Complete Revascularization of Multivessel Coronary Artery Disease Does Not Improve Clinical Outcome in ST-Segment Elevation Myocardial Infarction Patients with Reduced Left Ventricular Ejection Fraction
Abstract
:1. Introduction
2. Methods
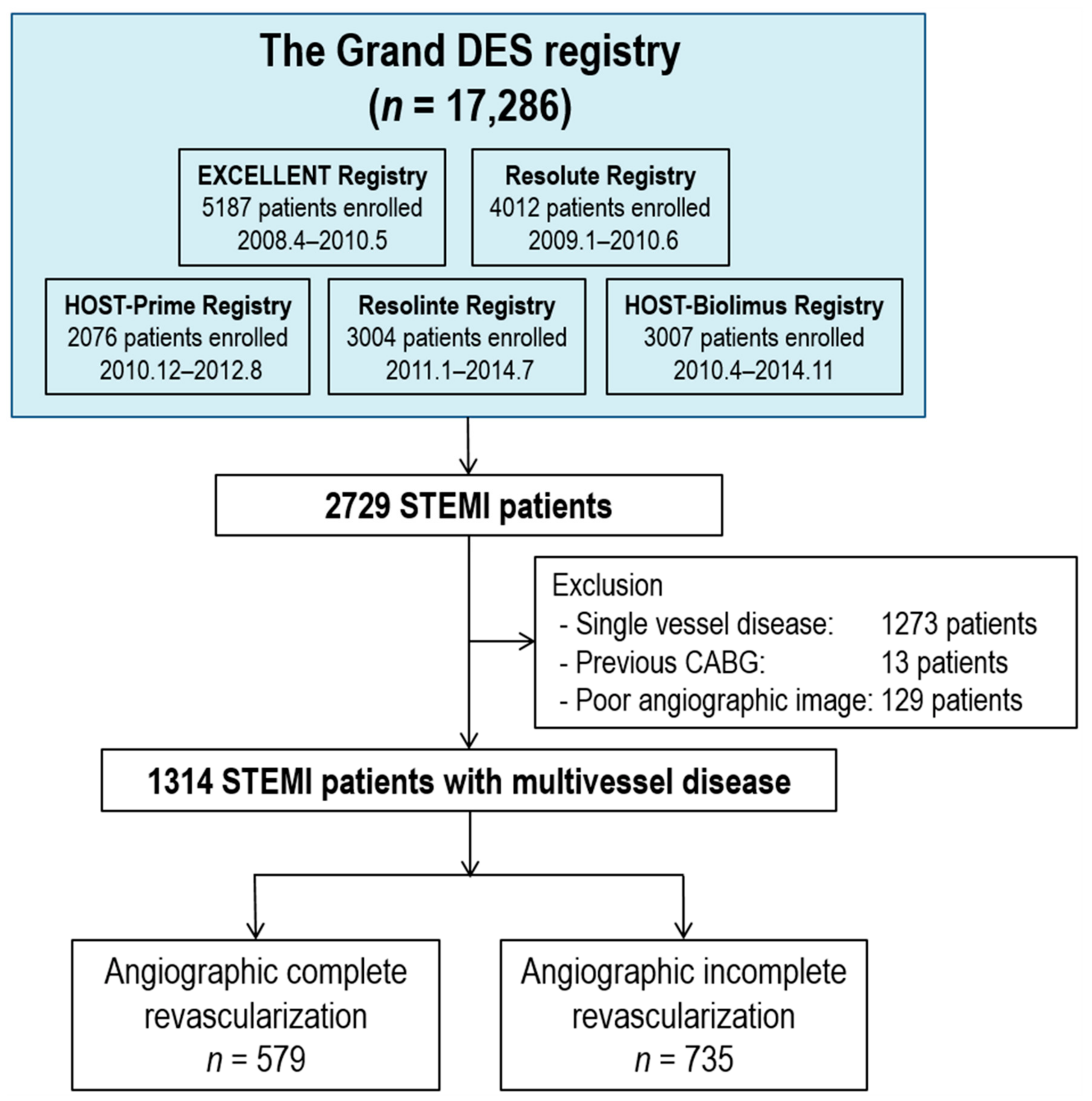
2.1. Study Population
2.2. Completeness of Revascularization and Calculation of the SYNTAX Score
2.3. Echocardiographic Study
2.4. End Points
2.5. Statistical Analyses
3. Results
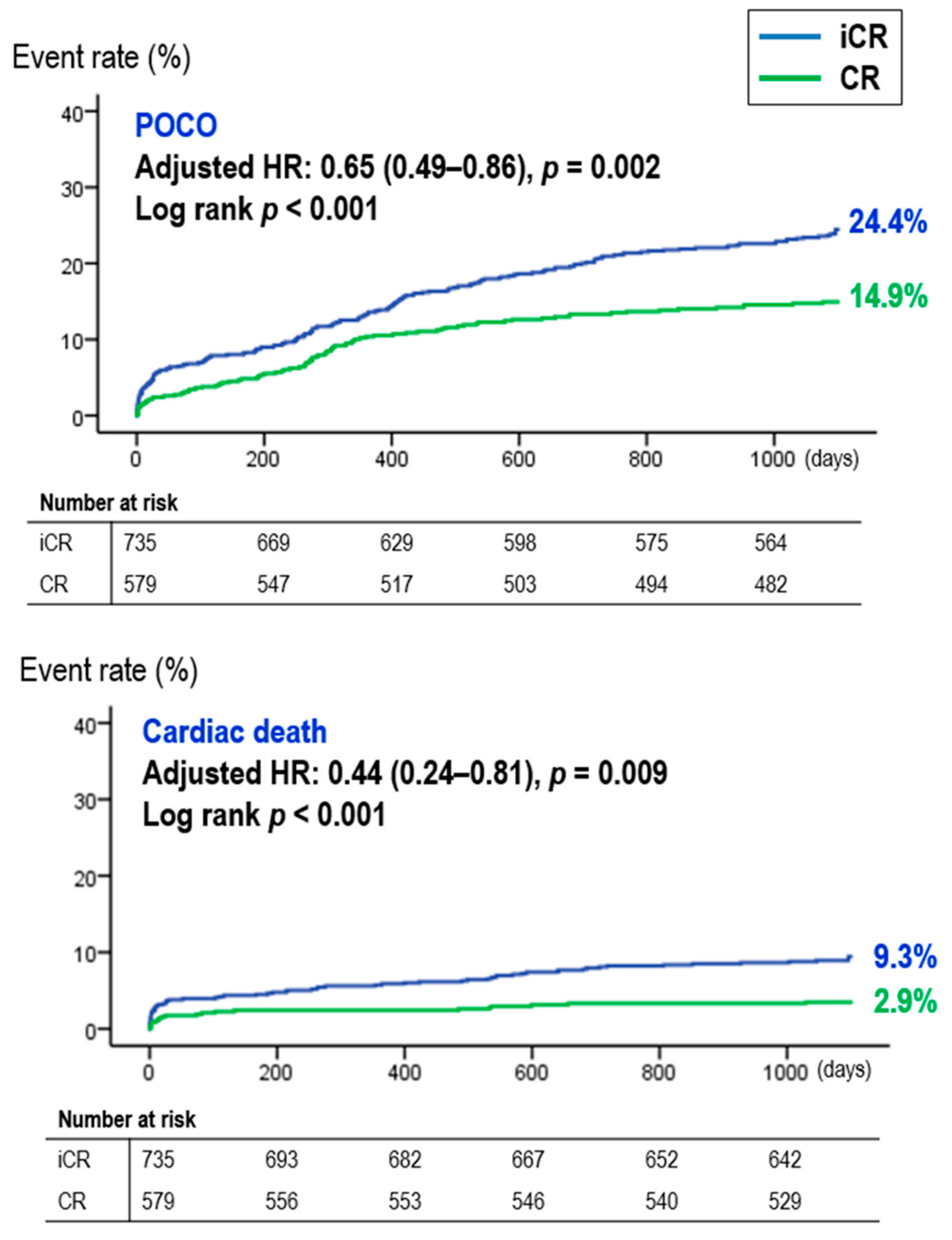
3.1. The Beneficial Effect of CR in STEMI Patients
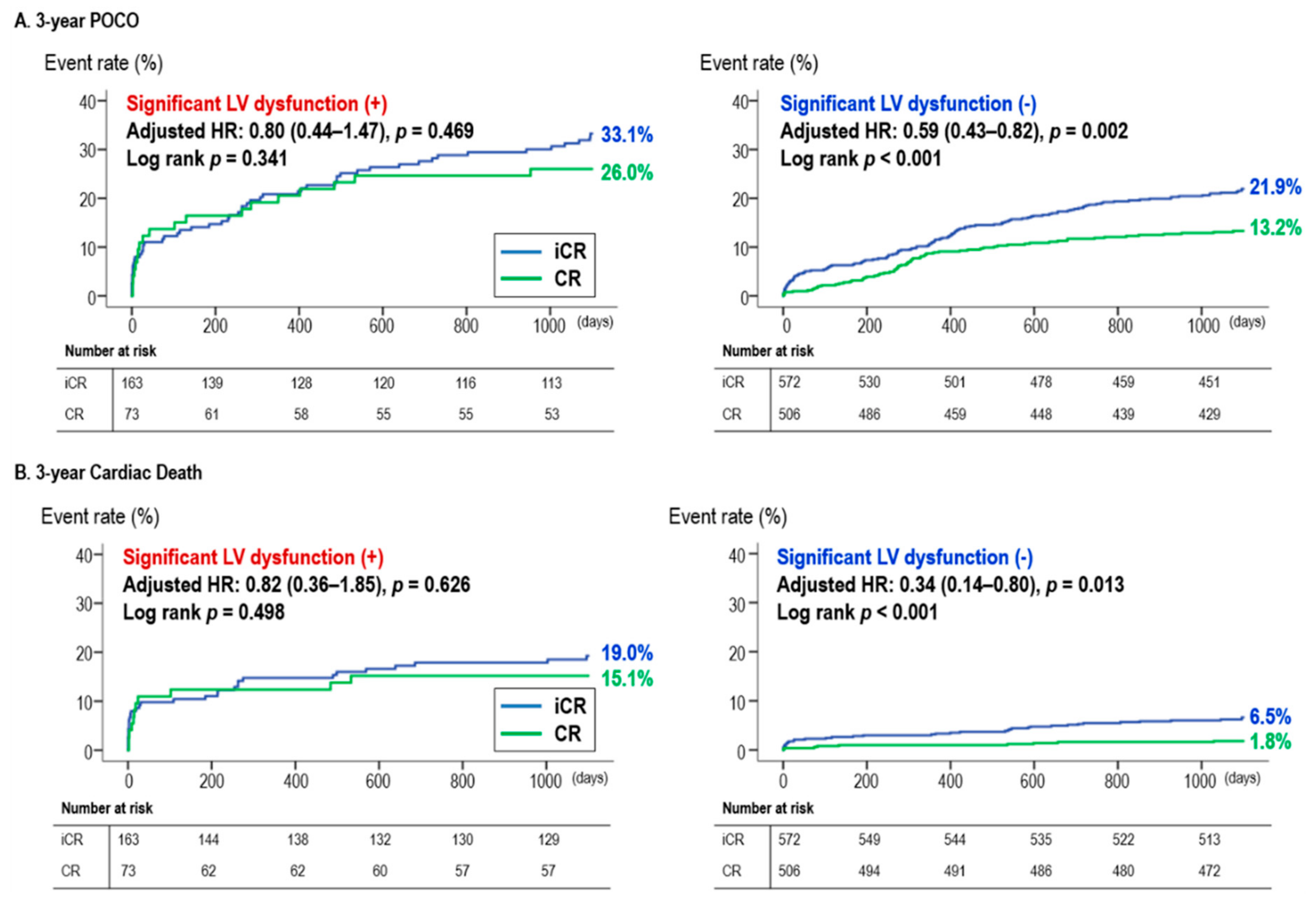
3.2. CR vs. iCR According to Baseline LVEF
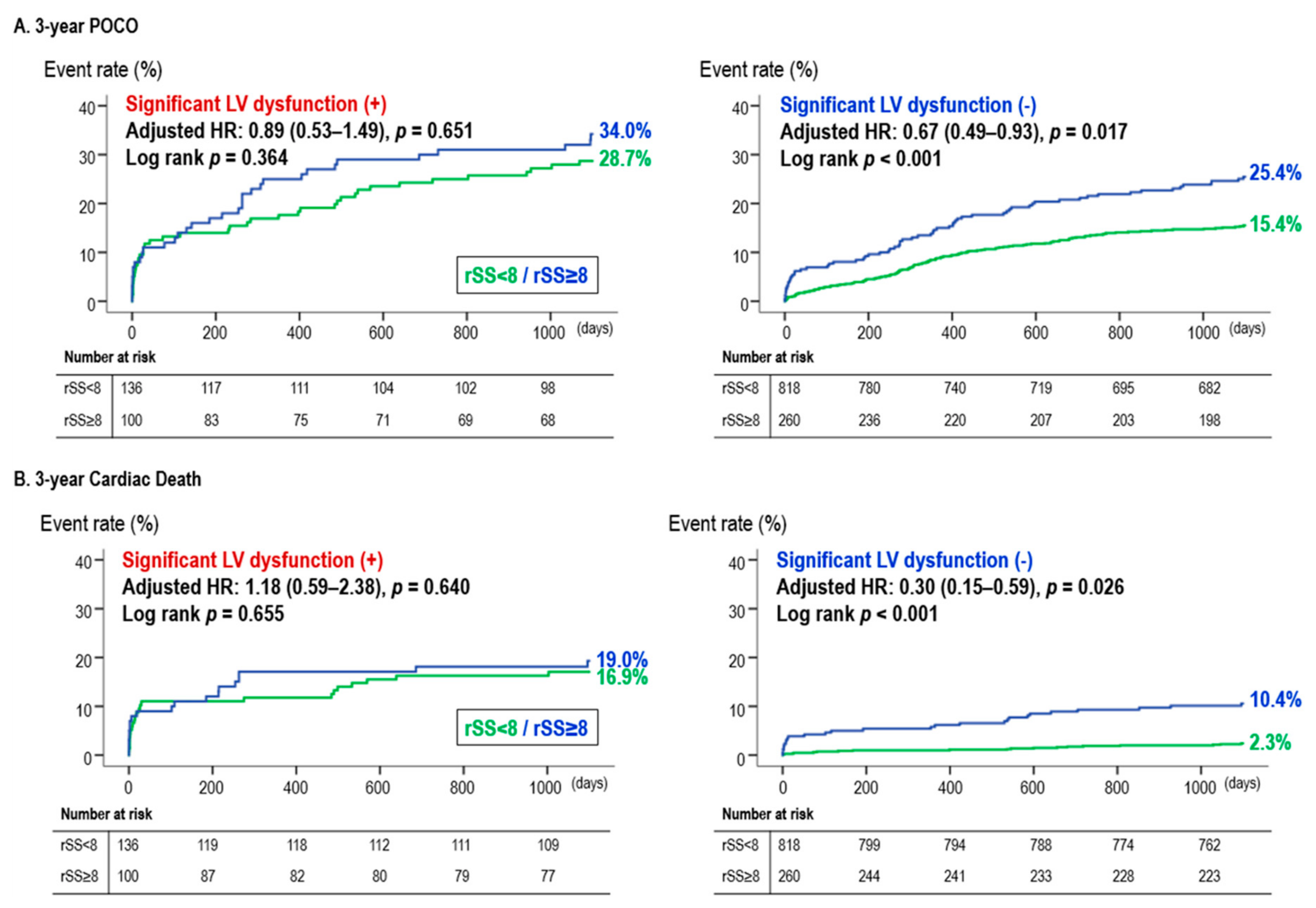
3.3. Corroboration Using SS-Based CR
4. Discussion
4.1. CR in STEMI Patients
4.2. CR in STEMI Patients with Reduced LVEF (Moderate to Severe LV Dysfunction)
4.3. Confirmation of the Effect of CR Using a SYNTAX Score-Based Definition of CR
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 esc guidelines for the management of acute myocardial infarction in patients presenting with st-segment elevation. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 1082. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2015 acc/aha/scai focused update on primary percutaneous coronary intervention for patients with st-elevation myocardial infarction: An update of the 2011 accf/aha/scai guideline for percutaneous coronary intervention and the 2013 accf/aha guideline for the management of st-elevation myocardial infarction. J. Am. Coll. Cardiol. 2016, 67, 1235–1250. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 esc guidelines for the management of acute myocardial infarction in patients presenting with st-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with st-segment elevation of the european society of cardiology (esc). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed] [Green Version]
- Wald, D.S.; Morris, J.K.; Wald, N.J.; Chase, A.J.; Edwards, R.J.; Hughes, L.O.; Berry, C.; Oldroyd, K.G.; Investigators, P. Randomized trial of preventive angioplasty in myocardial infarction. N. Engl. J. Med. 2013, 369, 1115–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrom, T.; Kelbaek, H.; Helqvist, S.; Hofsten, D.E.; Klovgaard, L.; Holmvang, L.; Jorgensen, E.; Pedersen, F.; Saunamaki, K.; Clemmensen, P.; et al. Complete revascularisation versus treatment of the culprit lesion only in patients with st-segment elevation myocardial infarction and multivessel disease (danami-3-primulti): An open-label, randomised controlled trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef]
- Gershlick, A.H.; Khan, J.N.; Kelly, D.J.; Greenwood, J.P.; Sasikaran, T.; Curzen, N.; Blackman, D.J.; Dalby, M.; Fairbrother, K.L.; Banya, W.; et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for stemi and multivessel disease: The cvlprit trial. J. Am. Coll. Cardiol. 2015, 65, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Smits, P.C.; Abdel-Wahab, M.; Neumann, F.J.; Boxma-de Klerk, B.M.; Lunde, K.; Schotborgh, C.E.; Piroth, Z.; Horak, D.; Wlodarczak, A.; Ong, P.J.; et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N. Engl. J. Med. 2017, 376, 1234–1244. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; de Waha-Thiele, S.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Nordbeck, P.; Geisler, T.; Landmesser, U.; et al. One-year outcomes after pci strategies in cardiogenic shock. N. Engl. J. Med. 2018, 379, 1699–1710. [Google Scholar] [CrossRef]
- Burns, R.J.; Gibbons, R.J.; Yi, Q.; Roberts, R.S.; Miller, T.D.; Schaer, G.L.; Anderson, J.L.; Yusuf, S.; Investigators, C.S. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J. Am. Coll. Cardiol. 2002, 39, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Ng, V.G.; Lansky, A.J.; Meller, S.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.; Shah, R.; Mehran, R.; Stone, G.W. The prognostic importance of left ventricular function in patients with st-segment elevation myocardial infarction: The horizons-ami trial. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Hannan, E.L.; Wu, C.; Walford, G.; Holmes, D.R.; Jones, R.H.; Sharma, S.; King, S.B., 3rd. Incomplete revascularization in the era of drug-eluting stents: Impact on adverse outcomes. JACC Cardiovasc. Interv. 2009, 2, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Park, K.W.; Han, J.K.; Yang, H.M.; Kang, H.J.; Koo, B.K.; Kim, H.S. Usefulness of the baseline syntax score to predict 3-year outcome after complete revascularization by percutaneous coronary intervention. Am. J. Cardiol. 2016, 118, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Farooq, V.; Serruys, P.W.; Bourantas, C.V.; Zhang, Y.; Muramatsu, T.; Feldman, T.; Holmes, D.R.; Mack, M.; Morice, M.C.; Stahle, E.; et al. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (syntax) trial validation of the residual syntax score. Circulation 2013, 128, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Sutton, N.R.; Li, S.; Thomas, L.; Wang, T.Y.; de Lemos, J.A.; Enriquez, J.R.; Shah, R.U.; Fonarow, G.C. The association of left ventricular ejection fraction with clinical outcomes after myocardial infarction: Findings from the acute coronary treatment and intervention outcomes network (action) registry-get with the guidelines (gwtg) medicare-linked database. Am. Heart J. 2016, 178, 65–73. [Google Scholar] [CrossRef]
- Delewi, R.; Zijlstra, F.; Piek, J.J. Left ventricular thrombus formation after acute myocardial infarction. Heart 2012, 98, 1743–1749. [Google Scholar] [CrossRef] [Green Version]
- Henkel, D.M.; Witt, B.J.; Gersh, B.J.; Jacobsen, S.J.; Weston, S.A.; Meverden, R.A.; Roger, V.L. Ventricular arrhythmias after acute myocardial infarction: A 20-year community study. Am. Heart J. 2006, 151, 806–812. [Google Scholar] [CrossRef]
- Russo, A.M.; Stainback, R.F.; Bailey, S.R.; Epstein, A.E.; Heidenreich, P.A.; Jessup, M.; Kapa, S.; Kremers, M.S.; Lindsay, B.D.; Stevenson, L.W. Accf/hrs/aha/ase/hfsa/scai/scct/scmr 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: A report of the american college of cardiology foundation appropriate use criteria task force, heart rhythm society, american heart association, american society of echocardiography, heart failure society of america, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, and society for cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2013, 61, 1318–1368. [Google Scholar]
- De Rosa, S.; Polimeni, A.; Sabatino, J.; Indolfi, C. Long-term outcomes of coronary artery bypass grafting versus stent-pci for unprotected left main disease: A meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 240. [Google Scholar] [CrossRef] [Green Version]
- Allman, K.C.; Shaw, L.J.; Hachamovitch, R.; Udelson, J.E. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: A meta-analysis. J. Am. Coll. Cardiol. 2002, 39, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Shantsila, E.; Lip, G.Y. The risk of thromboembolism in heart failure: Does it merit anticoagulation therapy? Am. J. Cardiol. 2011, 107, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; Lopez-Sendon, J.; Faxon, D.P.; et al. Complete revascularization with multivessel pci for myocardial infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.W.; Kang, J.; Kang, S.H.; Ahn, H.S.; Kang, H.J.; Koo, B.K.; Chae, I.H.; Youn, T.J.; Oh, B.H.; Park, Y.B.; et al. The impact of residual coronary lesions on clinical outcomes after percutaneous coronary intervention: Residual syntax score after percutaneous coronary intervention in patients from the efficacy of xience/promus versus cypher in reducing late loss after stenting (excellent) registry. Am. Heart J. 2014, 167, 384–392. [Google Scholar] [PubMed]
- Genereux, P.; Palmerini, T.; Caixeta, A.; Rosner, G.; Green, P.; Dressler, O.; Xu, K.; Parise, H.; Mehran, R.; Serruys, P.W.; et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: The residual syntax (synergy between pci with taxus and cardiac surgery) score. J. Am. Coll. Cardiol. 2012, 59, 2165–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolfo, D.; Cinquetti, M.; Merlo, M.; Santangelo, S.; Barbati, G.; Alonge, M.; Vitrella, G.; Rakar, S.; Salvi, A.; Perkan, A.; et al. St-elevation myocardial infarction with reduced left ventricular ejection fraction: Insights into persisting left ventricular dysfunction. A ppci-registry analysis. Int. J. Cardiol. 2016, 215, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Chew, D.S.; Heikki, H.; Schmidt, G.; Kavanagh, K.M.; Dommasch, M.; Bloch Thomsen, P.E.; Sinnecker, D.; Raatikainen, P.; Exner, D.V. Change in left ventricular ejection fraction following first myocardial infarction and outcome. JACC Clin. Electrophysiol. 2018, 4, 672–682. [Google Scholar] [CrossRef]
| Total (n = 1314) | CR (n = 579) | iCR (n = 735) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years old) | 63.3 ± 12.1 | 62.1 ± 11.8 | 64.2 ± 12.3 | 0.001 |
| Male sex, n (%) | 996 (75.8%) | 452 (78.1%) | 544 (74.0%) | 0.101 |
| Body mass index (kg/m2) | 23.9 ± 3.0 | 24.0 ± 3.0 | 23.9 ± 3.0 | 0.540 |
| Diabetes mellitus, n (%) | 427 (32.5%) | 195 (33.7%) | 232 (31.6%) | 0.451 |
| Hypertension, n (%) | 688 (52.4%) | 281 (48.5%) | 407 (55.4%) | 0.016 |
| Dyslipidemia, n (%) | 607 (46.2%) | 263 (45.4%) | 344 (46.8%) | 0.658 |
| Current smoking, n (%) | 633 (48.2%) | 286 (49.4%) | 347 (47.2%) | 0.465 |
| Previous stroke, n (%) | 87 (6.6%) | 36 (6.2%) | 51 (6.9%) | 0.682 |
| Congestive heart failure, n (%) | 20 (1.5%) | 8 (1.4%) | 12 (1.6%) | 0.887 |
| Chronic renal failure, n (%) | 486 (38.6%) | 194 (34.5%) | 292 (42.0%) | 0.007 |
| Peripheral vascular disease, n (%) | 14 (1.1%) | 9 (1.6%) | 5 (0.7%) | 0.207 |
| Prior MI, n (%) | 93 (7.1%) | 41 (7.1%) | 52 (7.1%) | 1.000 |
| Prior PCI, n (%) | 129 (9.8%) | 63 (10.9%) | 66 (9.0%) | 0.291 |
| Family history of CAD, n (%) | 74 (5.6%) | 30 (5.2%) | 44 (6.0%) | 0.611 |
| Angiographic findings | ||||
| Angiographic disease extent | ||||
| 2 vessel disease, n (%) | 795 (60.5%) | 407 (70.3%) | 388 (52.8%) | <0.001 |
| 3 vessel disease, n (%) | 519 (39.5%) | 172 (29.7%) | 347 (47.2%) | |
| Left main disease, n (%) | 50 (3.8%) | 24 (4.1%) | 26 (3.5%) | 0.670 |
| Bifurcation lesion, n (%) | 490 (37.3%) | 246 (42.5%) | 244 (33.2%) | 0.001 |
| Type B2/C lesion, n (%) | 1117 (85.0%) | 499 (86.2%) | 618 (84.1%) | 0.326 |
| Calcified lesion, n (%) | 109 (8.3%) | 48 (8.3%) | 61 (8.3%) | 1.000 |
| Tortuous lesion, n (%) | 280 (21.3%) | 136 (23.5%) | 144 (19.6%) | 0.100 |
| Thrombus in lesion, n (%) | 454 (34.6%) | 206 (35.6%) | 248 (33.7%) | 0.524 |
| Previously treated lesion, n (%) | 106 (8.1%) | 46 (7.9%) | 60 (8.2%) | 0.966 |
| Culprit lesion, n (%) | 0.787 | |||
| LM, n (%) | 36 (2.7%) | 15 (2.6%) | 21 (2.9%) | |
| LAD, n (%) | 620 (47.2%) | 281 (48.5%) | 339 (46.1%) | |
| LCX, n (%) | 151 (11.5%) | 70 (12.1%) | 81 (11.0%) | |
| RCA, n (%) | 505 (38.4%) | 212 (36.6%) | 293 (39.9%) | |
| Stent diameter, mm | 3.1 ± 0.4 | 3.0 ± 0.4 | 3.1 ± 0.4 | 0.088 |
| Stent diameter <3 mm, n (%) | 508 (38.7%) | 224 (38.8%) | 284 (38.6%) | 1.000 |
| Min. stent diameter, mm | 3.0 ± 0.4 | 2.9 ± 0.4 | 3.0 ± 0.4 | <0.001 |
| Min. stent diameter <3 mm, n (%) | 625 (47.6%) | 296 (51.1%) | 329 (44.8%) | 0.025 |
| Total stent length, mm | 43.5 ± 25.9 | 49.9 ± 28.8 | 38.5 ± 22.1 | <0.001 |
| Total stent length ≥30 mm, n (%) | 803 (61.2%) | 398 (68.9%) | 405 (55.1%) | <0.001 |
| Total stent number | 1.8 ± 1.0 | 2.1 ± 1.1 | 1.5 ± 0.8 | <0.001 |
| Staged PCI (among CR patients), n (%) | NA | 97 (16.8%) | NA | NA |
| Second generation DES usage, n (%) | 944 (71.8%) | 417 (72.0%) | 527 (71.7%) | 0.947 |
| Contrast volume, mL | 272.8 ± 111.1 | 282.5 ± 107.6 | 263.6 ± 114.1 | 0.199 |
| GP IIb/IIIa inhibitor usage, n (%) | 143 (10.9%) | 59 (10.2%) | 84 (11.4%) | 0.531 |
| IVUS usage, n (%) | 397 (30.2%) | 201 (34.7%) | 196 (26.7%) | 0.002 |
| Device success, n (%) | 1290 (98.2%) | 569 (98.3%) | 721 (98.1%) | 0.975 |
| Lesion success, n (%) | 1284 (97.7%) | 564 (97.4%) | 720 (98.0%) | 0.634 |
| Procedural success, n (%) | 1281 (97.5%) | 562 (97.1%) | 719 (97.8%) | 0.487 |
| SYNTAX score at baseline | 18.2 ± 8.8 | 15.6 ± 8.0 | 20.3 ± 8.9 | <0.001 |
| SYNTAX score after PCI (residual) | 5.7 ± 6.4 | 1.7 ± 2.4 | 8.8 ± 6.8 | <0.001 |
| Delta SYNTAX score | 12.5 ± 7.5 | 13.8 ± 7.8 | 11.5 ± 7.2 | <0.001 |
| Laboratory data | ||||
| LVEF (%) | 50.7 ± 22.8 | 53.4 ± 31.7 | 48.7 ± 11.6 | 0.002 |
| WBC (/ul) | 10868 ± 3934 | 10715 ± 4075 | 10986 ± 3820 | 0.221 |
| Hemoglobin (g/dL) | 13.8 ± 2.1 | 13.9 ± 2.0 | 13.7 ± 2.2 | 0.071 |
| Anemia (Hb <12 g/dL) | 236 (18.2%) | 93 (16.3%) | 143 (19.6%) | 0.148 |
| Creatinine (mg/dL) | 1.1 ± 0.7 | 73.5 ± 28.9 | 69.8 ± 30.1 | 0.027 |
| Total Cholesterol (mg/dL) | 181.5 ± 45.3 | 181.2 ± 45.1 | 181.8 ± 45.6 | 0.824 |
| Triglyceride (mg/dL) | 129.7 ± 87.8 | 133.1 ± 87.3 | 127.1 ± 88.1 | 0.260 |
| HDL-cholesterol (mg/dL) | 42.0 ± 12.1 | 41.9 ± 12.0 | 42.2 ± 12.2 | 0.685 |
| LDL-cholesterol (mg/dL) | 115.9 ± 38.0 | 116.3 ± 39.5 | 115.5 ± 36.9 | 0.722 |
| Discharge medication | ||||
| Aspirin, n (%) | 1306 (99.4%) | 576 (99.5%) | 730 (99.3%) | 0.986 |
| Clopidogrel, n (%) | 1285 (97.8%) | 569 (98.3%) | 716 (97.4%) | 0.389 |
| DAPT, n (%) | 1283 (97.6%) | 568 (98.1%) | 715 (97.3%) | 0.429 |
| Beta blocker, n (%) | 1028 (78.2%) | 460 (79.4%) | 568 (77.3%) | 0.380 |
| ACE inhibitor or ARBs, n (%) | 1049 (79.8%) | 457 (78.9%) | 592 (80.5%) | 0.512 |
| Statin, n (%) | 1147 (87.3%) | 512 (88.4%) | 635 (86.4%) | 0.310 |
| Calcium channel blocker, n (%) | 106 (8.1%) | 51 (8.8%) | 55 (7.5%) | 0.439 |
| Total | CR | ICR | Unadjusted | Multivariable-Adjusted | PSM | IPTW | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||||
| Reduced LV function | n = 236 | n = 73 | n = 163 | ||||||||
| POCO | 73 (30.9%) | 19 (26.0%) | 54 (33.1%) | 0.78 (0.46–1.31) | 0.343 | 0.80 (0.44–1.47) | 0.469 | 1.03 (0.50–2.14) | 0.937 | 0.94 (0.66–1.36) | 0.778 |
| All cause death | 51 (21.6%) | 15 (20.5%) | 36 (22.1%) | 0.93 (0.51–1.70) | 0.811 | 1.05 (0.51–2.15) | 0.899 | 1.01 (0.42–2.44) | 0.975 | 1.11 (0.72–1.70) | 0.650 |
| Cardiac death | 42 (17.8%) | 11 (15.1%) | 31 (19.0%) | 0.79 (0.40–1.57) | 0.498 | 0.82 (0.36–1.85) | 0.626 | 0.54 (0.18–1.65) | 0.280 | 0.58 (0.34–1.01) | 0.052 |
| MI | 15 (6.4%) | 3 (4.1%) | 12 (7.4%) | 0.28 (0.04–2.22) | 0.227 | 0.35 (0.03–4.17) | 0.403 | 0.32 (0.01–70.87) | 0.680 | 0.72 (0.28–1.88) | 0.502 |
| TVMI | 12 (5.1%) | 3 (4.1%) | 9 (5.5%) | 0.45 (0.05–3.85) | 0.466 | 0.38 (0.01–24.31) | 0.646 | - | 0.982 | 1.00 (0.30–3.39) | 0.989 |
| Stent thrombosis | 7 (3.0%) | 3 (4.1%) | 4 (2.5%) | 1.65 (0.37–7.39) | 0.511 | 4.27 (0.54–33.80) | 0.169 | 6.20 (0.18–215.37) | 0.313 | 1.46 (0.28–7.62) | 0.652 |
| Any revascularization | 23 (9.7%) | 6 (8.2%) | 17 (10.4%) | 0.79 (0.31–2.00) | 0.615 | 0.60 (0.21–1.76) | 0.354 | 1.31 (0.31–5.64) | 0.716 | 0.64 (0.33–1.25) | 0.188 |
| TLR | 8 (3.4%) | 4 (5.5%) | 4 (2.5%) | 2.27 (0.57–9.08) | 0.246 | 3.07 (0.60–15.69) | 0.178 | - | 0.850 | 3.77 (1.16–12.22) | 0.027 |
| TLF | 46 (19.5%) | 11 (15.1%) | 35 (21.5%) | 0.70 (0.36–1.38) | 0.302 | 0.78 (0.35–1.72) | 0.531 | 1.03 (0.39–2.71) | 0.946 | 0.79 (0.50–1.27) | 0.332 |
| Any bleeding | 3 (1.3%) | 0 (0.0%) | 3 (1.8%) | - | 0.497 | - | 0.977 | - | 0.878 | - | 1.000 |
| Preserved LV function | n = 1078 | n = 506 | n = 572 | ||||||||
| POCO | 192 (17.8%) | 67 (13.2%) | 125 (21.9%) | 0.58 (0.43–0.78) | <0.001 | 0.59 (0.43–0.82) | 0.002 | 0.60 (0.43–0.83) | 0.002 | 0.66 (0.54–0.82) | <0.001 |
| All cause death | 82 (7.6%) | 24 (4.7%) | 58 (10.1%) | 0.46 (0.29–0.74) | 0.001 | 0.52 (0.30–0.89) | 0.017 | 0.46 (0.26–0.83) | 0.010 | 0.52 (0.51–0.99) | 0.049 |
| Cardiac death | 46 (4.3%) | 9 (1.8%) | 37 (6.5%) | 0.27 (0.13–0.56) | <0.001 | 0.34 (0.14–0.80) | 0.013 | 0.22 (0.07–0.64) | 0.006 | 0.50 (0.29–0.84) | 0.001 |
| MI | 32 (3.0%) | 10 (2.0%) | 22 (3.8%) | 0.39 (0.16–0.91) | 0.030 | 0.40 (0.16–0.98) | 0.045 | 0.33 (0.11–1.00) | 0.051 | 0.67 (0.38–1.17) | 0.154 |
| TVMI | 16 (1.5%) | 6 (1.2%) | 10 (1.7%) | 0.42 (0.11–1.57) | 0.196 | 0.50 (0.12–2.09) | 0.341 | 0.62 (0.15–2.67) | 0.523 | 1.13 (0.51–2.51) | 0.758 |
| Stent thrombosis | 12 (1.1%) | 5 (1.0%) | 7 (1.2%) | 0.80 (0.25–2.52) | 0.701 | 1.26 (0.26–6.08) | 0.770 | 1.35 (0.29–6.24) | 0.701 | 1.56 (0.61–3.99) | 0.348 |
| Any revascularization | 108 (10.0%) | 43 (8.5%) | 65 (11.4%) | 0.73 (0.49–1.07) | 0.102 | 0.69 (0.45–1.03) | 0.071 | 0.65 (0.41–1.01) | 0.056 | 0.65 (0.49–0.86) | 0.003 |
| TLR | 36 (3.3%) | 20 (4.0%) | 16 (2.8%) | 1.40 (0.72–2.70) | 0.318 | 1.68 (0.79–3.55) | 0.176 | 1.32 (0.63–2.76) | 0.467 | 1.76 (1.05–2.94) | 0.031 |
| TLF | 80 (7.4%) | 28 (5.5%) | 52 (9.1%) | 0.60 (0.38–0.95) | 0.029 | 0.75 (0.45–1.28) | 0.296 | 0.64 (0.36–1.13) | 0.124 | 0.93 (0.65–1.33) | 0.698 |
| Any bleeding | 27 (2.5%) | 10 (2.0%) | 17 (3.0%) | 0.65 (0.30–1.43) | 0.286 | 0.86 (0.35–2.09) | 0.735 | 0.62 (0.22–1.75) | 0.362 | 0.69 (0.36–1.33) | 0.265 |
| Residual SYNTAX Score <8 | Residual SYNTAX Score ≥8 | p-Value | Multivariable Adjusted | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | ||||
| Three-year POCO | |||||
| Total population | 165/954 (17.3%) | 100/360 (27.8%) | <0.001 | 0.71 (0.54–0.94) | 0.017 |
| Preserved LVEF | 126/818 (15.4%) | 66/260 (25.4%) | <0.001 | 0.67 (0.49–0.93) | 0.017 |
| Reduced LVEF | 39/136 (28.7%) | 34/100 (34.0%) | 0.382 | 0.89 (0.53–1.49) | 0.651 |
| Three-year Cardiac Death | |||||
| Total population | 42/954 (4.4%) | 46/360 (12.8%) | <0.001 | 0.57 (0.35–0.94) | 0.026 |
| Preserved LVEF | 19/818 (2.3%) | 27/260 (10.4%) | <0.001 | 0.30 (0.15–0.59) | 0.001 |
| Reduced LVEF | 23/136 (16.9%) | 19/100 (19.0%) | 0.679 | 1.18 (0.59–2.38) | 0.640 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Zheng, C.; Park, K.W.; Park, J.; Rhee, T.; Lee, H.S.; Han, J.-K.; Yang, H.-M.; Kang, H.-J.; Koo, B.-K.; et al. Complete Revascularization of Multivessel Coronary Artery Disease Does Not Improve Clinical Outcome in ST-Segment Elevation Myocardial Infarction Patients with Reduced Left Ventricular Ejection Fraction. J. Clin. Med. 2020, 9, 232. https://doi.org/10.3390/jcm9010232
Kang J, Zheng C, Park KW, Park J, Rhee T, Lee HS, Han J-K, Yang H-M, Kang H-J, Koo B-K, et al. Complete Revascularization of Multivessel Coronary Artery Disease Does Not Improve Clinical Outcome in ST-Segment Elevation Myocardial Infarction Patients with Reduced Left Ventricular Ejection Fraction. Journal of Clinical Medicine. 2020; 9(1):232. https://doi.org/10.3390/jcm9010232
Chicago/Turabian StyleKang, Jeehoon, Chengbin Zheng, Kyung Woo Park, Jiesuck Park, Taemin Rhee, Hak Seung Lee, Jung-Kyu Han, Han-Mo Yang, Hyun-Jae Kang, Bon-Kwon Koo, and et al. 2020. "Complete Revascularization of Multivessel Coronary Artery Disease Does Not Improve Clinical Outcome in ST-Segment Elevation Myocardial Infarction Patients with Reduced Left Ventricular Ejection Fraction" Journal of Clinical Medicine 9, no. 1: 232. https://doi.org/10.3390/jcm9010232
APA StyleKang, J., Zheng, C., Park, K. W., Park, J., Rhee, T., Lee, H. S., Han, J.-K., Yang, H.-M., Kang, H.-J., Koo, B.-K., & Kim, H.-S. (2020). Complete Revascularization of Multivessel Coronary Artery Disease Does Not Improve Clinical Outcome in ST-Segment Elevation Myocardial Infarction Patients with Reduced Left Ventricular Ejection Fraction. Journal of Clinical Medicine, 9(1), 232. https://doi.org/10.3390/jcm9010232






