Circulating PCSK9 Level and Risk of Cardiovascular Events and Death in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Definitions
2.3. Laboratory Measurements
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographic Characteristics and Laboratory Data
3.2. Determinant Factors for Circulating PCSK9 Level in HD Patients
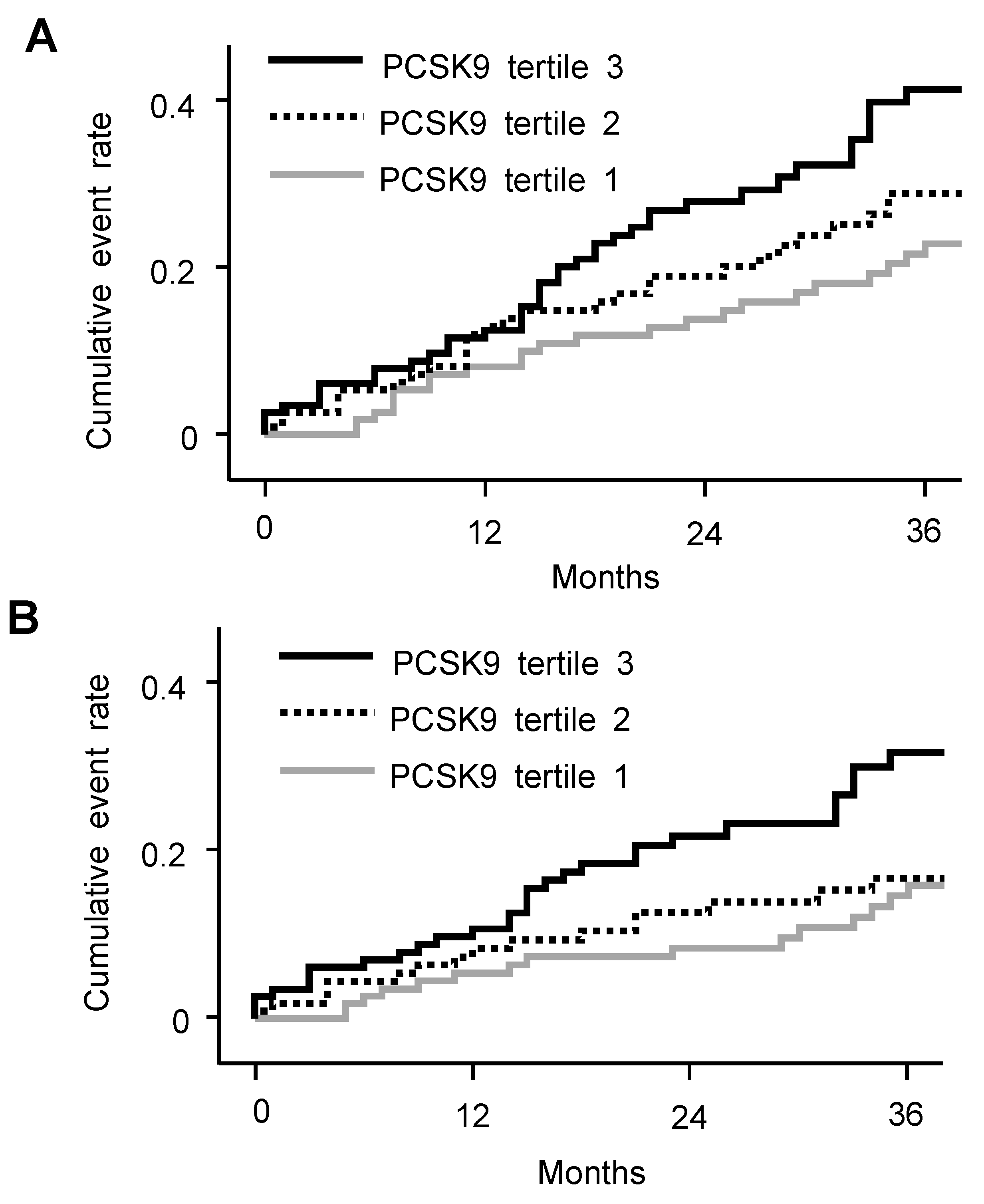
3.3. Risk of CV Events and Death in Different PCSK9 Level
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheung, A.K.; Sarnak, M.J.; Yan, G.; Dwyer, J.T.; Heyka, R.J.; Rocco, M.V.; Teehan, B.P.; Levey, A.S. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000, 58, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M. Alberta Kidney Disease Network. Cause of death in patients with reduced kidney function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, L.; Provenzano, M.; Chiodini, P.; D’Arrigo, G.; Tripepi, G.; Del Vecchio, L.; Conte, G.; Locatelli, F.; Zoccali, C.; Minutolo, R.; et al. Prognostic role of LDL cholesterol in non-dialysis chronic kidney disease: Multicenter prospective study in Italy. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Muntner, P.; Lloyd, A.; Manns, B.; Klarenbach, S.; Pannu, N.; James, M.; Hemmelgarn, B. Alberta Kidney Disease Network. Association between LDL-C and risk of myocardial infarction in CKD. J. Am. Soc. Nephrol. 2013, 24, 979–986. [Google Scholar] [CrossRef]
- Wanner, C.; Krane, V.; März, W.; Olschewski, M.; Mann, J.F.; Ruf, G.; Ritz, E. German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005, 353, 238–248. [Google Scholar] [CrossRef]
- Fellström, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.W.; Chevaile, A.; Cobbe, S.M.; Grönhagen-Riska, C.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef]
- Wanner, C.; Tonelli, M. Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014, 85, 1303–1309. [Google Scholar] [CrossRef]
- Tavori, H.; Fan, D.; Blakemore, J.L.; Yancey, P.G.; Ding, L.; Linton, M.F.; Fazio, S. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: Evidence for a reciprocal regulation. Circulation 2013, 127, 2403–2413. [Google Scholar] [CrossRef]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H., Jr.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Chapman, M.J.; Neff, D.R.; Voros, S.; Giugliano, R.P.; Davey Smith, G.; Fazio, S.; et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl. J. Med. 2016, 375, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Mata, P.; Muñiz, O.; Fuentes-Jimenez, F.; Díaz, J.L.; Zambón, D.; Tomás, M.; Martin, C.; Moyon, T.; Croyal, M.; et al. PCSK9 and lipoprotein (a) levels are two predictors of coronary artery calcification in asymptomatic patients with familial hypercholesterolemia. Atherosclerosis 2016, 254, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Leander, K.; Mälarstig, A.; Van’t Hooft, F.M.; Hyde, C.; Hellénius, M.L.; Troutt, J.S.; Konrad, R.J.; Öhrvik, J.; Hamsten, A.; de Faire, U. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. Circulation 2016, 133, 1230–1239. [Google Scholar] [CrossRef]
- Morena, M.; Le May, C.; Chenine, L.; Arnaud, L.; Dupuy, A.M.; Pichelin, M.; Leray-Moragues, H.; Chalabi, L.; Canaud, B.; Cristol, J.P.; et al. Plasma PCSK9 concentrations during the course of nondiabetic chronic kidney disease: Relationship with glomerular filtration rate and lipid metabolism. J. Clin. Lipidol. 2017, 11, 87–93. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Bøttcher, M.; Ivarsen, P.; Jørgensen, H.S.; Nyegaard, M.; Buttenschøn, H.; Gustafsen, C.; Glerup, S.; Bøtker, H.E.; Svensson, M.; et al. Association between circulating proprotein convertase subtilisin/kexin type 9 levels and prognosis in patients with severe chronic kidney disease. Nephrol. Dial. Transpl. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Konarzewski, M.; Szolkiewicz, M.; Sucajtys-Szulc, E.; Blaszak, J.; Lizakowski, S.; Swierczynski, J.; Rutkowski, B. Elevated circulating PCSK-9 concentration in renal failure patients is corrected by renal replacement therapy. Am. J. Nephrol. 2014, 40, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Park, B.S.; Kim, Y.W.; Vaziri, N.D. Plasma PCSK9 in nephrotic syndrome and in peritoneal dialysis: A cross-sectional study. Am. J. Kidney Dis. 2014, 63, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Gotch, F.A. Kinetic modeling in hemodialysis. In Clinical Dialysis, 2nd ed.; Nissenson, A.R., Fine, R.N., Gentile, D.E., Eds.; Appleton and Lange: Norwalk, CA, USA, 1995; pp. 156–188. [Google Scholar]
- Abujrad, H.; Mayne, J.; Ruzicka, M.; Cousins, M.; Raymond, A.; Cheesman, J.; Taljaard, M.; Sorisky, A.; Burns, K.; Ooi, T.C. Chronic kidney disease on hemodialysis is associated with decreased serum PCSK9 levels. Atherosclerosis 2014, 233, 123–129. [Google Scholar] [CrossRef]
- Guardiola, M.; Plana, N.; Ibarretxe, D.; Cabré, A.; González, M.; Ribalta, J.; Masana, L. Circulating PCSK9 levels are positively correlated with NMR-assessed atherogenic dyslipidaemia in patients with high cardiovascular risk. Clin. Sci. 2015, 128, 877–882. [Google Scholar] [CrossRef]
- Cohen, J.; Pertsemlidis, A.; Kotowski, I.K.; Graham, R.; Garcia, C.K.; Hobbs, H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005, 37, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Ancellin, N.; Charlton, F.; Comas, D.; Pilot, J.; Keech, A.; Patel, S.; Sullivan, D.R.; Cohn, J.S.; Rye, K.A.; et al. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin. Chem. 2008, 54, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Lakoski, S.G.; Lagace, T.A.; Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 2009, 94, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendía, L.E.; Guerrero-Romero, F.; Golledge, J.; Watts, G.F. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: A systematic review and meta-analysis of clinical trials. Diabetes Obes. Metab. 2015, 17, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, W.; Woo, J.S.; Lee, T.W.; Ihm, C.G.; Kim, Y.G.; Moon, J.Y.; Lee, S.H.; Jeong, M.H.; Jeong, K.H. Korea Acute Myocardial Infarction Registry Investigators. The Predictive Role of Serum Triglyceride to High-Density Lipoprotein Cholesterol Ratio According to Renal Function in Patients with Acute Myocardial Infarction. PLoS ONE 2016, 11, e0165484. [Google Scholar]
- Liberale, L.; Montecucco, F.; Camici, G.G.; Dallegri, F.; Vecchie, A.; Carbone, F.; Bonaventura, A. Treatment with Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors to Reduce Cardiovascular Inflammation and Outcomes. Curr. Med. Chem. 2017, 24, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.T.; Li, T.H.; Wang, Z.; Wei, D.H.; Liu, L.S.; Zheng, X.L.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef]
- Dwivedi, D.J.; Grin, P.M.; Khan, M.; Prat, A.; Zhou, J.; Fox-Robichaud, A.E.; Seidah, N.G.; Liaw, P.C. Differential Expression of PCSK9 Modulates Infection, Inflammation, and Coagulation in a Murine Model of Sepsis. Shock 2016, 46, 672–680. [Google Scholar] [CrossRef]
- Grune, J.; Meyborg, H.; Bezhaeva, T.; Kappert, K.; Hillmeister, P.; Kintscher, U.; Pieske, B.; Stawowy, P. PCSK9 regulates the chemokine receptor CCR2 on monocytes. Biochem. Biophys. Res. Commun. 2017, 485, 312–318. [Google Scholar] [CrossRef]
- Goettsch, C.; Hutcheson, J.D.; Hagita, S.; Rogers, M.A.; Creager, M.D.; Pham, T.; Choi, J.; Mlynarchik, A.K.; Pieper, B.; Kjolby, M.; et al. A single injection of gain-of-function mutant PCSK9 adeno-associated virus vector induces cardiovascular calcification in mice with no genetic modification. Atherosclerosis 2016, 251, 109–118. [Google Scholar] [CrossRef]
- Poggio, P.; Songia, P.; Cavallotti, L.; Barbieri, S.S.; Zanotti, I.; Arsenault, B.J.; Valerio, V.; Ferri, N.; Capoulade, R.; Camera, M. PCSK9 Involvement in Aortic Valve Calcification. J. Am. Coll. Cardiol. 2018, 72, 3225–3227. [Google Scholar] [CrossRef] [PubMed]

| Circulating PCSK9 Level | p | |||
|---|---|---|---|---|
| Tertile 1 (n = 118) | Tertile 2 (n = 117) | Tertile 3 (n = 118) | ||
| Age (years) | 62.1 ± 12.4 | 61.7 ± 13.7 | 62.8 ± 12.2 | 0.791 |
| Male (%) | 78 (66.1) | 80 (68.4) | 79 (66.9) | 0.932 |
| Body mass index (kg/m2) | 23.0 ± 3.7 | 22.6 ± 3.5 | 23.7 ± 4.5 | 0.079 |
| HD duration (months) | 70.0 ± 76.7 | 55.4 ± 53.8 | 47.8 ± 60.7 a | 0.029 |
| Diabetes (%) | 57 (48.3) | 60 (51.3) | 79 (66.9) a,b | 0.008 |
| History of CVE (%) | 52 (44.1) | 46 (39.3) | 60 (50.8) | 0.203 |
| Charlson comorbidity score | 3.9 ± 1.6 | 3.9 ± 1.5 | 4.3 ± 1.4 | 0.055 |
| Follow-up duration (months) | 30.9 ± 10.6 | 27.9 ± 12.7 | 28.0 ± 10.9 | 0.072 |
| Statin use (%) | 33 (28.0) | 55 (47.0) a | 76 (64.4) a,b | <0.001 |
| PCSK9 (ng/mL) | 17.8 ± 5.5 | 33.6 ± 4.1 a | 58.3 ± 18.6 a,b | <0.001 |
| Hemoglobin (g/dL) | 10.5 ± 1.0 | 10.3 ± 1.2 | 10.4 ± 1.4 | 0.396 |
| Serum glucose | 142.6 ± 51.3 | 146.8 ± 54.3 | 171.4 ± 75.6 a,b | 0.001 |
| Albumin (g/dL) | 4.0 ± 0.3 | 3.9 ± 0.4 a | 3.8 ± 0.4 a,b | 0.017 |
| Total cholesterol (mg/dL) | 134.5 ± 29.0 | 135.3 ± 30.3 | 137.9 ± 31.6 | 0.668 |
| Triglyceride (mg/dL) | 117.4 ± 78.7 | 108.3 ± 68.8 | 134.6 ± 80.4 b | 0.028 |
| LDL-cholesterol (mg/dL) | 77.1 ± 26.3 | 77.1 ± 24.8 | 77.1 ± 27.4 | 1.00 |
| HDL-cholesterol (mg/dL) | 45.5 ± 13.9 | 45.2 ± 13.2 | 43.4 ± 11.4 | 0.420 |
| hsCRP (mg/dL) | 3.5 ± 6.7 | 4.7 ± 9.7 | 3.5 ± 7.5 | 0.424 |
| i-PTH | 288.0 ± 252.3 | 280.8 ± 210.8 | 246.9 ± 193.3 | 0.312 |
| Predialysis SBP (mmHg) | 143.0 ± 20.3 | 143.0 ± 19.5 | 144.3 ± 21.0 | 0.855 |
| UF (L) | 2.2 ± 1.1 | 2.3 ± 1.1 | 2.2 ± 1.1 | 0.804 |
| spKt/V | 1.57 ± 0.29 | 1.56 ± 0.30 | 1.56 ± 0.31 | 0.967 |
| Catheter use (%) | 1 (0.8) | 5 (4.3) | 8 (6.8) | 0.066 |
| Unstandardized β | 95% CI | p | |
|---|---|---|---|
| HD duration (months) | −0.011 | −0.043, 0.021 | 0.507 |
| Glucose (mg/dL) | 0.047 | 0.014, 0.080 | 0.005 |
| Albumin (g/dL) | −8.292 | −14.477, −2.107 | 0.009 |
| Total cholesterol (mg/dL) | 0.134 | 0.023, 0.244 | 0.018 |
| Triglyceride (mg/dL) | 0.018 | −0.011, 0.047 | 0.216 |
| LDL-cholesterol (mg/dL) | −0.093 | −0.217, 0.031 | 0.141 |
| i-PTH (pg/mL) | −0.004 | −0.013, 0.006 | 0.435 |
| Statin use | 11.192 | 7.115, 15.269 | <0.001 |
| Circulating PCSK9 Level | p | |||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| All CV events (%) | 15 (12.7) | 16 (13.7) | 29 (24.6) | 0.027 |
| Coronary artery disease (%) | 9 (7.6) | 9 (7.7) | 14 (11.9) | 0.431 |
| Heart failure (%) | 3 (2.5) | 2 (1.7) | 4 (3.4) | 0.912 |
| Stroke (%) | 1 (0.8) | 0 | 6 (5.1) | 0.019 |
| Peripheral artery disease (%) | 0 | 1 (0.9) | 4 (3.4) | 0.092 |
| Cardiac arrest (%) | 2 (1.7) | 4 (3.4) | 1 (0.8) | 0.320 |
| No. of Events (%) | HR (95% CI), Crude | HR (95% CI), Adjusted a | |
|---|---|---|---|
| Composite events | |||
| PCSK9 tertile 1 | 23 (19.5) | Reference | Reference |
| PCSK9 tertile 2 | 28 (23.9) | 1.35 (0.78–2.35) | 1.33 (0.75–2.36) |
| PCSK9 tertile 3 | 39 (33.1) | 2.05 (1.22–3.43) | 1.97 (1.13–3.45) |
| PCSK9 increase per 1 ng/mL | 1.01 (1.001–1.019) | 1.02 (1.004–1.026) | |
| CV events | |||
| PCSK9 tertile 1 | 15 (12.7) | Reference | Reference |
| PCSK9 tertile 2 | 16 (13.7) | 1.18 (0.58–2.39) | 1.28 (0.61–2.66) |
| PCSK9 tertile 3 | 29 (24.6) | 2.31 (1.24–4.32) | 2.31 (1.17–4.59) |
| PCSK9 increase per 1 ng/mL | 1.01 (1.003–1.024) | 1.02 (1.004–1.030) | |
| Death | |||
| PCSK9 tertile 1 | 12 (10.2) | Reference | Reference |
| PCSK9 tertile 2 | 20 (17.1) | 1.88 (0.92–3.84) | 1.79 (0.85–3.78) |
| PCSK9 tertile 3 | 16 (13.6) | 1.48 (0.70–3.14) | 1.45 (0.65–3.27) |
| PCSK9 increase per 1 ng/mL | 1.01 (0.993–1.019) | 1.01 (0.997–1.032) |
| No. of Events/No. of Patients | HR (95% CI), Crude | HR (95% CI), Adjusted a | p for Interaction | |
|---|---|---|---|---|
| LDL-cholesterol | 0.370 | |||
| Low LDL and low PCSK9 | 28/114 (24.6) | Reference | Reference | |
| Low LDL and high PCSK9 | 18/61 (29.5) | 1.36 (0.75–2.46) | 1.30 (0.69–2.46) | |
| High LDL and low PCSK9 | 23/121 (19.0) | 0.83 (0.48–1.44) | 1.16 (0.59–2.27) | |
| High LDL and high PCSK9 | 21/57 (36.8) | 1.90 (1.08–3.36) | 2.59 (1.30–5.14) | |
| hsCRP | 0.443 | |||
| Low hsCRP and low PCSK9 | 23/124 (18.5) | Reference | Reference | |
| Low hsCRP and high PCSK9 | 19/54 (35.2) | 2.18 (1.19–4.00) | 2.03 (1.06–3.89) | |
| High hsCRP and low PCSK9 | 28/111 (25.2) | 1.38 (0.79–2.39) | 1.09 (0.60–1.99) | |
| High hsCRP and high PCSK9 | 20/64 (31.2) | 1.97 (1.08–3.58) | 1.56 (0.81–3.02) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.S.; Kim, J.S.; Kim, Y.G.; Lee, S.-Y.; Ahn, S.Y.; Lee, H.J.; Lee, D.-Y.; Lee, S.H.; Moon, J.Y.; Jeong, K.H. Circulating PCSK9 Level and Risk of Cardiovascular Events and Death in Hemodialysis Patients. J. Clin. Med. 2020, 9, 244. https://doi.org/10.3390/jcm9010244
Hwang HS, Kim JS, Kim YG, Lee S-Y, Ahn SY, Lee HJ, Lee D-Y, Lee SH, Moon JY, Jeong KH. Circulating PCSK9 Level and Risk of Cardiovascular Events and Death in Hemodialysis Patients. Journal of Clinical Medicine. 2020; 9(1):244. https://doi.org/10.3390/jcm9010244
Chicago/Turabian StyleHwang, Hyeon Seok, Jin Sug Kim, Yang Gyun Kim, So-Young Lee, Shin Young Ahn, Hong Joo Lee, Dong-Young Lee, Sang Ho Lee, Ju Young Moon, and Kyung Hwan Jeong. 2020. "Circulating PCSK9 Level and Risk of Cardiovascular Events and Death in Hemodialysis Patients" Journal of Clinical Medicine 9, no. 1: 244. https://doi.org/10.3390/jcm9010244
APA StyleHwang, H. S., Kim, J. S., Kim, Y. G., Lee, S.-Y., Ahn, S. Y., Lee, H. J., Lee, D.-Y., Lee, S. H., Moon, J. Y., & Jeong, K. H. (2020). Circulating PCSK9 Level and Risk of Cardiovascular Events and Death in Hemodialysis Patients. Journal of Clinical Medicine, 9(1), 244. https://doi.org/10.3390/jcm9010244





