Bionic Upper Limb Reconstruction: A Valuable Alternative in Global Brachial Plexus Avulsion Injuries—A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Demographic Data
2.2. Surgeries Performed to Improve the Biotechnological Interface
2.3. Outcome Measures
2.3.1. Functional Outcome Measures
2.3.2. Pain Assessment
2.3.3. Patient-Reported Quality of Life Assessment
2.3.4. Statistical Analysis
3. Results
3.1. Functional Outcome
3.2. Pain
3.3. Patient-Reported Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergerot, A.; Shortland, P.J.; Anand, P.; Hunt, S.P.; Carlstedt, T. Co-treatment with riluzole and GDNF is necessary for functional recovery after ventral root avulsion injury. Exp. Neurol. 2004, 187, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Bonney, G. Prognosis in traction lesions of the brachial plexus. J. Bone Jt. Surg. 1959, 41-B, 4–35. J. Bone Jt. Surg. 1959, 41-B, 4–35. [Google Scholar] [CrossRef]
- Moran, S.L.; Steinmann, S.P.; Shin, A.Y. Adult brachial plexus injuries: Mechanism, patterns of injury, and physical diagnosis. Hand Clin. 2005, 21, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, J.A.; Ghizoni, M.F. Reconstruction of complete palsies of the adult brachial plexus by root grafting using long grafts and nerve transfers to target nerves. J. Hand Surg. 2010, 35, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lao, J.; Gao, K.; Gu, Y.; Zhao, X. Functional outcome of nerve transfers for traumatic global brachial plexus avulsion. Injury 2013, 44, 655–660. [Google Scholar] [CrossRef]
- Bertelli, J.A.; Ghizoni, M.F. Results and current approach for Brachial Plexus reconstruction. J. Brachial Plex. Peripher. Nerve Inj. 2011, 6, e54–e61. [Google Scholar] [CrossRef]
- Terzis, J.K.; Kostopoulos, V.K. The surgical treatment of brachial plexus injuries in adults. Plast. Reconstr. Surg. 2007, 119, 73e–92e. [Google Scholar] [CrossRef]
- Tung, T.H.; Novak, C.B.; Mackinnon, S.E. Nerve transfers to the biceps and brachialis branches to improve elbow flexion strength after brachial plexus injuries. J. Neurosurg. 2003, 98, 313–318. [Google Scholar] [CrossRef]
- Tung, T.H.H.; Moore, A.M. Brachial Plexus Injuries in Nerve Surgery; Mackinnon, S.E., Ed.; Thieme Medical Publishers: New York, NY, USA, 2015. [Google Scholar]
- Rasulic, L.; Savic, A.; Zivkovic, B.; Vitosevic, F.; Micovic, M.; Bascarevic, V.; Puzovic, V.; Novakovic, N.; Lepic, M.; Samardzic, M.; et al. Outcome after brachial plexus injury surgery and impact on quality of life. Acta Neurochir. (Wien.) 2017, 159, 1257–1264. [Google Scholar] [CrossRef]
- Carlstedt, T. Central Nerve Plexus Injury; World Scientific Publishing: Hackensack, NJ, USA; Imperial College Press: London, UK, 2007; p. 184. [Google Scholar]
- Davidson, J. A comparison of upper limb amputees and patients with upper limb injuries using the Disability of the Arm, Shoulder and Hand (DASH). Disabil. Rehabil. 2004, 26, 917–923. [Google Scholar] [CrossRef]
- Franzblau, L.; Chung, K.C. Psychosocial outcomes and coping after complete avulsion traumatic brachial plexus injury. Disabil. Rehabil. 2015, 37, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.G. Brachial plexus injury: Deafferentation pain and dorsal root entry zone (DREZ) coagulation. Clin. Neurol. Neurosurg. 1993, 95, 48–49. [Google Scholar] [CrossRef]
- Hruby, L.A.; Sturma, A.; Mayer, J.A.; Pittermann, A.; Salminger, S.; Aszmann, O.C. Algorithm for bionic hand reconstruction in patients with global brachial plexopathies. J. Neurosurg. 2017, 127, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Aszmann, O.C.; Roche, A.D.; Salminger, S.; Paternostro-Sluga, T.; Herceg, M.; Sturma, A.; Hofer, C.; Farina, D. Bionic reconstruction to restore hand function after brachial plexus injury: A case series of three patients. Lancet 2015, 385, 2183–2189. [Google Scholar] [CrossRef]
- Ashley, Z.; Sutherland, H.; Russold, M.F.; Lanmuller, H.; Mayr, W.; Jarvis, J.C.; Salmons, S. Therapeutic stimulation of denervated muscles: The influence of pattern. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2008, 38, 875–886. [Google Scholar] [CrossRef]
- Bergmeister, K.D.; Vujaklija, I.; Muceli, S.; Sturma, A.; Hruby, L.A.; Prahm, C.; Riedl, O.; Salminger, S.; Manzano-Szalai, K.; Aman, M.; et al. Broadband Prosthetic Interfaces: Combining Nerve Transfers and Implantable Multichannel EMG Technology to Decode Spinal Motor Neuron Activity. Front. Neurosci. 2017, 11, 421. [Google Scholar] [CrossRef]
- Salminger, S.; Sturma, A.; Roche, A.D.; Mayer, J.A.; Gstoettner, C.; Aszmann, O.C. Outcomes, challenges and pitfalls after targeted muscle reinnervation in high level amputees. Is it worth the effort? Plast. Reconstr. Surg. 2019, 144, 1037e–1043e. [Google Scholar] [CrossRef]
- Bouwsema, H.; Kyberd, P.J.; Hill, W.; van der Sluis, C.K.; Bongers, R.M. Determining skill level in myoelectric prosthesis use with multiple outcome measures. J. Rehabil. Res. Dev. 2012, 49, 1331–1348. [Google Scholar] [CrossRef]
- Lyle, R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar]
- Kersten, P.; White, P.J.; Tennant, A. Is the pain visual analogue scale linear and responsive to change? An exploration using Rasch analysis. PLoS ONE 2014, 9, e99485. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar]
- Functional Assessment of Case No. 5 with the Plexus Arm, the Hybrid Arm and the Final Prosthetic Arm. Available online: https://zenodo.org/record/3541121#.Xcx49DJKg_U (accessed on 13 November 2019).
- Pondaag, W.; van Driest, F.Y.; Groen, J.L.; Malessy, M.J.A. Early nerve repair in traumatic brachial plexus injuries in adults: Treatment algorithm and first experiences. J. Neurosurg. 2018, 130, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.D.; Novak, C.B.; Mackinnon, S.E.; Kline, D.G. Quality of life and functional outcome following brachial plexus injury. J. Hand Surg. 1997, 22, 605–612. [Google Scholar] [CrossRef]
- Hruby, L.A.; Sturma, A.; Aszmann, O.C. Surface Electromyographic Biofeedback as a Rehabilitation Tool for Patients with Global Brachial Plexus Injury Receiving Bionic Reconstruction. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Sturma, A.; Hruby, L.A.; Farina, D.; Aszmann, O.C. Structured Motor Rehabilitation After Selective Nerve Transfers. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Sturma, A.; Hruby, L.A.; Prahm, C.; Mayer, J.A.; Aszmann, O.C. Rehabilitation of Upper Extremity Nerve Injuries Using Surface EMG Biofeedback: Protocols for Clinical Application. Front. Neurosci. 2018, 12, 906. [Google Scholar] [CrossRef]
- Wright, T.W.; Hagen, A.D.; Wood, M.B. Prosthetic usage in major upper extremity amputations. J. Hand Surg. 1995, 20, 619–622. [Google Scholar] [CrossRef]
- Biddiss, E.; Chau, T. Upper-limb prosthetics: Critical factors in device abandonment. Am. J. Phys. Med. Rehabil. 2007, 86, 977–987. [Google Scholar] [CrossRef]
- Farina, D.; Aszmann, O. Bionic limbs: Clinical reality and academic promises. Sci. Transl. Med. 2014, 6, 257ps12. [Google Scholar] [CrossRef]
- Biddiss, E.A.; Chau, T.T. Upper limb prosthesis use and abandonment: A survey of the last 25 years. Prosthet. Orthot. Int. 2007, 31, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Probsting, E.; Blumentritt, S.; Kannenberg, A. Changes in the Locomotor System as a Consequence of Amputation of a Lower Limb. Z. Orthop. Unfall 2017, 155, 77–91. [Google Scholar] [PubMed]
- Bertels, T.; Schmalz, T.; Ludwigs, E. Biomechanical influences of shoulder disarticulation prosthesis during standing and level walking. Prosthet. Orthot. Int. 2012, 36, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Hattori, Y.; Ikeda, K.; Dhawan, V. Significance of shoulder function in the reconstruction of prehension with double free-muscle transfer after complete paralysis of the brachial plexus. Plast. Reconstr. Surg. 2003, 112, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Hruby, L.A.; Pittermann, A.; Sturma, A.; Aszmann, O.C. The Vienna psychosocial assessment procedure for bionic reconstruction in patients with global brachial plexus injuries. PLoS ONE 2018, 13, e0189592. [Google Scholar] [CrossRef]

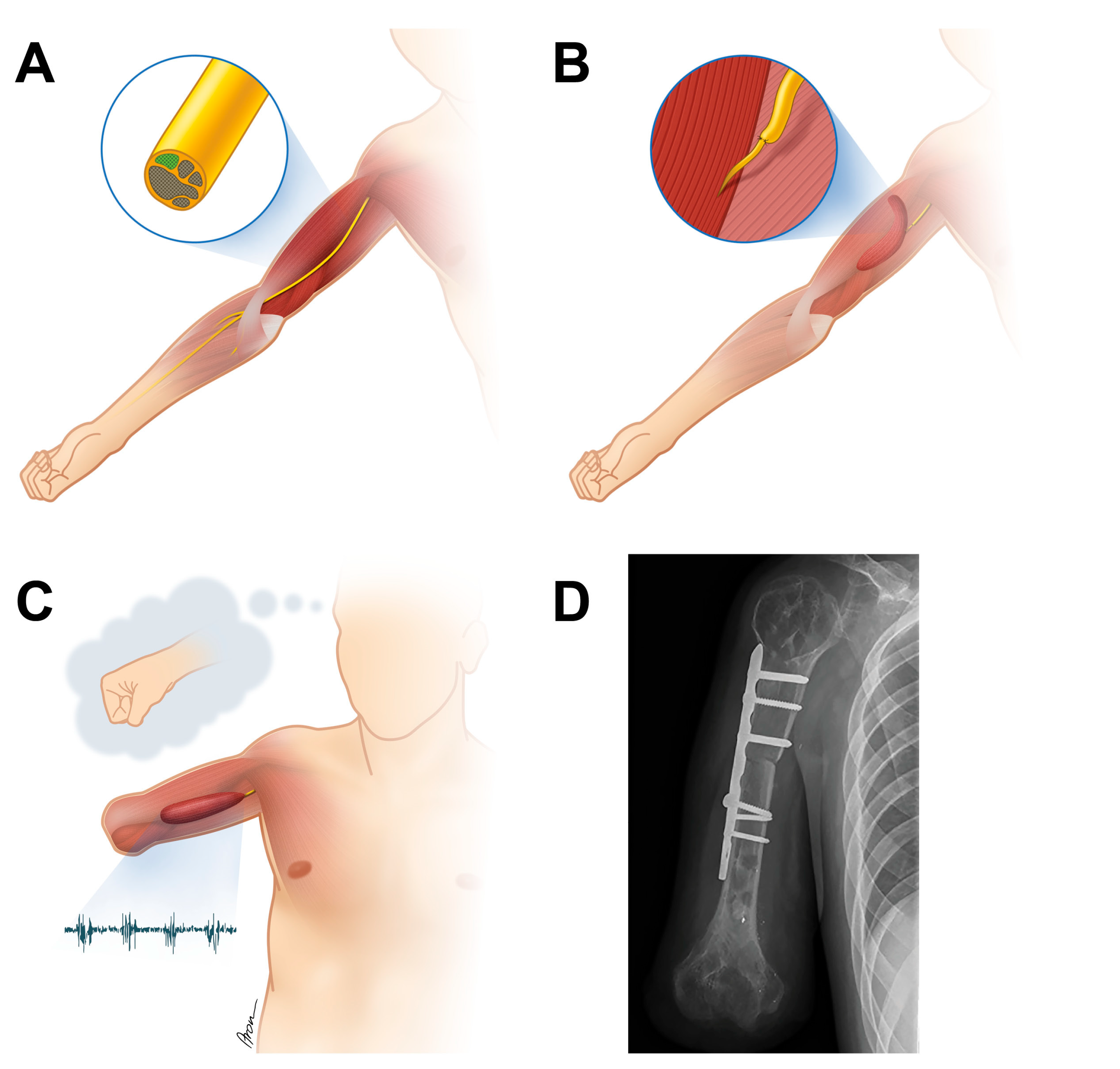
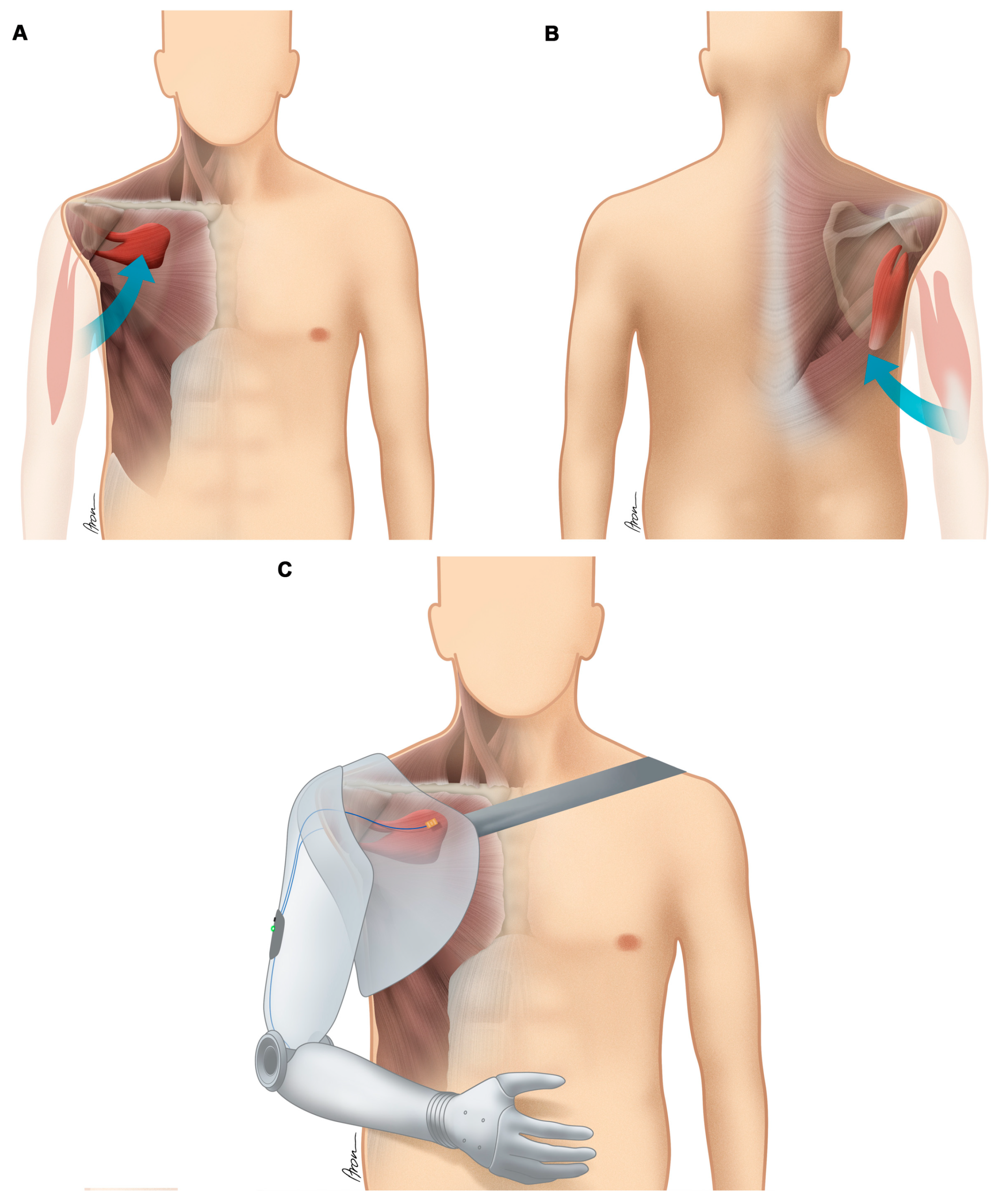
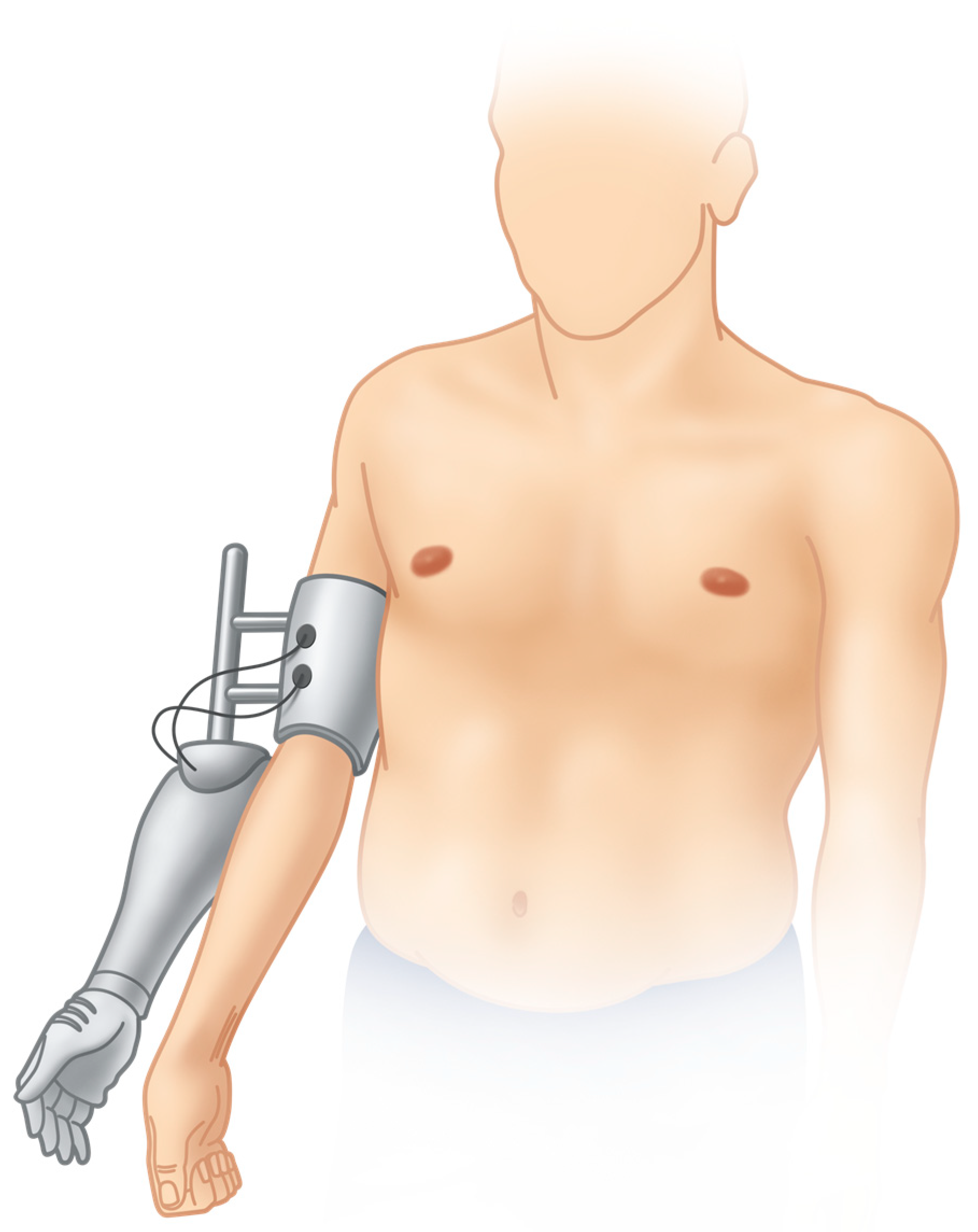
| Case No. | Type of Accident | Type of Lesion, Side | Primary Reconstructive Surgeries aimed at Restoration of Shoulder and Elbow Function |
|---|---|---|---|
| 1 | Motorcycle | Avulsion of roots C5-T1, right | Transfer of accessory nerve to suprascapular nerve and hypoglossal nerve to MCN resulted in a stable shoulder; elbow function did not recover (surgery performed elsewhere five months after injury) |
| 2 | Work-related injury | Rupture of roots C5-C6 and avulsion of C7-T1, left | Sural nerve grafts were used to bridge the defects of C5 and C6 to restore elbow flexion and shoulder stability; motor recovery was unable to move the biological arm (surgery performed elsewhere five months after injury) |
| 3 | Motorcycle | Avulsion of roots C6-T1, right | Restoration of elbow function was attempted with ICN transfers to MCN, however no recovery was achieved (surgery performed elsewhere five months after injury) |
| 4 | Motorcycle | Avulsion of roots C5-T1, left | Transfer of phrenic to suprascapular nerve and ICN transfers to MCN and axillary nerve resulted in a stable shoulder; elbow function did not recover (surgery performed elsewhere four months after injury) |
| 5 | Motorcycle | Avulsion of roots C6-T1, right | Restoration of elbow function was attempted with nerve grafts from C4 and C5 to MCN; ICN transfers to median nerve; motor recovery was unable to move the biological arm (surgery performed elsewhere three months after injury) |
| Case No. | sEMG Signal Sites at Initial Consultation | Surgeries Performed to Improve the Biotechnological Interface | Level of Amputation |
|---|---|---|---|
| 1 | biceps m. + triceps m. | free gracilis muscle transferred to medial upper arm and neurotization of median nerve to obturator nerve to generate a third EMG signal; humerus shortening osteotomy | elbow ex-articulation (with humeral shortening osteotomy) |
| 2 | no detectable myoactivity in the upper arm | free gracilis muscle transferred to dorsal upper arm and neurotization of thoracodorsal nerve to obturator nerve; free adductor longus muscle transferred to medial upper arm and neurotization of median nerve to obturator nerve | transhumeral |
| 3 | biceps m. + triceps m. | transfer of pedicled biceps and triceps muscles to infaclavicular fossa and infraspinatus fossa | glenohumeral |
| 4 | infraspinatus m. + pectoralis major m. | ND | glenohumeral |
| 5 | pectoralis major m. + biceps m. + brachioradialis m. | transfer of pedicled brachioradialis muscle to dorsal upper arm to preserve this signal site upon elective transhumeral amputation | transhumeral |
| ARAT | SHAP | DASH | VAS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Before | Hybrid | After | Before | Hybrid | After | Before | After | Before | After |
| 1 | 0 | 17 | ND | 7 | 10 | ND | 57.5 | 36.7 | 10 | 9.8 |
| 2 | 3 | 16 | ND | 6 | 18 | ND | 37.5 | 15 | 8.2 | 6.9 |
| 3 | 0 | 0 | 17 | 0 | 24 | 30 | 49.2 | 34.2 | 7.8 | 6.5 |
| 4 | 0 | 11 | 19 | 0 | 0 | 12 | 60 | 30 | 7.5 | 4 |
| 5 | 0 | 11 | 16 | 7 | 17 | 24 | 58.3 | 40 | 9.1 | 6.4 |
| MEAN ± SD | 0.6 ± 1.3 | 11.0 ± 6.7 | 17.3 ± 1.5 | 4.0 ± 3.7 | 13.8 ± 9.2 | 22.0 ± 9.2 | 52.5 ± 9.4 | 31.2 ± 9.8 | 8.5 ± 1.0 | 6.7 ± 2.1 |
| Case No. | Physical Functioning | Role Physical | Bodily Pain | General Health | Vitality | Social Functioning | Role Emotional | Mental Health | Physical Comp. Sum. Scale | Mental Comp. Sum. Scale | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before amputation | 1 | 28 | 10 | 18 | 56 | 38 | 32 | 36 | 48 | 37.8 | 48.2 |
| 2 | 42 | 20 | 26 | 64 | 44 | 41 | 12 | 50 | 38.6 | 44.3 | |
| 3 | 39 | 29 | 34 | 54 | 44 | 48 | 39 | 52 | 36.3 | 54.5 | |
| 4 | 28 | 22 | 34 | 17 | 26 | 10 | 28 | 20 | 26.3 | 30.8 | |
| 5 | 0 | 0 | 14 | 38 | 24 | 24 | 18 | 35 | 25.6 | 40.1 | |
| MEAN ± SD | 27.4 ± 16.6 | 16.2 ± 11.3 | 25.2 ± 9.1 | 45.8 ± 18.7 | 35.2 ± 9.7 | 31.0 ± 14.8 | 26.6 ± 11.5 | 41.0 ± 13.5 | 32.9 ± 6.4 | 43.6 ± 8.9 | |
| After bionic reconstruction | 1 | 44 | 52 | 33 | 56 | 40 | 54 | 53 | 52 | 49.7 | 54.2 |
| 2 | 47 | 54 | 40 | 50 | 58 | 55 | 53 | 55 | 46.1 | 59.4 | |
| 3 | 44 | 38 | 37 | 62 | 52 | 55 | 53 | 60 | 40.7 | 62.9 | |
| 4 | 40 | 22 | 37 | 20 | 32 | 26 | 54 | 42 | 25 | 49.4 | |
| 5 | 14 | 52 | 33 | 44 | 48 | 46 | 53 | 60 | 40.5 | 60.8 | |
| MEAN ± SD | 37.8 ± 13.5 | 43.6 ± 13.7 | 36.0 ± 3.0 | 46.4 ± 16.2 | 46.0 ± 10.2 | 47.2 ± 12.4 | 53.2 ± 0.4 | 58.8 ± 7.4 | 40.4 ± 9.4 | 57.3 ± 5.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hruby, L.A.; Gstoettner, C.; Sturma, A.; Salminger, S.; Mayer, J.A.; Aszmann, O.C. Bionic Upper Limb Reconstruction: A Valuable Alternative in Global Brachial Plexus Avulsion Injuries—A Case Series. J. Clin. Med. 2020, 9, 23. https://doi.org/10.3390/jcm9010023
Hruby LA, Gstoettner C, Sturma A, Salminger S, Mayer JA, Aszmann OC. Bionic Upper Limb Reconstruction: A Valuable Alternative in Global Brachial Plexus Avulsion Injuries—A Case Series. Journal of Clinical Medicine. 2020; 9(1):23. https://doi.org/10.3390/jcm9010023
Chicago/Turabian StyleHruby, Laura A., Clemens Gstoettner, Agnes Sturma, Stefan Salminger, Johannes A. Mayer, and Oskar C. Aszmann. 2020. "Bionic Upper Limb Reconstruction: A Valuable Alternative in Global Brachial Plexus Avulsion Injuries—A Case Series" Journal of Clinical Medicine 9, no. 1: 23. https://doi.org/10.3390/jcm9010023
APA StyleHruby, L. A., Gstoettner, C., Sturma, A., Salminger, S., Mayer, J. A., & Aszmann, O. C. (2020). Bionic Upper Limb Reconstruction: A Valuable Alternative in Global Brachial Plexus Avulsion Injuries—A Case Series. Journal of Clinical Medicine, 9(1), 23. https://doi.org/10.3390/jcm9010023






