Safety of P28GST, a Protein Derived from a Schistosome Helminth Parasite, in Patients with Crohn’s Disease: A Pilot Study (ACROHNEM)
Abstract
1. Introduction
2. Materials and Methods
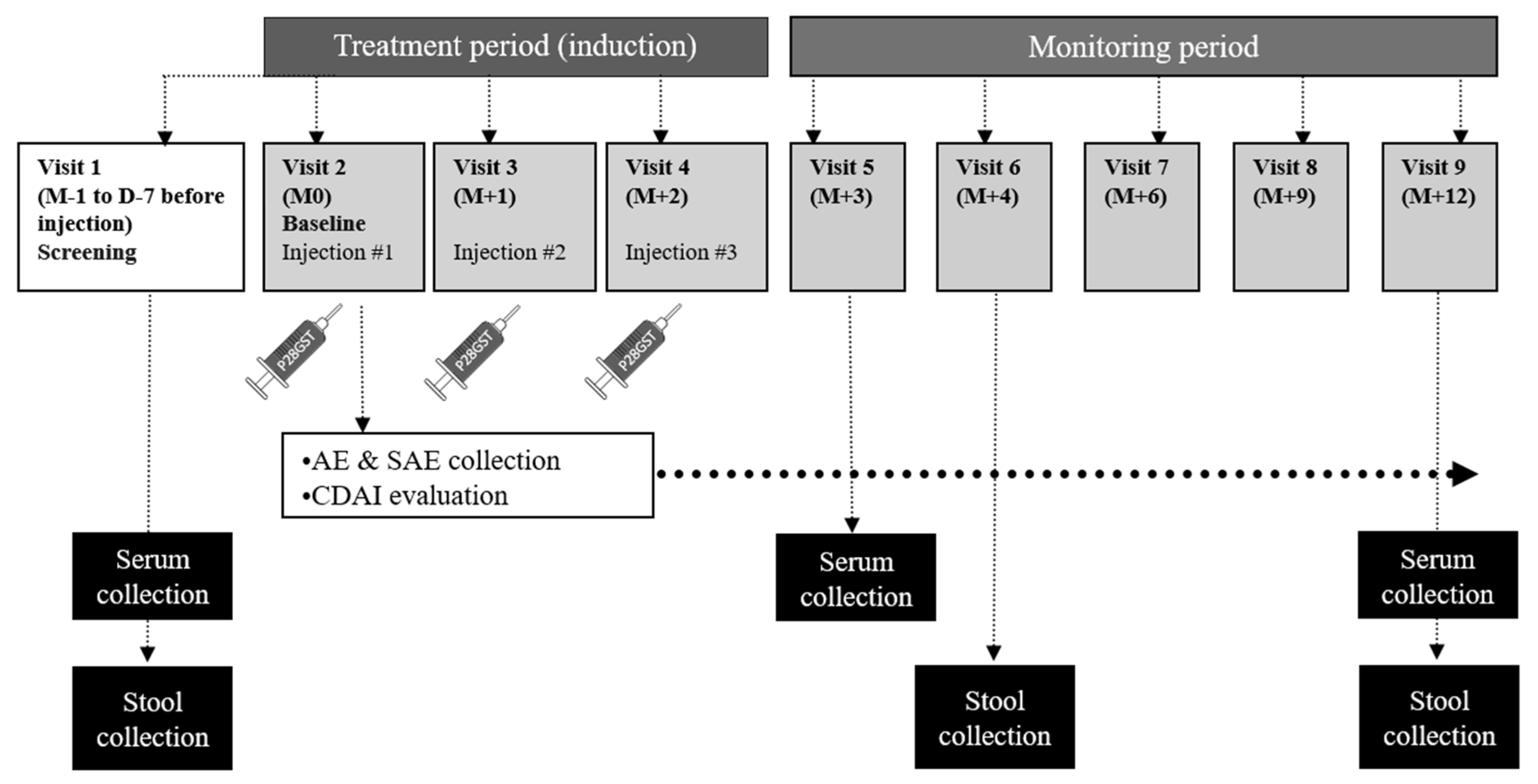
2.1. Study Design
2.2. Experimental Treatment
2.3. Safety Outcomes
2.4. Secondary Outcomes
2.5. Statistical Analyses
3. Results
3.1. Patients
3.2. Safety Outcomes
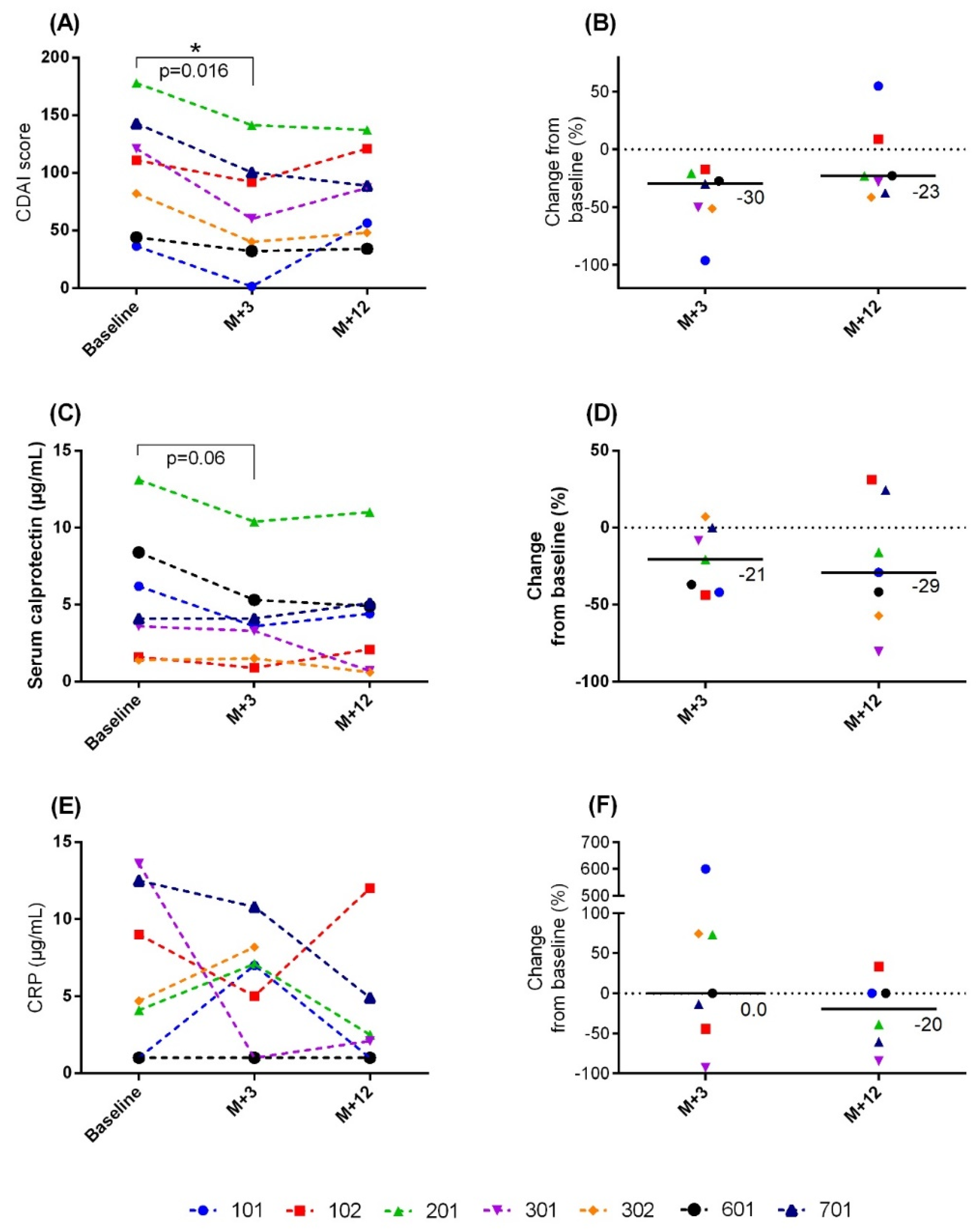
3.3. Secondary Outcomes
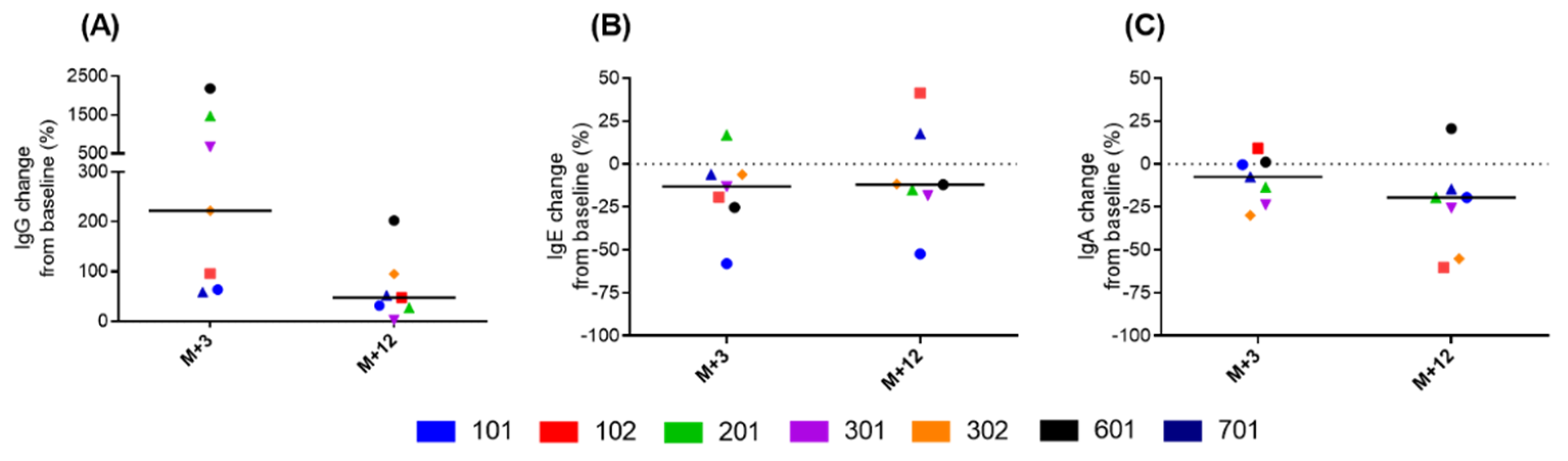
3.4. Immunological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panés, J.; D’Haens, G.R.; Higgins, P.D.R.; Mele, L.; Moscariello, M.; Chan, G.; Wang, W.; Niezychowski, W.; Su, C.; Maller, E. Long-term safety and tolerability of oral tofacitinib in patients with Crohn’s disease: Results from a phase 2, open-label, 48-week extension study. Aliment. Pharmacol. Ther. 2019, 49, 265–276. [Google Scholar] [CrossRef]
- Wammes, L.J.; Mpairwe, H.; Elliott, A.M.; Yazdanbakhsh, M. Helminth therapy or elimination: Epidemiological, immunological, and clinical considerations. Lancet Infect. Dis. 2014, 14, 1150–1162. [Google Scholar] [CrossRef]
- Eberl, G. Immunity by equilibrium. Nat. Rev. Immunol. 2016, 16, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, A.M.; Baptista, D.M. Helminths as an alternative therapy for intestinal diseases. World J. Gastroenterol. 2017, 23, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Driss, V.; El Nady, M.; Delbeke, M.; Rousseaux, C.; Dubuquoy, C.; Sarazin, A.; Gatault, S.; Dendooven, A.; Riveau, G.; Colombel, J.F.; et al. The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils. Mucosal. Immunol. 2016, 9, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Riveau, G.; Deplanque, D.; Remoué, F.; Schacht, A.-M.; Vodougnon, H.; Capron, M.; Thiry, M.; Martial, J.; Libersa, C.; Capron, A. Safety and Immunogenicity of rSh28GST Antigen in Humans: Phase 1 Randomized Clinical Study of a Vaccine Candidate against Urinary Schistosomiasis. PLoS Negl. Trop. Dis. 2012, 6, e1704. [Google Scholar] [CrossRef] [PubMed]
- Riveau, G.; Schacht, A.-M.; Dompnier, J.-P.; Deplanque, D.; Seck, M.; Waucquier, N.; Senghor, S.; Delcroix-Genete, D.; Hermann, E.; Idris-Khodja, N.; et al. Safety and efficacy of the rSh28GST urinary schistosomiasis vaccine: A phase 3 randomized, controlled trial in Senegalese children. PLoS Negl. Trop. Dis. 2018, 12, e0006968. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, A.; Dendooven, A.; Delbeke, M.; Gatault, S.; Pagny, A.; Standaert, A.; Rousseaux, C.; Desreumaux, P.; Dubuquoy, L.; Capron, M. Treatment with P28GST, a schistosome-derived enzyme, after acute colitis induction in mice: Decrease of intestinal inflammation associated with a down regulation of Th1/Th17 responses. PLoS ONE 2018, 13, e0209681. [Google Scholar] [CrossRef]
- Foligné, B.; Plé, C.; Titécat, M.; Dendooven, A.; Pagny, A.; Daniel, C.; Singer, E.; Pottier, M.; Bertin, B.; Neut, C.; et al. Contribution of the Gut Microbiota in P28GST-Mediated Anti-Inflammatory Effects: Experimental and Clinical Insights. Cells 2019, 8, 577. [Google Scholar] [CrossRef]
- Bégaud, B.; Evreux, J.C.; Jouglard, J.; Lagier, G. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 1985, 40, 111–118. [Google Scholar]
- Best, W.R.; Becktel, J.M.; Singleton, J.W. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology 1979, 77, 843–846. [Google Scholar] [CrossRef]
- Kostas, A.; Siakavellas, S.I.; Kosmidis, C.; Takou, A.; Nikou, J.; Maropoulos, G.; Vlachogiannakos, J.; Papatheodoridis, G.V.; Papaconstantinou, I.; Bamias, G. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- Korolkova, O.Y.; Myers, J.N.; Pellom, S.T.; Wang, L.; M’koma, A.E. Characterization of Serum Cytokine Profile in Predominantly Colonic Inflammatory Bowel Disease to Delineate Ulcerative and Crohn’s Colitides. Clin. Med. Insights Gastroenterol. 2015, 8, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W.; Elliott, D.E.; Urban, J.F.; Thompson, R.A.; Weinstock, J.V. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology 2005, 128, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Schölmerich, J.; Fellermann, K.; Seibold, F.W.; Rogler, G.; Langhorst, J.; Howaldt, S.; Novacek, G.; Petersen, A.M.; Bachmann, O.; Matthes, H.; et al. A Randomised, Double-blind, Placebo-controlled Trial of Trichuris suis ova in Active Crohn’s disease. J. Crohn’s Colitis 2016, 11, 390–399. [Google Scholar]
- Fousekis, F.S.; Saridi, M.; Albani, E.; Daniel, F.; Katsanos, K.H.; Kastanioudakis, I.G.; Christodoulou, D.K. Ear Involvement in Inflammatory Bowel Disease: A Review of the Literature. J. Clin. Med. Res. 2018, 10, 609–614. [Google Scholar] [CrossRef]
- Greco, A.; Macri, G.F.; Gallo, A.; Fusconi, M.; De Virgilio, A.; Pagliuca, G.; Marinelli, C.; de Vincentiis, M. Is Vestibular Neuritis an Immune Related Vestibular Neuropathy Inducing Vertigo? J. Immunol. Res. 2014, 2014, 459048. [Google Scholar] [CrossRef]
- Principi, M.; Cassano, N.; Contaldo, A.; Iannone, A.; Losurdo, G.; Barone, M.; Mastrolonardo, M.; Vena, G.A.; Ierardi, E.; Di Leo, A. Hydradenitis suppurativa and inflammatory bowel disease: An unusual, but existing association. World J. Gastroenterol. 2016, 22, 4802–4811. [Google Scholar] [CrossRef]
- Kamal, N.; Cohen, B.L.; Buche, S.; Delaporte, E.; Colombel, J.-F. Features of Patients with Crohn’s Disease and Hidradenitis Suppurativa. Clin. Gastroenterol. Hepatol. 2016, 14, 71–79. [Google Scholar] [CrossRef]
- Rajadhyaksha, V. Conducting feasibilities in clinical trials: An investment to ensure a good study. Perspect. Clin. Res. 2010, 1, 106–109. [Google Scholar] [PubMed]
- Meuwis, M.-A.; Vernier-Massouille, G.; Grimaud, J.C.; Bouhnik, Y.; Laharie, D.; Piver, E.; Seidel, L.; Colombel, J.F.; Louis, E. Serum calprotectin as a biomarker for Crohn’s disease. J. Crohn’s Colitis 2013, 7, e678–e683. [Google Scholar] [CrossRef] [PubMed]
| Anthropometric Characteristics (n = 8) | Median (range) |
|---|---|
| Age (years) | 32 (22–44) |
| BMI (kg/m2) | 24 (20–29) |
| Crohn’s disease indicators (n = 8) | |
| Age of onset (years) | 22 (16–35) |
| Disease duration (months) | 7 (2–16) |
| CDAI | 96 (11–178) |
| Events | Number of Patients | Number of AEs |
|---|---|---|
| Treatment-Emergent AEs, All Causalities * | ||
| AESI | 4 | 12 |
| Not related to the study | 0 | 0 |
| Possibly related to the study | 1 | 1 |
| Probably related to the study | 0 | 0 |
| Certainly related to the study | 3 | 11 |
| Other AE | 8 | 27 |
| Not related to the study | 7 | 14 |
| Possibly related to the study | 4 | 13 |
| Probably related to the study | 0 | 0 |
| Certainly related to the study | 0 | 0 |
| SAE | 1 | 2 |
| Not related to the study | 0 | 0 |
| Possibly related to the study | 1 | 2 |
| Probably related to the study | 0 | 0 |
| Certainly related to the study | 0 | 0 |
| Treatment-Emergent AEs that Led to Study Discontinuation | ||
| AESI | 0 | 0 |
| Other AE | 0 | 0 |
| SAE | 1 | 1 |
| Type of Event | Grade, Specific AESIs | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| AESIs certainly related to the study | ||||
| General disorders and administration site conditions | Injection site erythema | 2 (1) | 1 (1) * | 0 (0) |
| Injection site hypoaesthesia | 0 (0) | 2 (1) * | 0 (0) | |
| Injection site pain | 1 (1) | 2 (1) * | 0 (0) | |
| Injection site reaction | 1 (1) | 2 (1) * | 0 (0) | |
| AESIs possibly related to the study | ||||
| Psychiatric disorders | Sleep disorder | 0 (0) | 1 (1) | 0 (0) |
| Type of Event | Grade, Specific AEs (Number of Patients with AEs) | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| AEs possibly related to the study | ||||
| General disorders and administration site conditions | Asthenia | 1 (1) | 1 (1) | 0 (0) |
| Ear and labyrinth disorders | Vertigo | 1 (1) | 0 (0) | 0 (0) |
| Eye disorders | Blurred vision | 2 (2) | 0 (0) | 0 (0) |
| Gastrointestinal disorders | Abdominal pain | 2 (2) | 0 (0) | 0 (0) |
| Diarrhea | 1 (1) | 0 (0) | 0 (0) | |
| Vomiting | 1 (1) | 0 (0) | 0 (0) | |
| Nervous system disorders | Headache | 1 (1) | 0 (0) | 0 (0) |
| Migraine | 0 (0) | 1 (1) | 0 (0) | |
| Reproductive system and breast disorders | Hematospermia | 1 (1) | 0 (0) | 0 (0) |
| Skin and subcutaneous tissue disorders | Hidradenitis | 0 (0) | 1 (1) | 0 (0) |
| SAEs possibly related to the study | ||||
| Ear and labyrinth disorders | Vestibular neuronitis * | 0 (0) | 1 (1) | 0 (0) |
| Renal and urinary disorders | Renal failure * | 0 (0) | 1 (1) | 0 (0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capron, M.; Béghin, L.; Leclercq, C.; Labreuche, J.; Dendooven, A.; Standaert, A.; Delbeke, M.; Porcherie, A.; Nachury, M.; Boruchowicz, A.; et al. Safety of P28GST, a Protein Derived from a Schistosome Helminth Parasite, in Patients with Crohn’s Disease: A Pilot Study (ACROHNEM). J. Clin. Med. 2020, 9, 41. https://doi.org/10.3390/jcm9010041
Capron M, Béghin L, Leclercq C, Labreuche J, Dendooven A, Standaert A, Delbeke M, Porcherie A, Nachury M, Boruchowicz A, et al. Safety of P28GST, a Protein Derived from a Schistosome Helminth Parasite, in Patients with Crohn’s Disease: A Pilot Study (ACROHNEM). Journal of Clinical Medicine. 2020; 9(1):41. https://doi.org/10.3390/jcm9010041
Chicago/Turabian StyleCapron, Monique, Laurent Béghin, Céline Leclercq, Julien Labreuche, Arnaud Dendooven, Annie Standaert, Marie Delbeke, Adeline Porcherie, Maria Nachury, Arnaud Boruchowicz, and et al. 2020. "Safety of P28GST, a Protein Derived from a Schistosome Helminth Parasite, in Patients with Crohn’s Disease: A Pilot Study (ACROHNEM)" Journal of Clinical Medicine 9, no. 1: 41. https://doi.org/10.3390/jcm9010041
APA StyleCapron, M., Béghin, L., Leclercq, C., Labreuche, J., Dendooven, A., Standaert, A., Delbeke, M., Porcherie, A., Nachury, M., Boruchowicz, A., Dupas, J.-L., Fumery, M., Paupard, T., Catteau, S., Deplanque, D., Colombel, J.-F., & Desreumaux, P. (2020). Safety of P28GST, a Protein Derived from a Schistosome Helminth Parasite, in Patients with Crohn’s Disease: A Pilot Study (ACROHNEM). Journal of Clinical Medicine, 9(1), 41. https://doi.org/10.3390/jcm9010041






