Continuous Fentanyl Background Infusion Regimen Optimised by Patient-Controlled Analgesia for Acute Postoperative Pain Management: A Randomised Controlled Trial
Abstract
1. Introduction
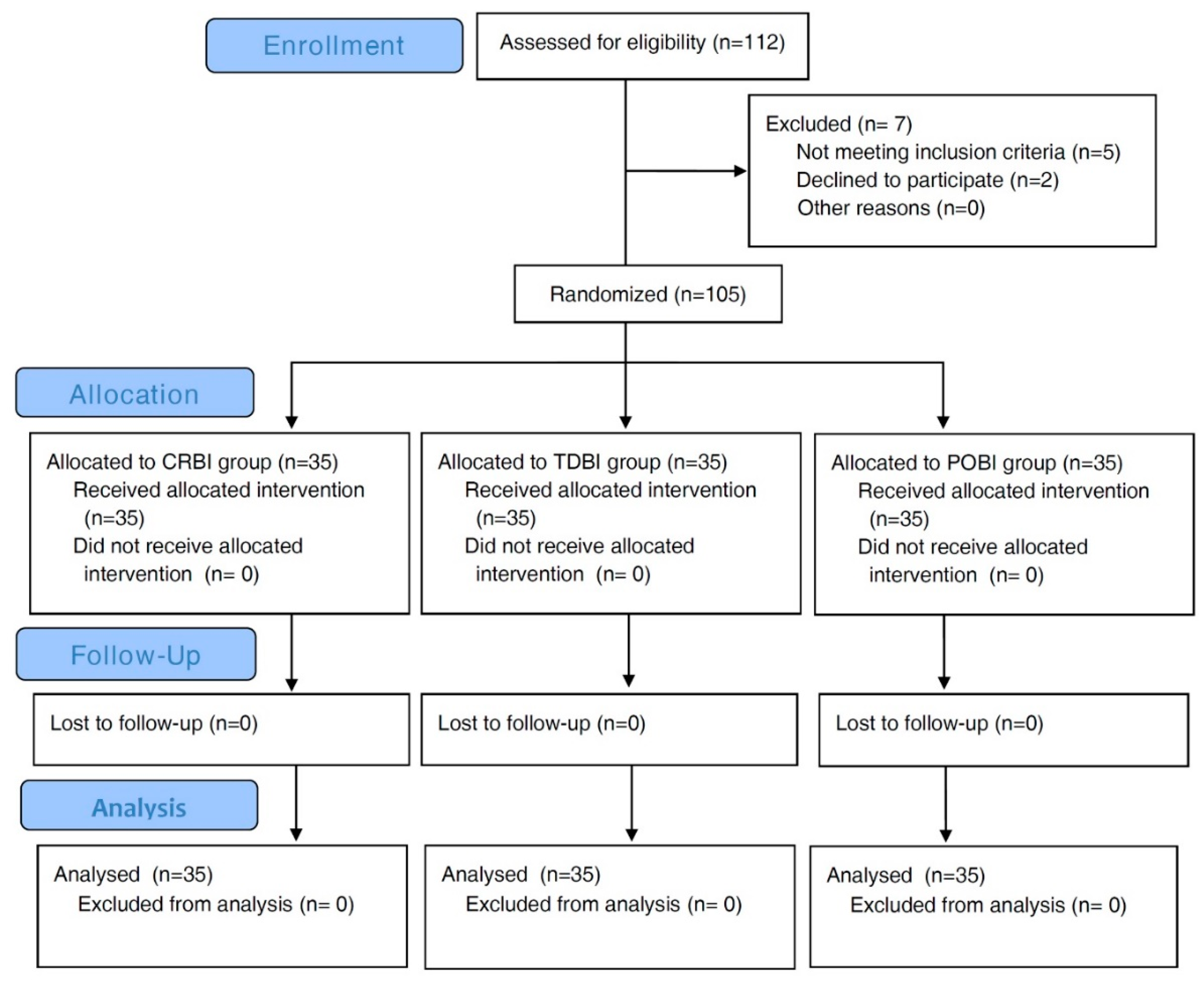
2. Methods
2.1. Study Design and Setting
2.2. Subjects
2.3. Anaesthesia
2.4. Study Interventions
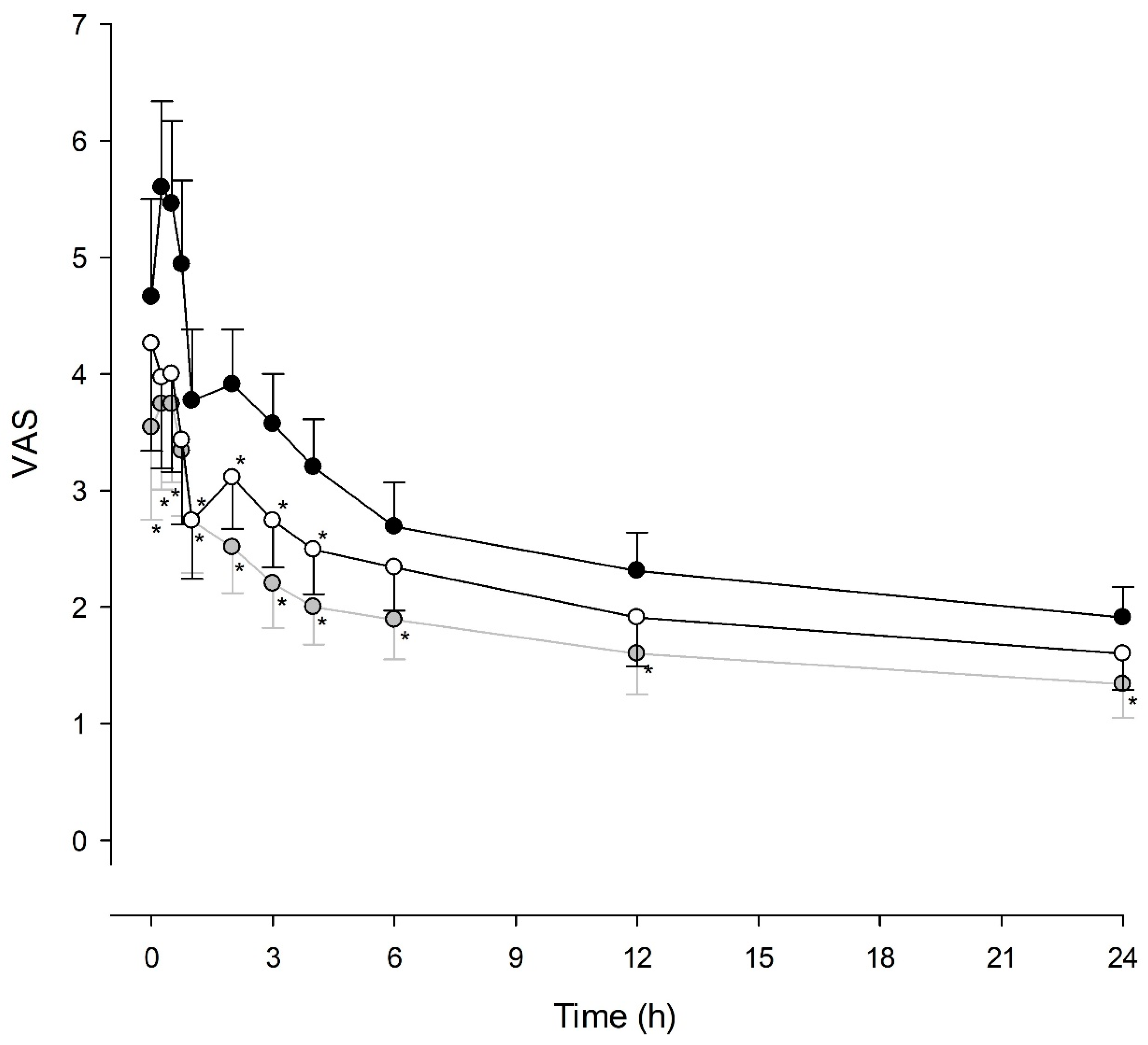
2.5. Assessment of Outcomes
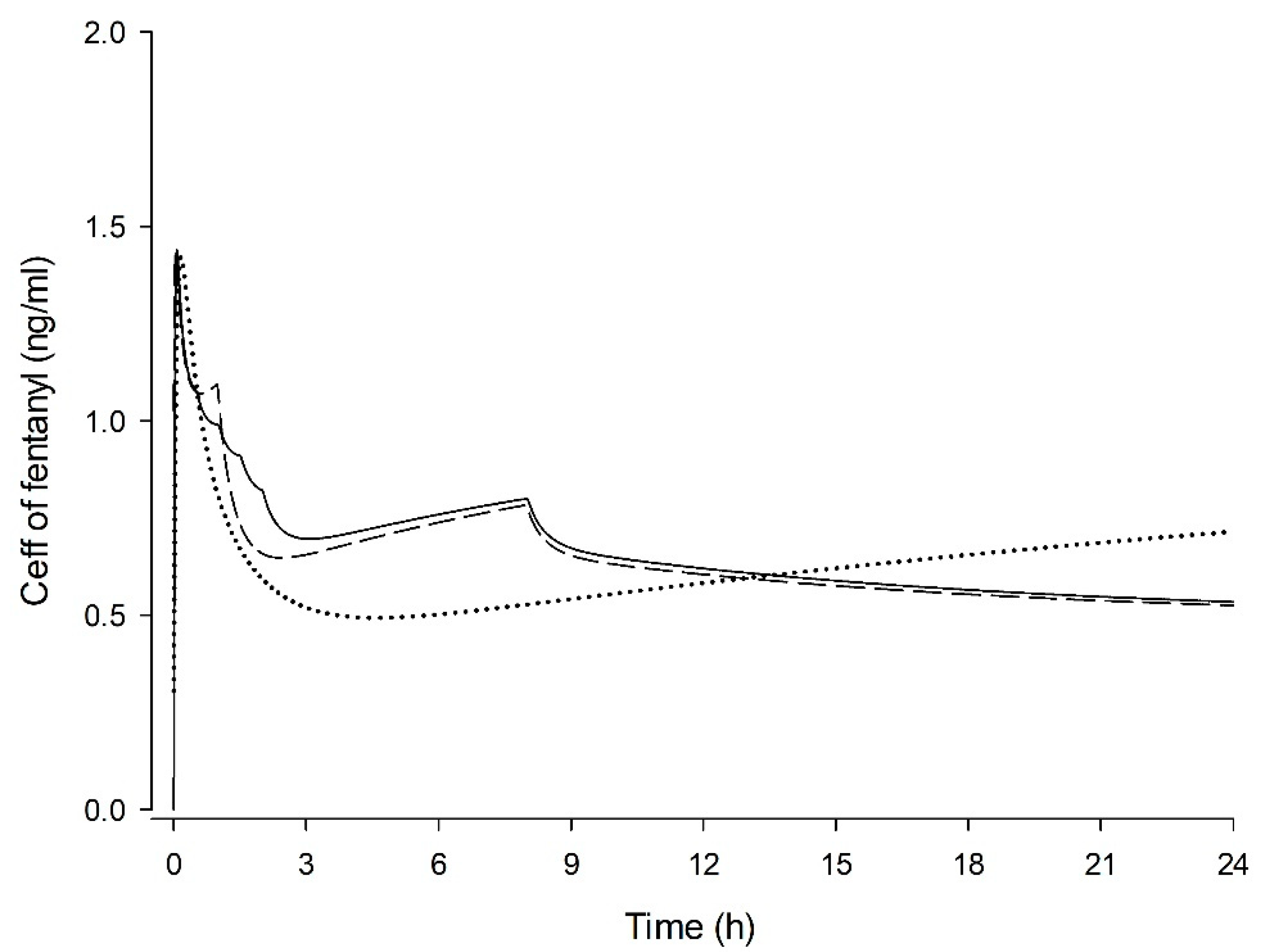
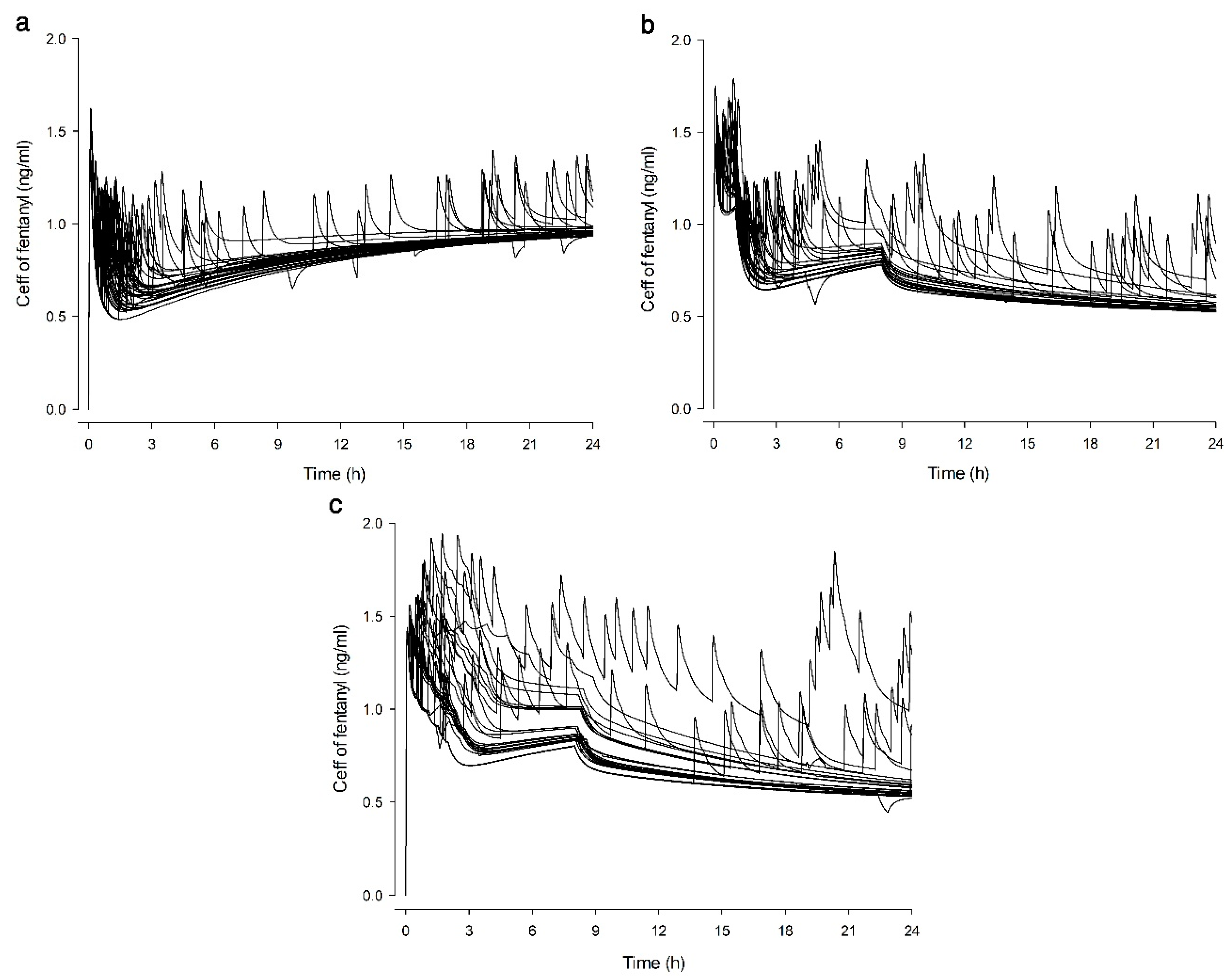
2.6. Simulation of Ceff
2.7. Sample Size and Statistics
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kehlet, H.; Holte, K. Effect of postoperative analgesia on surgical outcome. Br. J. Anaesth. 2001, 87, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Wu, C.L. Effect of postoperative analgesia on major postoperative complications: A systematic update of the evidence. Anesth. Analg. 2007, 104, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, A.; Hobbes, A.F.; Mather, L.E.; Gibson, M. A comparison of morphine, pethidine and fentanyl in the postsurgical patient-controlled analgesia environment. Pain 1996, 64, 115–121. [Google Scholar] [CrossRef]
- Hutchison, R.W.; Chon, E.H.; Tucker, W.F.; Gilder, R.; Moss, J.; Daniel, P. A Comparison of a Fentanyl, Morphine, and Hydromorphone Patient-Controlled Intravenous Delivery for Acute Postoperative Analgesia: A Multicenter Study of Opioid-Induced Adverse Reactions. Hosp. Pharm. 2006, 41, 659–663. [Google Scholar] [CrossRef]
- Peng, P.W.; Sandler, A.N. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 1999, 90, 576–599. [Google Scholar] [CrossRef]
- Shin, S.; Min, K.T.; Shin, Y.S.; Joo, H.M.; Yoo, Y.C. Finding the ‘Ideal’ Regimen for Fentanyl-Based Intravenous Patient-Controlled Analgesia: How to Give and What to Mix? Yonsei Med. J. 2014, 55, 800–806. [Google Scholar] [CrossRef]
- Butkovic, D.; Kralik, S.; Matolic, M.; Kralik, M.; Toljan, S.; Radesic, L. Postoperative analgesia with intravenous fentanyl PCA vs epidural block after thoracoscopic pectus excavatum repair in children. Br. J. Anaesth 2007, 98, 677–681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, J.-H.; Lee, C.; Shin, Y.; An, J.-H.; Ban, J.-S.; Lee, J.-H. Comparison of oxycodone and fentanyl for postoperative patient-controlled analgesia after laparoscopic gynecological surgery. Korean J. Anesth. 2015, 68, 153–158. [Google Scholar] [CrossRef]
- Grant, R.P.; Dolman, J.F.; Harper, J.A.; White, S.A.; Parsons, D.G.; Evans, K.G.; Merrick, C.P. Patient-controlled lumbar epidural fentanyl compared with patient-controlled intravenous fentanyl for post-thoracotomy pain. Can. J. Anaesth. 1992, 39, 214–219. [Google Scholar] [CrossRef]
- Gourlay, G.K.; Kowalski, S.R.; Plummer, J.L.; Cousins, M.J.; Armstrong, P.J. Fentanyl blood concentration-analgesic response relationship in the treatment of postoperative pain. Anesth. Analg. 1988, 67, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Gurbet, A.; Goren, S.; Sahin, S.; Uckunkaya, N.; Korfali, G. Comparison of analgesic effects of morphine, fentanyl, and remifentanil with intravenous patient-controlled analgesia after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 755–758. [Google Scholar] [CrossRef]
- Shibutani, K.; Inchiosa, M.A., Jr.; Sawada, K.; Bairamian, M. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br. J. Anaesth. 2005, 95, 377–383. [Google Scholar] [CrossRef]
- Choi, S.H.; Koo, B.N.; Nam, S.H.; Lee, S.J.; Kim, K.J.; Kil, H.K.; Lee, K.Y.; Jeon, D.H. Comparison of remifentanil and fentanyl for postoperative pain control after abdominal hysterectomy. Yonsei Med. J. 2008, 49, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Ha, M.H.; Jung, S.H.; Song, N.W. The efficiency of IV PCA with remifentanil and ketorolac after laparoscopic-assisted vaginal hysterectomy. Korean J. Anesth. 2011, 61, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.Y.; Chang, H.S.; Nam, S.K.; Min, S.K. The efficacy of the time-scheduled decremental continuous infusion of fentanyl for postoperative patient-controlled analgesia after total intravenous anesthesia. Korean J. Anesth. 2013, 65, 544–551. [Google Scholar] [CrossRef]
- Coghill, R.C. Individual differences in the subjective experience of pain: New insights into mechanisms and models. Headache 2010, 50, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Moote, C.A. The prevention of postoperative pain. Can. J. Anaesth. 1994, 41, 527. [Google Scholar] [CrossRef][Green Version]
- Gepts, E.; Camu, F.; Cockshott, I.D.; Douglas, E.J. Disposition of propofol administered as constant rate intravenous infusions in humans. Anesth. Analg. 1987, 66, 1256–1263. [Google Scholar] [CrossRef]
- Minto, C.F.; Schnider, T.W.; Egan, T.D.; Youngs, E.; Lemmens, H.B.J.; Gambus, P.L.; Billard, V.; Hoke, J.F.; Moore, K.H.; Hermann, D.J.; et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology 1997, 86, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Cho, E.J. A randomized, double-blind trial of palonosetron compared with ondansetron in preventing postoperative nausea and vomiting after gynaecological laparoscopic surgery. J. Int. Med. Res. 2011, 39, 399–407. [Google Scholar] [CrossRef]
- Kovac, A.L.; Eberhart, L.; Kotarski, J.; Clerici, G.; Apfel, C. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth. Analg. 2008, 107, 439–444. [Google Scholar] [CrossRef]
- Camu, F.; Van Aken, H.; Bovill, J.G. Postoperative analgesic effects of three demand-dose sizes of fentanyl administered by patient-controlled analgesia. Anesth. Analg. 1998, 87, 890–895. [Google Scholar] [PubMed]
- Shafer, S.L.; Varvel, J.R.; Aziz, N.; Scott, J.C. Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology 1990, 73, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.C.; Stanski, D.R. Decreased fentanyl and alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. J. Pharm. Exp. 1987, 240, 159–166. [Google Scholar] [CrossRef]
- Shafer, S.L.; Varvel, J.R. Pharmacokinetics, pharmacodynamics, and rational opioid selection. Anesthesiology 1991, 74, 53–63. [Google Scholar] [CrossRef]
- McNicol, E.D.; Ferguson, M.C.; Hudcova, J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst. Rev. 2015, CD003348. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.; Wheatley, B. Patient-controlled analgesia. BJA CEPD Reviews 2002, 2, 79–82. [Google Scholar] [CrossRef]
- Mather, L.E. Clinical Pharmacokinetics of Fentanyl and its Newer Derivatives. Clin. Pharmacokinet. 1983, 8, 422–446. [Google Scholar] [CrossRef]
- Hug, C.C.; Murphy, M.R. Tissue redistribution of fentanyl and termination of its effects in rats. Anesthesiology 1981, 55, 369–375. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, Y.; Lee, H.; Lim, J.A.; Park, S.; Kim, S.O. The evaluation of implementing smart patient controlled analgesic pump with a different infusion rate for different time duration on postoperative pain management. J. Dent. Anesth. Pain Med. 2016, 16, 289–294. [Google Scholar] [CrossRef]
- Cartwright, P.; Prys-Roberts, C.; Gill, K.; Dye, A.; Stafford, M.; Gray, A. Ventilatory depression related to plasma fentanyl concentrations during and after anesthesia in humans. Anesth. Analg. 1983, 62, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Hudcova, J.; McNicol, E.; Quah, C.; Lau, J.; Carr, D.B. Patient controlled intravenous opioid analgesia versus conventional opioid analgesia for postoperative pain control: A quantitative systematic review. Acute Pain 2005, 7, 115–132. [Google Scholar] [CrossRef]
| CRBI Group (n = 35) | TDBI Group (n = 35) | POBI Group (n = 35) | |
|---|---|---|---|
| Age (years) | 46.7 ± 4.0 | 47.9 ± 7.3 | 48.5 ± 9.4 |
| Weight (kg) | 60.2 ± 7.0 | 61.0 ± 8.9 | 60.0 ± 9.2 |
| Height (cm) | 159.5 ± 4.1 | 158.3 ± 5.6 | 157.7 ± 5.8 |
| Duration of surgery (min) | 98.0 ± 34.1 | 88.5 ± 35.0 | 85.1 ± 46.5 |
| Duration of anaesthesia (min) | 128.0 ± 34.3 | 118.3 ± 37.5 | 116.7 ± 53.7 |
| CRBI Group (n = 35) | TDBI Group (n = 35) | POBI Group (n = 35) | P-Value | |
|---|---|---|---|---|
| Insufficient analgesia | ||||
| At PACU | 33 (94.3) | 24 (68.6) a | 18 (51.4) b | <0.001 |
| At Ward | 14 (40.0) | 5 (14.3) | 1 (2.9) b | <0.001 |
| Total fentanyl dose (μg) | 722.5 (632.7–815.6) | 600.6 (508.8–671.6) a | 587.1 (490.2–667.7) b | 0.002 |
| Number of PCA demands | 4.0 (2.8–5.0) | 3.0 (1.0–6.0) | 1.0 (1.0–4.0) b | 0.009 |
| Number of Rescue analgesics | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (0.0–3.0) c | 0.027 |
| Cessation of PCA | 8 (22.9) | 5 (14.3) | 7 (20.0) | 0.649 |
| Side effects | ||||
| PONV | 23 (65.7) | 17 (48.6) | 15 (42.9) | 0.137 |
| Itching | 11 (31.4) | 0 (0.0) a | 1 (2.9) b | <0.001 |
| Headache | 10 (28.6) | 6 (17.1) | 4 (11.4) | 0.177 |
| Dizziness | 30 (85.7) | 21 (60) a | 18 (51.4) b | 0.007 |
| Patient satisfaction (0–10) | 8 (5–9) | 9 (7–10) | 9 (8–10) b | 0.013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Min, S.K.; Chae, Y.J.; Lim, G.M.; Joe, H.B. Continuous Fentanyl Background Infusion Regimen Optimised by Patient-Controlled Analgesia for Acute Postoperative Pain Management: A Randomised Controlled Trial. J. Clin. Med. 2020, 9, 211. https://doi.org/10.3390/jcm9010211
Hwang J, Min SK, Chae YJ, Lim GM, Joe HB. Continuous Fentanyl Background Infusion Regimen Optimised by Patient-Controlled Analgesia for Acute Postoperative Pain Management: A Randomised Controlled Trial. Journal of Clinical Medicine. 2020; 9(1):211. https://doi.org/10.3390/jcm9010211
Chicago/Turabian StyleHwang, Jihoon, Sang Kee Min, Yun Jeong Chae, Gang Mee Lim, and Han Bum Joe. 2020. "Continuous Fentanyl Background Infusion Regimen Optimised by Patient-Controlled Analgesia for Acute Postoperative Pain Management: A Randomised Controlled Trial" Journal of Clinical Medicine 9, no. 1: 211. https://doi.org/10.3390/jcm9010211
APA StyleHwang, J., Min, S. K., Chae, Y. J., Lim, G. M., & Joe, H. B. (2020). Continuous Fentanyl Background Infusion Regimen Optimised by Patient-Controlled Analgesia for Acute Postoperative Pain Management: A Randomised Controlled Trial. Journal of Clinical Medicine, 9(1), 211. https://doi.org/10.3390/jcm9010211





