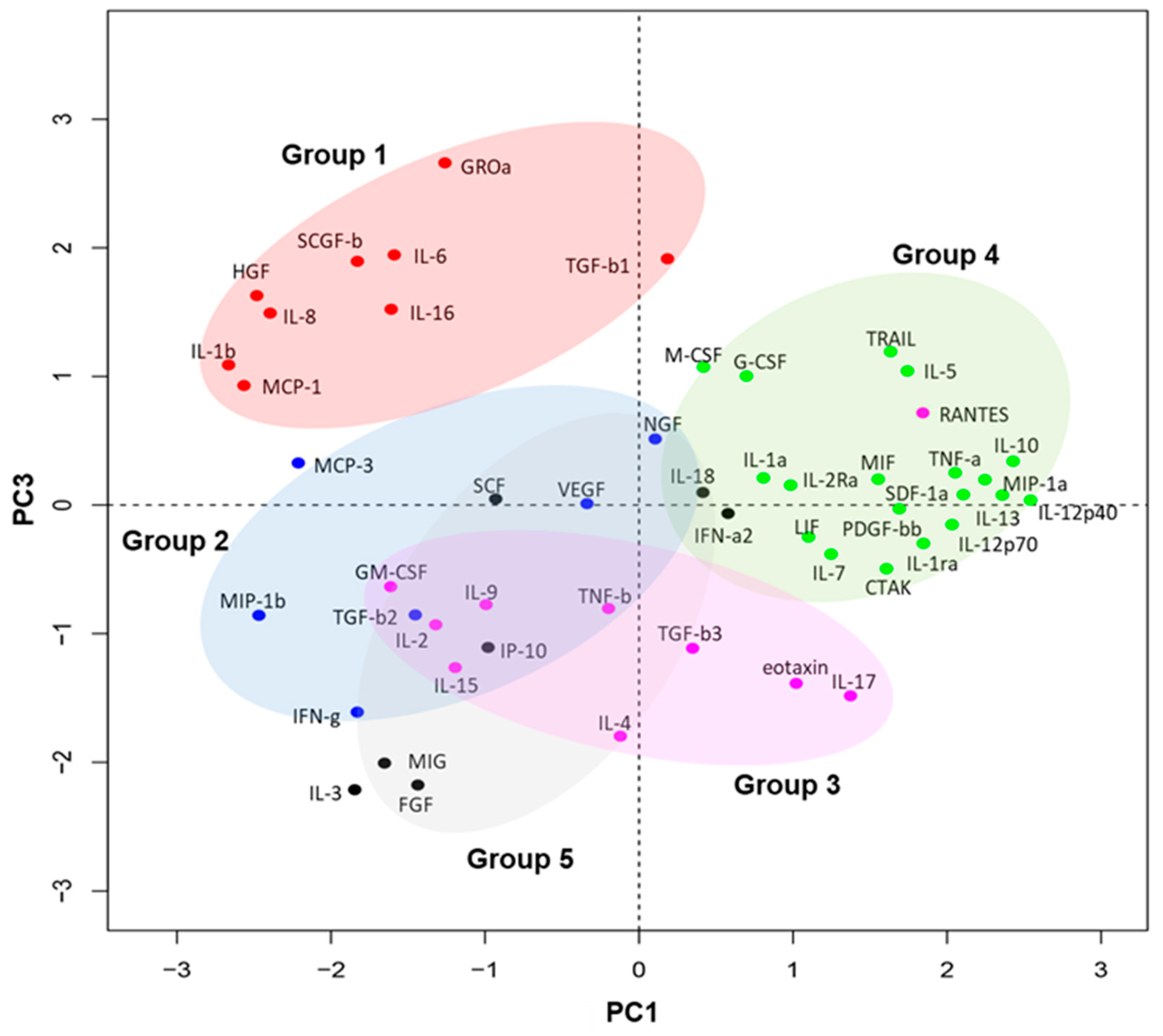
2.4. Principal Component Analysis of Tear Protein Expression Kinetics
2.5. Measurement of Tear Substance P
2.6. Statistical Analysis
3. Results
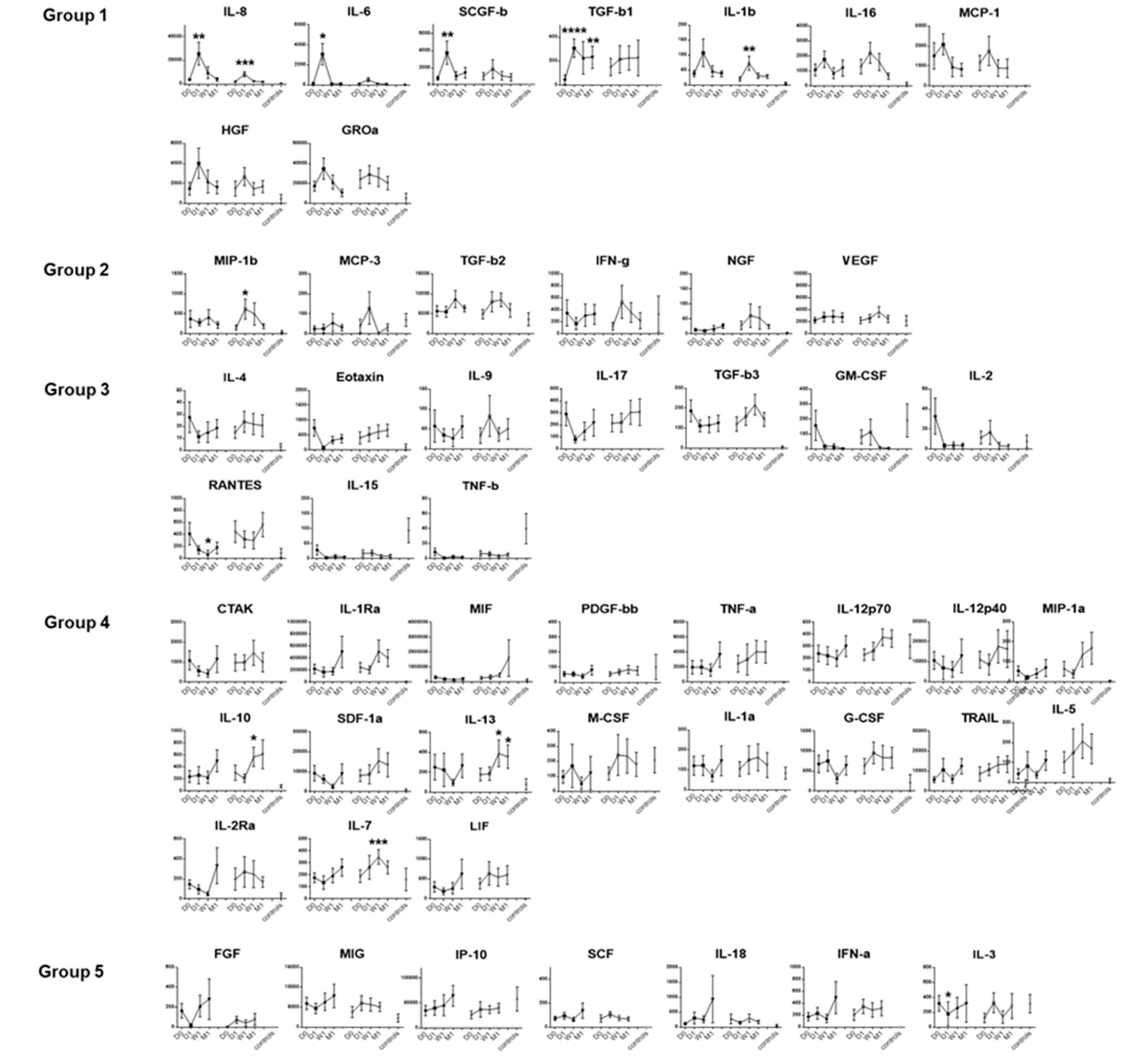
3.1. Dynamic Changes in Tear Fluid Cytokines in Both Eyes after Endothelial Keratoplasty
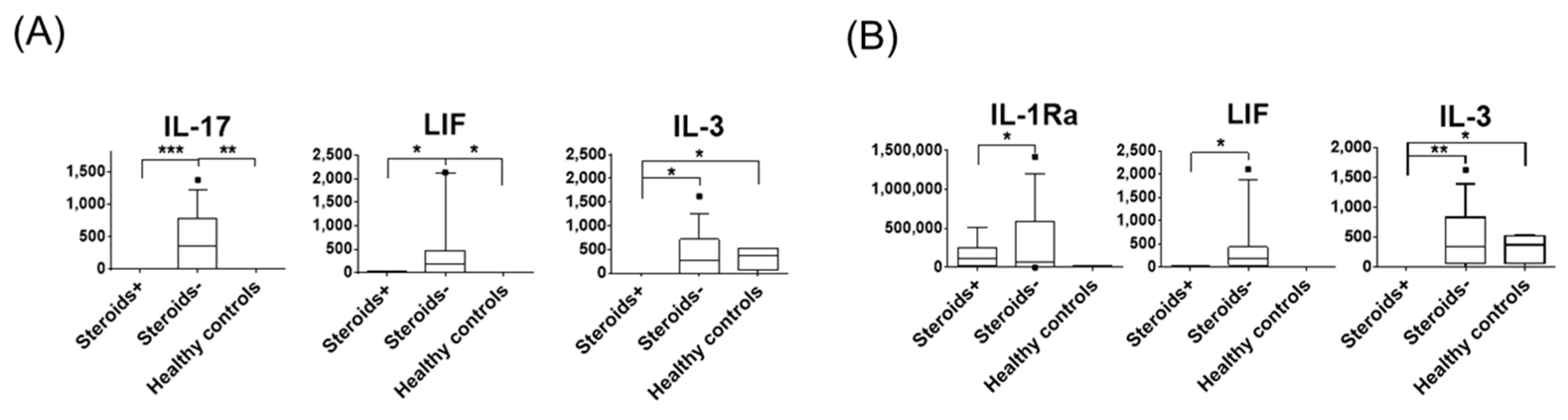
3.2. The Effect of Corticosteroids on Tear Protein Expression
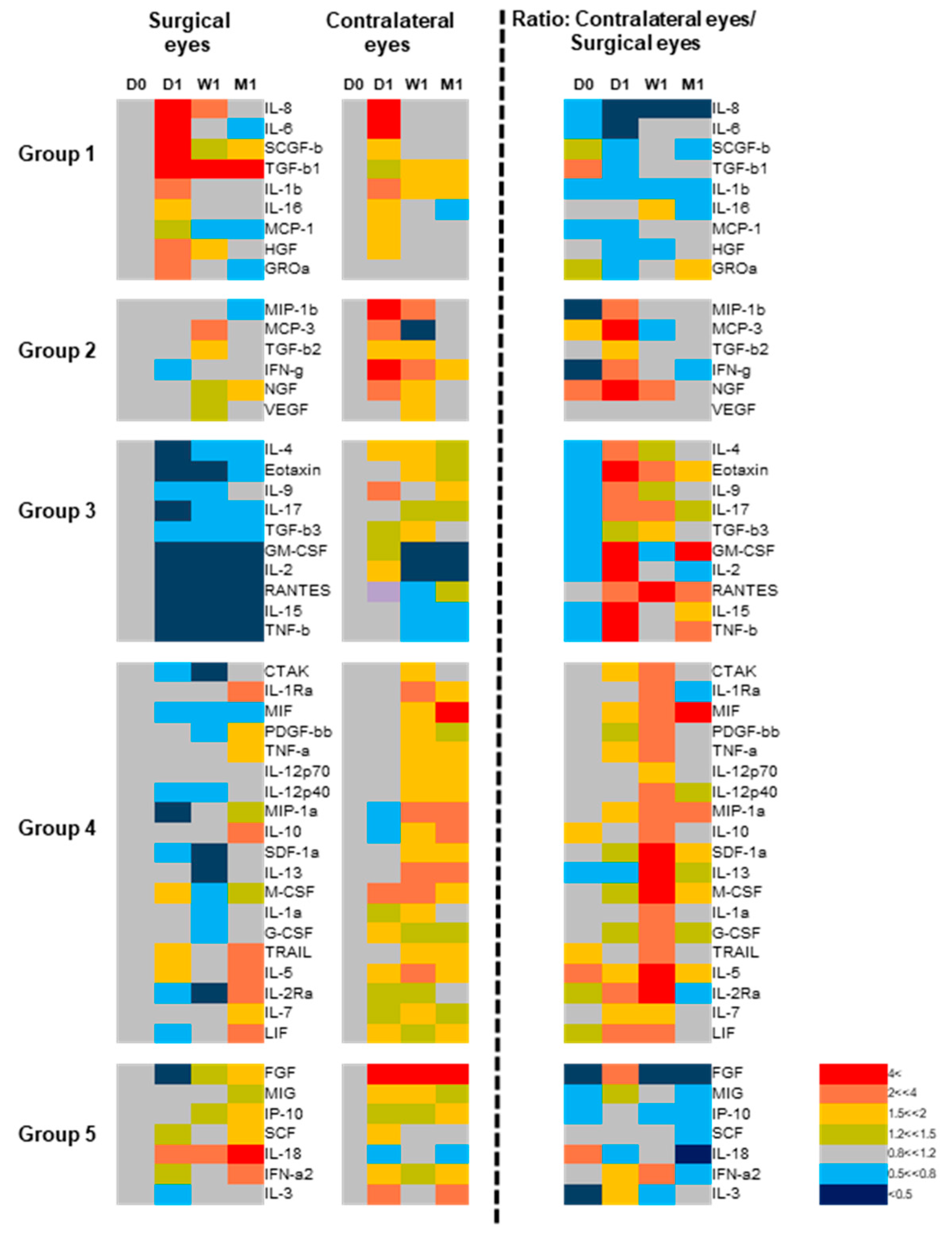
3.3. Bilateral Increase in Tear Cytokines in Patients with Unilateral Corneal Haze
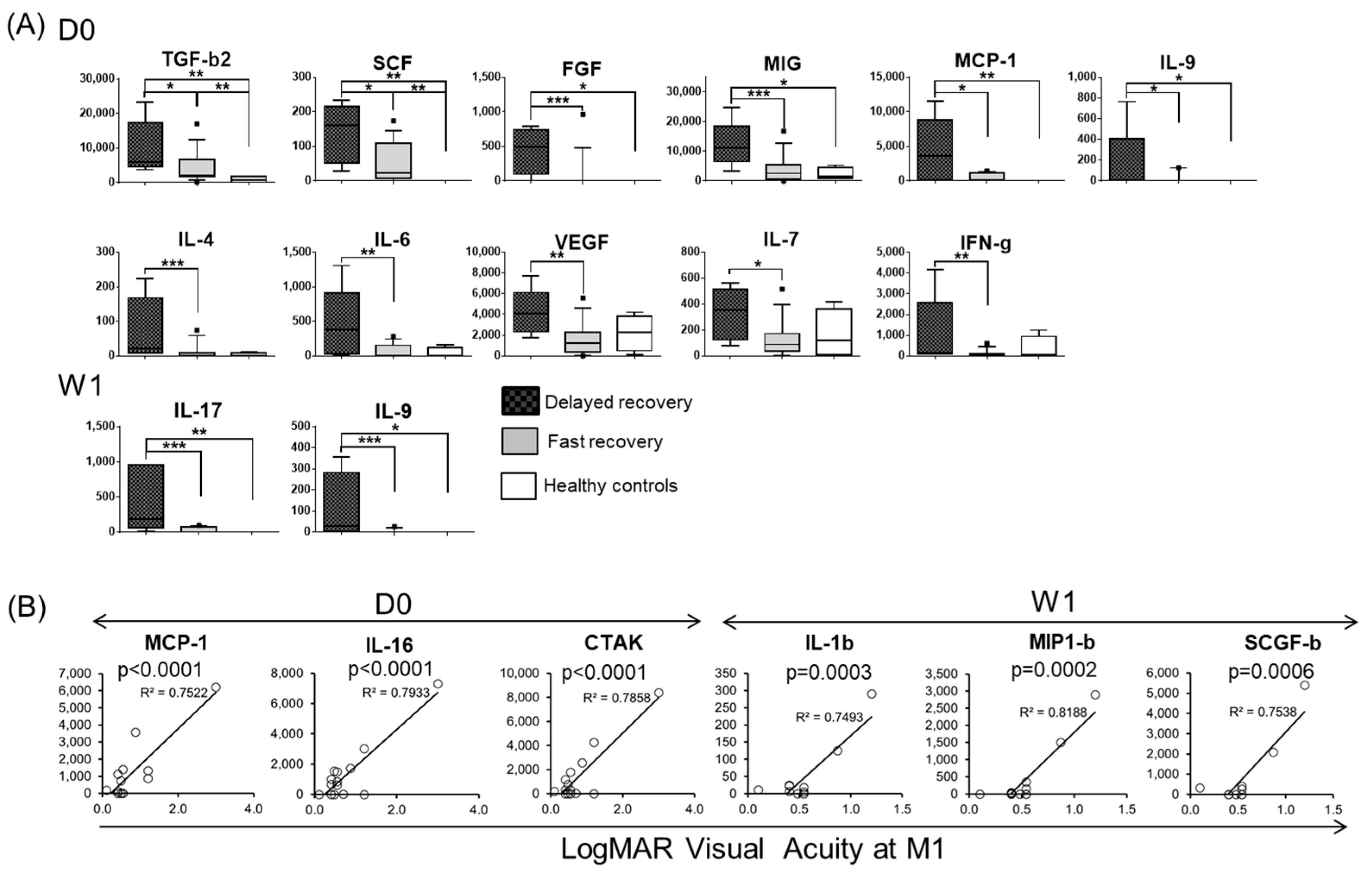
3.4. Baseline and One Week-Post Transplant Cytokine Levels Are Major Determinants of Corneal Haze at One Month after Endothelial Keratoplasty
3.5. Tear Protein at Baseline and One Week after DSAEK Are Associated with Visual Acuity at One Month Post-Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Price, M.O.; Gorovoy, M.; Price, F.W., Jr.; Benetz, B.A.; Menegay, H.J.; Lass, J.H. Descemet’s stripping automated endothelial keratoplasty: Three-year graft and endothelial cell survival compared with penetrating keratoplasty. Ophthalmology 2013, 120, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ezon, I.; Shih, C.Y.; Rosen, L.M.; Suthar, T.; Udell, I.J. Immunologic graft rejection in descemet’s stripping endothelial keratoplasty and penetrating keratoplasty for endothelial disease. Ophthalmology 2013, 120, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. Immunology of Corneal Allografts: Insights from Animal Models. J. Clin. Exp. Ophthalmol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Treacy, O.; Fahy, G.; Ritter, T.; O’Flynn, L. Corneal Immunosuppressive Mechanisms, Anterior Chamber-Associated Immune Deviation (ACAID) and Their Role in Allograft Rejection. Methods Mol. Biol. 2016, 1371, 205–214. [Google Scholar]
- Torricelli, A.A.; Wilson, S.E. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp. Eye Res. 2014, 129, 151–160. [Google Scholar] [CrossRef]
- Nicholls, S.M.; Banerjee, S.; Figueiredo, F.C.; Crome, S.; Mistry, S.; Easty, D.L.; Dick, A.D. Differences in leukocyte phenotype and interferon-gamma expression in stroma and endothelium during corneal graft rejection. Exp. Eye Res. 2006, 83, 339–347. [Google Scholar] [CrossRef]
- Chong, E.M.; Dana, M.R. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int. Ophthalmol. 2008, 28, 209–222. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Lim, R.R.; Tan, A.; Liu, Y.C.; Barathi, V.A.; Mohan, R.R.; Mehta, J.S.; Chaurasia, S.S. ITF2357 transactivates Id3 and regulate TGFbeta/BMP7 signaling pathways to attenuate corneal fibrosis. Sci. Rep. 2016, 6, 20841. [Google Scholar] [CrossRef]
- Wilson, S.E.; Mohan, R.R.; Ambrosio, R., Jr.; Hong, J.; Lee, J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Daley-Bauer, L.P.; Wynn, G.M.; Mocarski, E.S. Cytomegalovirus impairs antiviral CD8+ T cell immunity by recruiting inflammatory monocytes. Immunity 2012, 37, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Glasser, A.; Hu, Y.S.; McDermott, A.M. The effect of interleukin-1 on cytokine gene expression by human corneal epithelial cells. Exp. Eye Res. 2005, 80, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, K.H.; Nakao, S.; Nakamura, T.; Oshima, T.; Qiao, H.; Hisatomi, T.; Kinoshita, S.; Ishibashi, T. Cellular events in the normal and inflamed cornea. Cornea 2005, 24, S50–S54. [Google Scholar] [CrossRef]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.; Day, A.J.; Milner, C.M. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Mehta, J.S.; Lim, F.; Bose, S.; Htoon, H.M.; Tan, D. Endothelial cell loss and graft survival after Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology 2012, 119, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Yawata, N.; Selva, K.J.; Liu, Y.C.; Tan, K.P.; Lee, A.W.; Siak, J.; Lan, W.; Vania, M.; Arundhati, A.; Tong, L.; et al. Dynamic change in natural killer cell type in the human ocular mucosa in situ as means of immune evasion by adenovirus infection. Mucosal Immunol. 2016, 9, 159–170. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Ishii, N.; Yamaguchi, T.; Yazu, H.; Satake, Y.; Yoshida, A.; Shimazaki, J. Factors associated with graft survival and endothelial cell density after Descemet’s stripping automated endothelial keratoplasty. Sci. Rep. 2016, 6, 25276. [Google Scholar] [CrossRef]
- Dickman, M.M.; Cheng, Y.Y.; Berendschot, T.T.; van den Biggelaar, F.J.; Nuijts, R.M. Effects of graft thickness and asymmetry on visual gain and aberrations after descemet stripping automated endothelial keratoplasty. JAMA Ophthalmol. 2013, 131, 737–744. [Google Scholar] [CrossRef]
- Huang, M.; Sharma, S.; Zhu, L.X.; Keane, M.P.; Luo, J.; Zhang, L.; Burdick, M.D.; Lin, Y.Q.; Dohadwala, M.; Gardner, B.; et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J. Clin. Investig. 2002, 109, 931–937. [Google Scholar] [CrossRef]
- Qin, S.Y.; Lu, D.H.; Guo, X.Y.; Luo, W.; Hu, B.L.; Huang, X.L.; Chen, M.; Wang, J.X.; Ma, S.J.; Yang, X.W.; et al. A deleterious role for Th9/IL-9 in hepatic fibrogenesis. Sci. Rep. 2016, 6, 18694. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Perri, P.; Rimondi, E.; Melloni, E.; Lamberti, G.; Milani, D.; Secchiero, P.; Zauli, G. Kinetic Profiles of Inflammatory Mediators in the Conjunctival Sac Fluid of Patients upon Photorefractive Keratectomy. Mediat. Inflamm. 2015, 2015, 942948. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Hamrah, P.; Zhang, Q.; Liu, Y.; Huq, S.; Dana, M.R. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol. Vis. 2005, 11, 632–640. [Google Scholar] [PubMed]
- Zhu, S.; Dekaris, I.; Duncker, G.; Dana, M.R. Early expression of proinflammatory cytokines interleukin-1 and tumor necrosis factor-alpha after corneal transplantation. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 1999, 19, 661–669. [Google Scholar] [CrossRef]
- Yin, X.T.; Zobell, S.; Jarosz, J.G.; Stuart, P.M. Anti-IL-17 therapy restricts and reverses late-term corneal allorejection. J. Immunol. 2015, 194, 4029–4038. [Google Scholar] [CrossRef]
- Cruzat, A.; Schrems, W.A.; Schrems-Hoesl, L.M.; Cavalcanti, B.M.; Baniasadi, N.; Witkin, D.; Pavan-Langston, D.; Dana, R.; Hamrah, P. Contralateral Clinically Unaffected Eyes of Patients With Unilateral Infectious Keratitis Demonstrate a Sympathetic Immune Response. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6612–6620. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Calvacanti, B.M.; Cruzat, A.; Qazi, Y.; Ishikawa, S.; Osuka, A.; Lederer, J.; Hamrah, P. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7457–7466. [Google Scholar] [CrossRef]
- Ferrari, G.; Bignami, F.; Giacomini, C.; Capitolo, E.; Comi, G.; Chaabane, L.; Rama, P. Ocular surface injury induces inflammation in the brain: In vivo and ex vivo evidence of a corneal-trigeminal axis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6289–6300. [Google Scholar] [CrossRef]
- Paunicka, K.J.; Mellon, J.; Robertson, D.; Petroll, M.; Brown, J.R.; Niederkorn, J.Y. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2015, 15, 1490–1501. [Google Scholar] [CrossRef]
- Gao, B.; Sun, W.; Wang, X.; Jia, X.; Ma, B.; Chang, Y.; Zhang, W.; Xue, D. Whole genome expression profiling and screening for differentially expressed cytokine genes in human bone marrow endothelial cells treated with humoral inhibitors in liver cirrhosis. Int. J. Mol. Med. 2013, 32, 1204–1214. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hamrah, P.; Shimazaki, J. Bilateral Alterations in Corneal Nerves, Dendritic Cells, and Tear Cytokine Levels in Ocular Surface Disease. Cornea 2016, 35 (Suppl. 1), S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Twardy, B.S.; Channappanavar, R.; Suvas, S. Substance P in the corneal stroma regulates the severity of herpetic stromal keratitis lesions. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8604–8613. [Google Scholar] [CrossRef] [PubMed]
- Lema, I.; Duran, J.A. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology 2005, 112, 654–659. [Google Scholar] [CrossRef]
- Riemens, A.; Stoyanova, E.; Rothova, A.; Kuiper, J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol. Vis. 2012, 18, 797–802. [Google Scholar] [PubMed]
- VanDerMeid, K.R.; Su, S.P.; Krenzer, K.L.; Ward, K.W.; Zhang, J.Z. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol. Vis. 2011, 17, 1056–1063. [Google Scholar]
- Landsend, E.C.S.; Utheim, O.A.; Pedersen, H.R.; Aass, H.C.D.; Lagali, N.; Dartt, D.A.; Baraas, R.C.; Utheim, T.P. The Level of Inflammatory Tear Cytokines is Elevated in Congenital Aniridia and Associated with Meibomian Gland Dysfunction. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2197–2204. [Google Scholar] [CrossRef]
- Shoji, J.; Aso, H.; Inada, N. Clinical Usefulness of Simultaneous Measurement of the Tear Levels of CCL17, CCL24, and IL-16 for the Biomarkers of Allergic Conjunctival Disorders. Curr. Eye Res. 2017, 42, 677–684. [Google Scholar] [CrossRef]
- Shoji, J.; Inada, N.; Sawa, M. Antibody array-generated cytokine profiles of tears of patients with vernal keratoconjunctivitis or giant papillary conjunctivitis. Jpn. J. Ophthalmol. 2006, 50, 195–204. [Google Scholar] [CrossRef]

| Age | Gender | Indication | History of Corneal Transplantation | Presurgery Topical Treatment | IOL | History of Glaucoma Treatment | Neuroretinal Damage | Recovery | Rejection in This Study | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rejection | Steroids | Others | ||||||||||
| 1 | 72 | F | BK | Timolol | + | + | + | Delayed | ||||
| 2 | 74 | F | BK | PKP | + | + | Delayed | + | ||||
| 3 | 76 | F | BK | + | Fast | |||||||
| 4 | 85 | M | BK | + | NA | |||||||
| 5 | 91 | F | BK | + | Fast | |||||||
| 6 | 81 | F | FED | DSAEK (contralateral eye) | Fast | |||||||
| 7 | 58 | M | BK | DSAEK | + | + | + | Fast | ||||
| 8 | 75 | F | BK | Fast | ||||||||
| 9 | 65 | F | FED | DSAEK (contralateral eye) | + | Fast | ||||||
| 10 | 72 | M | BK | DSAEK | + | Timolol, Brimonidine | + | Delayed | ||||
| 11 | 50 | M | BK | Timolol | + | Fast | + | |||||
| 12 | 73 | M | BK | + | Delayed | |||||||
| 13 | 83 | M | BK | + | Fast | |||||||
| 14 | 75 | F | BK | Fast | ||||||||
| 15 | 78 | F | BK | + | + | Fast | ||||||
| 16 | 38 | M | BK | + | + | + | Fast | |||||
| 17 | 30 | F | BK | + | + | Fast | ||||||
| 18 | 64 | M | BK | + | Fast | |||||||
| 19 | 61 | M | BK | + | Delayed | |||||||
| 20 | 63 | F | BK | + | Fast | |||||||
| 21 | 87 | F | BK | DSAEK | + | Fast | ||||||
| Cytokines | Chemokines | Growth Factors | |||
|---|---|---|---|---|---|
| Wound Healing | Adaptive Immunity | Innate Immunity | |||
| Proinflammatory | Immunoregulatory | Proinflammatory | |||
| IL-1a * | IL-2 | IL-4 | IL-6 | Eotaxin | SCGF-b |
| IL-1b * | IL-2Ra | IL-5 | IL-8 | IP-10 | SCF |
| IL-1Ra * | IL-9 | IL-10 | IL-12 p40 | MCP-1 | B-NGF |
| TGF-b1 * | IL-16 | IL-13 | IL-12 p70 | MIP-1a | LIF |
| TGF-b2 * | IL-17 | TRAIL | IL-15 | MIP-1b | IL-3 |
| TGF-b3 * | MIF | IL-18 | RANTES | IL-7 | |
| G-CSF | IFN-γ | MCP-3 | GM-CSF | ||
| M-CSF | IFN-a2 | MIG | |||
| VEGF * | TNF-a | CTAK | |||
| FGF * | TNF-b | GRO-a | |||
| PDGF-bb * | SDF-1a | ||||
| HGF # | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yawata, N.; Awate, S.; Liu, Y.-C.; Yuan, S.; Woon, K.; Siak, J.; Kawano, Y.-I.; Sonoda, K.-H.; Mehta, J.S.; Yawata, M. Kinetics of Tear Fluid Proteins after Endothelial Keratoplasty and Predictive Factors for Recovery from Corneal Haze. J. Clin. Med. 2020, 9, 63. https://doi.org/10.3390/jcm9010063
Yawata N, Awate S, Liu Y-C, Yuan S, Woon K, Siak J, Kawano Y-I, Sonoda K-H, Mehta JS, Yawata M. Kinetics of Tear Fluid Proteins after Endothelial Keratoplasty and Predictive Factors for Recovery from Corneal Haze. Journal of Clinical Medicine. 2020; 9(1):63. https://doi.org/10.3390/jcm9010063
Chicago/Turabian StyleYawata, Nobuyo, Sunita Awate, Yu-Chi Liu, Shi Yuan, Kaing Woon, Jay Siak, Yoh-Ichi Kawano, Koh-Hei Sonoda, Jodhbir S. Mehta, and Makoto Yawata. 2020. "Kinetics of Tear Fluid Proteins after Endothelial Keratoplasty and Predictive Factors for Recovery from Corneal Haze" Journal of Clinical Medicine 9, no. 1: 63. https://doi.org/10.3390/jcm9010063
APA StyleYawata, N., Awate, S., Liu, Y.-C., Yuan, S., Woon, K., Siak, J., Kawano, Y.-I., Sonoda, K.-H., Mehta, J. S., & Yawata, M. (2020). Kinetics of Tear Fluid Proteins after Endothelial Keratoplasty and Predictive Factors for Recovery from Corneal Haze. Journal of Clinical Medicine, 9(1), 63. https://doi.org/10.3390/jcm9010063






