Bone Marrow-Derived Mesenchymal Stromal Cells (MSCs) Modulate the Inflammatory Character of Alveolar Macrophages from Sarcoidosis Patients
Abstract
1. Introduction
2. Methods
2.1. Clinical Procedure
2.2. Cell Culture
2.3. Osteogenic and Adipogenic Differentiation Assay of MSCs
2.4. ELISA
2.5. Flow Cytometry
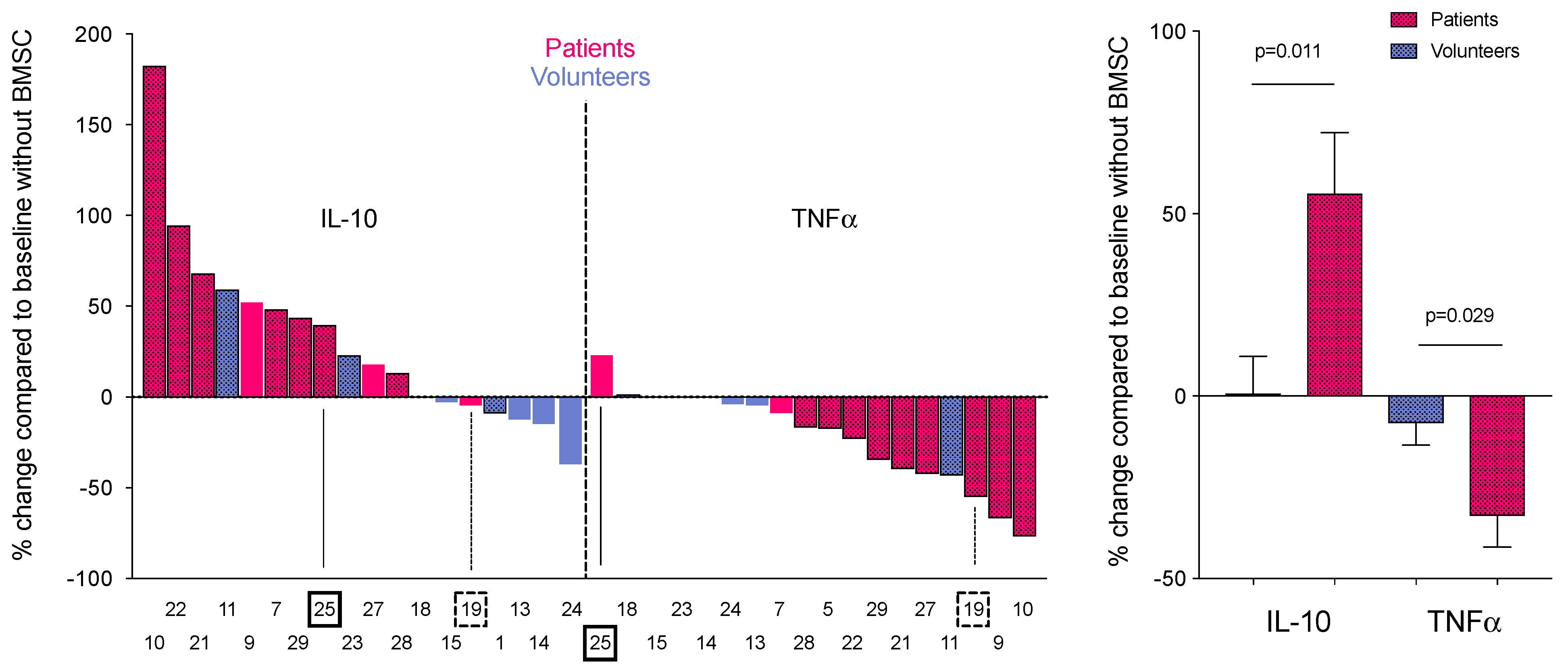
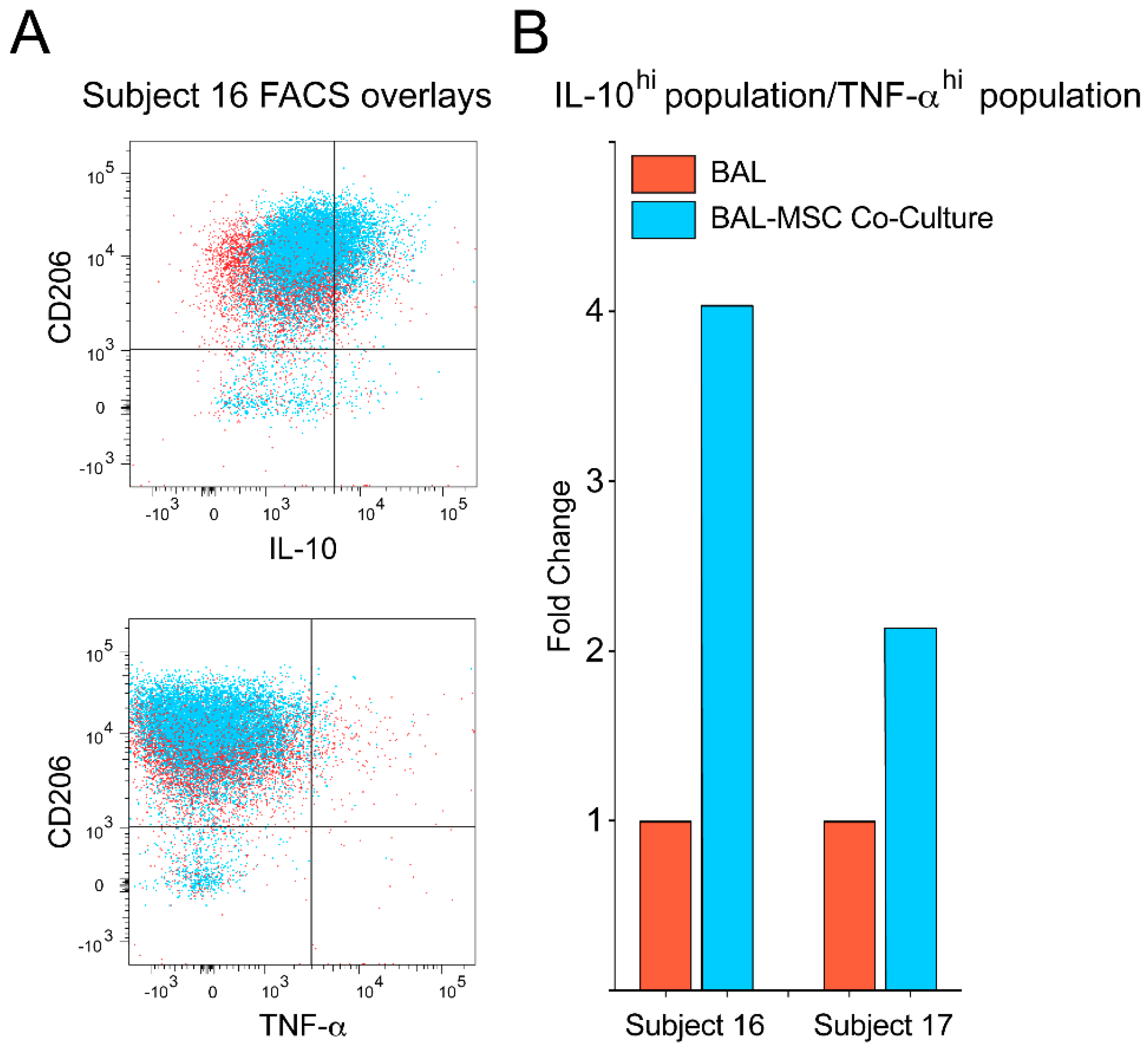
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phinney, D.G. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J. Cell Biochem. 2012, 113, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Cao, Y.; Ding, Z.; Han, C.; Shi, H.; Cui, L.; Lin, R. Efficacy of Mesenchymal Stromal Cells for Fistula Treatment of Crohn’s Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2017, 62, 851–860. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef]
- Zhao, R.; Su, Z.; Wu, J.; Ji, H.L. Serious adverse events of cell therapy for respiratory diseases: A systematic review and meta-analysis. Oncotarget 2017, 8, 30511–30523. [Google Scholar] [CrossRef]
- Le Blanc, K.; Davies, L.C. MSCs-cells with many sides. Cytotherapy 2018, 20, 273–278. [Google Scholar] [CrossRef]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef]
- Kim, J.; Hematti, P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol. 2009, 37, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Galipeau, J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr. Mol. Med. 2013, 13, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, H.; Zissel, G.; Goldmann, T.; Tschernig, T.; Vollmer, E.; Pabst, R.; Muller-Quernheim, J. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur. Respir. J. 2003, 21, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, M.C.; Fontana, J.R. Sarcoidosis: Clinical presentation, immunopathogenesis, and therapeutics. JAMA 2011, 305, 391–399. [Google Scholar] [CrossRef]
- Patterson, K.C.; Chen, E.S. The Pathogenesis of Pulmonary Sarcoidosis and Implications for Treatment. Chest 2017. [Google Scholar] [CrossRef]
- Grunewald, J.; Olerup, O.; Persson, U.; Ohrn, M.B.; Wigzell, H.; Eklund, A. T-cell receptor variable region gene usage by CD4+ and CD8+ T cells in bronchoalveolar lavage fluid and peripheral blood of sarcoidosis patients. Proc. Natl. Acad. Sci. USA 1994, 91, 4965–4969. [Google Scholar] [CrossRef]
- Katchar, K.; Wahlstrom, J.; Eklund, A.; Grunewald, J. Highly activated T-cell receptor AV2S3(+) CD4(+) lung T-cell expansions in pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 163, 1540–1545. [Google Scholar] [CrossRef]
- Hofmann, S.; Franke, A.; Fischer, A.; Jacobs, G.; Nothnagel, M.; Gaede, K.I.; Schurmann, M.; Muller-Quernheim, J.; Krawczak, M.; Rosenstiel, P.; et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat. Genet. 2008, 40, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Valentonyte, R.; Hampe, J.; Huse, K.; Rosenstiel, P.; Albrecht, M.; Stenzel, A.; Nagy, M.; Gaede, K.I.; Franke, A.; Haesler, R.; et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat. Genet. 2005, 37, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kjellin, H.; Silva, E.; Branca, R.M.; Eklund, A.; Jakobsson, P.J.; Grunewald, J.; Lehtio, J.; Wheelock, A.M. Alterations in the membrane-associated proteome fraction of alveolar macrophages in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2016, 33, 17–28. [Google Scholar]
- Facco, M.; Cabrelle, A.; Teramo, A.; Olivieri, V.; Gnoato, M.; Teolato, S.; Ave, E.; Gattazzo, C.; Fadini, G.P.; Calabrese, F.; et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 2011, 66, 144–150. [Google Scholar] [CrossRef] [PubMed]
- James, W.E.; Baughman, R. Treatment of sarcoidosis: Grading the evidence. Expert Rev. Clin. Pharm. 2018, 11, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Scadding, J.G. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br. Med. J. 1961, 2, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Mayer, B.; Sworder, B.J.; Kuznetsov, S.A.; Mezey, E. A practical guide to culturing mouse and human bone marrow stromal cells. Curr. Protoc. Immunol. 2013, 102. [Google Scholar] [CrossRef]
- Mitsi, E.; Kamng’ona, R.; Rylance, J.; Solorzano, C.; Jesus Reine, J.; Mwandumba, H.C.; Ferreira, D.M.; Jambo, K.C. Human alveolar macrophages predominately express combined classical M1 and M2 surface markers in steady state. Respir. Res. 2018, 19, 66. [Google Scholar] [CrossRef]
- Yu, Y.R.; Hotten, D.F.; Malakhau, Y.; Volker, E.; Ghio, A.J.; Noble, P.W.; Kraft, M.; Hollingsworth, J.W.; Gunn, M.D.; Tighe, R.M. Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues. Am. J. Respir. Cell Mol. Biol. 2016, 54, 13–24. [Google Scholar] [CrossRef]
- Zheng, L.; Teschler, H.; Guzman, J.; Hubner, K.; Striz, I.; Costabel, U. Alveolar macrophage TNF-alpha release and BAL cell phenotypes in sarcoidosis. Am. J. Respir. Crit. Care Med. 1995, 152, 1061–1066. [Google Scholar] [CrossRef]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, H.; Galun, E.; Rachmilewitz, J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Gur-Wahnon, D.; Borovsky, Z.; Beyth, S.; Liebergall, M.; Rachmilewitz, J. Contact-dependent induction of regulatory antigen-presenting cells by human mesenchymal stem cells is mediated via STAT3 signaling. Exp. Hematol. 2007, 35, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Crouser, E.D. Potential immunotherapies for sarcoidosis. Expert Opin. Biol. Ther. 2018, 18, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Culver, D.A.; Jankovi, V.; Fischkoff, S.; Brockway, G.; Lower, E.E. Placenta-derived mesenchymal-like cells (PDA-001) as therapy for chronic pulmonary sarcoidosis: A phase 1 study. Sarcoidosis Vasc. Diffus. Lung Dis. 2015, 32, 106–114. [Google Scholar]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Mezey, E.; Nemeth, K. Mesenchymal stem cells and infectious diseases: Smarter than drugs. Immunol. Lett. 2015, 168, 208–214. [Google Scholar] [CrossRef]
- Broekman, W.; Khedoe, P.; Schepers, K.; Roelofs, H.; Stolk, J.; Hiemstra, P.S. Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 2018, 73, 565–574. [Google Scholar] [CrossRef]
- Kruk, D.; Heijink, I.H.; Slebos, D.J.; Timens, W.; Ten Hacken, N.H. Mesenchymal Stromal Cells to Regenerate Emphysema: On the Horizon? Respiration 2018. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Gholamrezanezhad, A.; Mirpour, S.; Bagheri, M.; Mohamadnejad, M.; Alimoghaddam, K.; Abdolahzadeh, L.; Saghari, M.; Malekzadeh, R. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl. Med. Biol. 2011, 38, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Ankrum, J.A.; Kamhieh-Milz, J.; Bieback, K.; Ringden, O.; Volk, H.D.; Geissler, S.; Reinke, P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol. Med. 2019, 25, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Thomspon, M.; Wolfe, D.; Champagne, J.; Mei, S.H.; Lalu, M.; Fergusson, D.; Winston, B.; Marshall, J.; Walley, K.; English, S.; et al. Safety of cell therapy with mesenchymal stromal cells): An updated systematic review and meta-analysis of randomized controlled trials (safecell update). Cytotherapy 2018, 20, S53–S54. [Google Scholar] [CrossRef]
- Moll, G.; Geissler, S.; Catar, R.; Ignatowicz, L.; Hoogduijn, M.J.; Strunk, D.; Bieback, K.; Ringden, O. Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy? Adv. Exp. Med. Biol. 2016, 951, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhou, Y.; Xian, L.; Li, C.; Xu, T.; Plunkett, B.; Huang, S.K.; Wan, M.; Cao, X. Functional effects of TGF-beta1 on mesenchymal stem cell mobilization in cockroach allergen-induced asthma. J. Immunol. 2014, 192, 4560–4570. [Google Scholar] [CrossRef]
- Glassberg, M.K.; Minkiewicz, J.; Toonkel, R.L.; Simonet, E.S.; Rubio, G.A.; DiFede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 2017, 151, 971–981. [Google Scholar] [CrossRef]
- Chambers, D.C.; Enever, D.; Ilic, N.; Sparks, L.; Whitelaw, K.; Ayres, J.; Yerkovich, S.T.; Khalil, D.; Atkinson, K.M.; Hopkins, P.M. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 2014, 19, 1013–1018. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Paspaliaris, V.; Koliakos, G.; Ntolios, P.; Bouros, E.; Oikonomou, A.; Zissimopoulos, A.; Boussios, N.; Dardzinski, B.; Gritzalis, D.; et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 2013, 11, 1–13. [Google Scholar] [CrossRef]
- Pechkovsky, D.V.; Prasse, A.; Kollert, F.; Engel, K.M.; Dentler, J.; Luttmann, W.; Friedrich, K.; Muller-Quernheim, J.; Zissel, G. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin. Immunol. 2010, 137, 89–101. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Toonkel, R.; Karampitsakos, T.; Medapalli, K.; Ninou, I.; Aidinis, V.; Bouros, D.; Glassberg, M.K. Mesenchymal Stem Cells for the Treatment of Idiopathic Pulmonary Fibrosis. Front. Med. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.A.; Antunes, M.A.; Dos Santos, C.C.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Kardia, E.; Yusoff, N.M.; Zakaria, Z.; Yahaya, B. Aerosol-based delivery of fibroblast cells for treatment of lung diseases. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 30–34. [Google Scholar] [CrossRef]
- Aver’yanov, A.V.; Konoplyannikov, A.G.; Antonov, N.S.; Osipova, G.L.; Vasil’eva, O.S.; Sakharova, M.G.; Tatarskii, A.R.; Kobylyansky, V.I. Survival of Mesenchymal Stem Cells in Different Methods of Nebulization. Bull. Exp. Biol. Med. 2018, 164, 576–578. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Copland, I.B.; Garcia, M.A.; Petersen, C.T.; Lewis, C.N.; Waller, E.K.; Kirk, A.D.; Galipeau, J. Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNgamma Licensing. Stem Cells 2016, 34, 2429–2442. [Google Scholar] [CrossRef]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.; McKenna, D.H.; Rocco, P.R.; Weiss, D.J. Freshly thawed and continuously cultured human bone marrow-derived mesenchymal stromal cells comparably ameliorate allergic airways inflammation in immunocompetent mice. Stem Cells Transl. Med. 2015, 4, 615–624. [Google Scholar] [CrossRef]


| Parameter | Controls (n = 7) | Sarcoidosis (n = 15) | p-Value |
|---|---|---|---|
| Mean Value | Mean Value | ||
| Total Participants | |||
| Female (number)/(%) | 4/(50%) | 11/(73.3%) | NS |
| Age (years) | 45.4 | 53.1 | NS |
| Race | |||
| Black (number)/(%) | 5/(62.5) | 8/(53.3) | NS |
| White (number)/(%) | 2/(25) | 5/(33.3) | NS |
| Asian (number)/(%) | 1/(12.5) | 0 | NS |
| Multiracial (number)/(%) | 0 | 2/(13.3) | NS |
| Height (cm), (SD) | 174, (0.91) | 168, (0.36) | NS |
| Modification of MRC Dyspnea Scale | 0 | 0.9, (1.36) | 0.027 |
| Inhaled Steroid (number/(%)), (SD) | 0 | 5/(33.3%), (0.49) | 0.019 |
| Prednisone (number / (%)), (SD) | 0 | 2/(20%), (0.35) | NS |
| Parameter | Controls (n = 7) | Sarcoidosis (n = 15) | p-Value | ||
|---|---|---|---|---|---|
| Mean Value | SD | Mean Value | SD | ||
| BAL Cell count (× 107) | 21 | 9.7 | 22.6 | 14.8 | NS |
| BAL Lymphocytes (%) | 6.57 | 6.4 | 16.96 | 12.5 | 0.02 |
| BAL Macrophage (%) | 80.25 | 33.2 | 70.21 | 23.7 | NS |
| BALF % return (%) | 48.6 | 8.2 | 51.67 | 12.2 | NS |
| Viability of cells (%) | 80.57 | 15.7 | 84.6 | 8.0 | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClain Caldwell, I.; Hogden, C.; Nemeth, K.; Boyajian, M.; Krepuska, M.; Szombath, G.; MacDonald, S.; Abshari, M.; Moss, J.; Vitale-Cross, L.; et al. Bone Marrow-Derived Mesenchymal Stromal Cells (MSCs) Modulate the Inflammatory Character of Alveolar Macrophages from Sarcoidosis Patients. J. Clin. Med. 2020, 9, 278. https://doi.org/10.3390/jcm9010278
McClain Caldwell I, Hogden C, Nemeth K, Boyajian M, Krepuska M, Szombath G, MacDonald S, Abshari M, Moss J, Vitale-Cross L, et al. Bone Marrow-Derived Mesenchymal Stromal Cells (MSCs) Modulate the Inflammatory Character of Alveolar Macrophages from Sarcoidosis Patients. Journal of Clinical Medicine. 2020; 9(1):278. https://doi.org/10.3390/jcm9010278
Chicago/Turabian StyleMcClain Caldwell, Ian, Christopher Hogden, Krisztian Nemeth, Michael Boyajian, Miklos Krepuska, Gergely Szombath, Sandra MacDonald, Mehrnoosh Abshari, Joel Moss, Lynn Vitale-Cross, and et al. 2020. "Bone Marrow-Derived Mesenchymal Stromal Cells (MSCs) Modulate the Inflammatory Character of Alveolar Macrophages from Sarcoidosis Patients" Journal of Clinical Medicine 9, no. 1: 278. https://doi.org/10.3390/jcm9010278
APA StyleMcClain Caldwell, I., Hogden, C., Nemeth, K., Boyajian, M., Krepuska, M., Szombath, G., MacDonald, S., Abshari, M., Moss, J., Vitale-Cross, L., Fontana, J. R., & Mezey, E. (2020). Bone Marrow-Derived Mesenchymal Stromal Cells (MSCs) Modulate the Inflammatory Character of Alveolar Macrophages from Sarcoidosis Patients. Journal of Clinical Medicine, 9(1), 278. https://doi.org/10.3390/jcm9010278





