Does Beta-Trace Protein (BTP) Outperform Cystatin C as a Diagnostic Marker of Acute Kidney Injury Complicating the Early Phase of Acute Pancreatitis?
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Patients
- -
- consecutive adult patients admitted to surgery department with symptoms of AP lasting no longer than 24 hours before admission were asked to join the study, and those who signed the informed consent were included in the study;
- -
- the diagnosis of AP was based on revised 2012 Atlanta classification [28], i.e., AP was diagnosed when two of three diagnostic criteria were met, i.e., characteristic abdominal pain, characteristic signs in abdominal imaging (magnetic resonance imaging, contrast-enhanced computed tomography or ultrasonography); serum amylase or lipase exceeding the upper reference limit more than three times;
- -
- patients with chronic pancreatitis, active cancer, or chronic liver diseases (viral hepatitis, liver cirrhosis) were excluded.
2.2. Laboratory Tests
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Orenes-Piñero, E.; Manzano-Fernández, S.; López-Cuenca, Á.; Marín, F.; Valdés, M.; Januzzi, J.L. β-Trace protein: From GFR marker to cardiovascular risk predictor. Clin. J. Am. Soc. Nephrol. 2013, 8, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Meco, C.; Oberascher, G.; Arrer, E.; Moser, G.; Albegger, K. β-trace protein test: New guidelines for the reliable diagnosis of cerebrospinal fluid fistula. Otolaryngol.-Head Neck. Surg. 2003, 129, 508–517. [Google Scholar] [CrossRef]
- Bachmann, G.; Petereit, H.; Djenabi, U.; Michel, O. Predictive Values of β-Trace Protein (Prostaglandin D Synthase) by Use of Laser-Nephelometry Assay for the Identification of Cerebrospinal Fluid. Neurosurgery 2002, 50, 571–577. [Google Scholar]
- Joo, M.; Sadikot, R.T. PGD Synthase and PGD 2 in Immune Response. Mediators Inflamm. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Donadio, C.; Lucchesi, A.; Ardini, M.; Donadio, E.; Giordani, R. Serum levels of beta-trace protein and glomerular filtration rate--preliminary results. J. Pharm. Biomed. Anal. 2003, 32, 1099–1104. [Google Scholar] [CrossRef]
- Donadio, C. Serum and urinary markers of early impairment of GFR in chronic kidney disease patients: Diagnostic accuracy of urinary β-trace protein. Am. J. Physiol. Renal Physiol. 2010, 299, F1407–F1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanaus, K.S.; Kollerits, B.; Ritz, E.; Hersberger, M.; Kronenberg, F.; Von Eckardstein, A. Serum creatinine, cystatin C, and β-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin. Chem. 2010, 56, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C.; Bozzoli, L. Urinary β-trace protein. A unique biomarker to screen early glomerular filtration rate impairment. Medicine 2016, 95, e5553. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.R.; Cavallari, M.R.; de Rozier-Alves, R.M.; Alves, B.D.C.A.; Fonseca, F.L.A. The impact of lipocalin-type-prostaglandin-D-synthase as a predictor of kidney disease in patients with type 2 diabetes. Drug Des. Devel. Ther. 2015, 9, 3179–3182. [Google Scholar] [PubMed] [Green Version]
- Manzano-Fernández, S.; López-Cuenca, Á.; Januzzi, J.L.; Parra-Pallares, S.; Mateo-Martínez, A.; Sánchez-Martínez, M.; Pérez-Berbel, P.; Orenes-Piñero, E.; Romero-Aniorte, A.I.; Avilés-Plaza, F.; et al. Usefulness of β-trace protein and cystatin C for the prediction of mortality in non ST segment elevation acute coronary syndromes. Am. J. Cardiol. 2012, 110, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Fernández, S.; Januzzi, J.L.; Boronat-Garcia, M.; Bonaque-González, J.C.; Truong, Q.A.; Pastor-Pérez, F.J.; Muñoz-Esparza, C.; Pastor, P.; Albaladejo-Otón, M.D.; Casas, T.; et al. β-trace protein and cystatin C as predictors of long-term outcomes in patients with acute heart failure. J. Am. Coll. Cardiol. 2011, 57, 849–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012, 143, 1179–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Párniczky, A.; Kui, B.; Szentesi, A.; Balázs, A.; Szűcs, Á.; Mosztbacher, D.; Czimmer, J.; Sarlós, P.; Bajor, J.; Gódi, S.; et al. Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS ONE 2016, 11, e0165309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. Acute Kidney Injury Advisory Group of the American Society of Nephrology World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Druml, W.; Lenz, K.; Laggner, A.N. Our paper 20 years later: From acute renal failure to acute kidney injury—The metamorphosis of a syndrome. Intensive Care Med. 2015, 41, 1941–1949. [Google Scholar] [CrossRef]
- Ostermann, M.; Liu, K. Pathophysiology of AKI. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 305–314. [Google Scholar] [CrossRef]
- Wajda, J.; Dumnicka, P.; Maraj, M.; Ceranowicz, P.; Kuźniewski, M.; Kuśnierz-Cabala, B. Potential prognostic markers of acute kidney injury in the early phase of acute pancreatitis. Int. J. Mol. Sci. 2019, 20, 3714. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-Y.; Lai, J.-I.; Lai, Y.-C.; Lin, P.-C.; Chang, S.-C.; Tang, G.-J. Acute renal failure in severe pancreatitis: A population-based study. Ups. J. Med. Sci. 2011, 116, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Nassar, T.I.; Qunibi, W.Y. AKI Associated with Acute Pancreatitis. Clin. J. Am. Soc. Nephrol. 2019, 14, 106–1115. [Google Scholar] [CrossRef] [PubMed]
- Beker, B.M.; Corleto, M.G.; Fieiras, C.; Musso, C.G. Novel acute kidney injury biomarkers: Their characteristics, utility and concerns. Int. Urol. Nephrol. 2018, 50, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury—Pathophysiological basis and clinical performance. Acta Physiol. 2017, 219, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mase, M.; Inui, T.; Shimoda, M.; Isomura, K.; Oda, H.; Yamada, K.; Urade, Y. Pharmacokinetics of recombinant human lipocalin-type prostaglandin D synthase/β-trace in canine. Neurosci. Res. 2008, 61, 289–293. [Google Scholar] [CrossRef]
- Chen, H.H. β-trace protein versus cystatin C: which is a better surrogate marker of renal function versus prognostic indicator in cardiovascular diseases? J. Am. Coll. Cardiol. 2011, 57, 859–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sporek, M.; Dumnicka, P.; Gala-Błądzińska, A.; Ceranowicz, P.; Warzecha, Z.; Dembiński, A.; Stępień, E.; Walocha, J.; Drożdż, R.; Kuźniewski, M.; et al. Angiopoietin-2 is an early indicator of acute pancreatic-renal syndrome in patients with acute pancreatitis. Mediators Inflamm. 2016, 5780903, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolber, W.; Kuśnierz-Cabala, B.; Dumnicka, P.; Maraj, M.; Mazur-Laskowska, M.; Pędziwiatr, M.; Ceranowicz, P. Serum Urokinase-Type Plasminogen Activator Receptor Does Not Outperform C-Reactive Protein and Procalcitonin as an Early Marker of Severity of Acute Pancreatitis. J. Clin. Med. 2018, 7, 305. [Google Scholar] [CrossRef] [Green Version]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Tabak, Y.; Conwell, D.L.; Banks, P. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008, 57, 1698–1703. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Dumnicka, P.; Kuśnierz-Cabala, B.; Sporek, M.; Mazur-Laskowska, M.; Gil, K.; Kuźniewski, M.; Ceranowicz, P.; Warzecha, Z.; Dembiński, A.; Bonior, J.; et al. Serum concentrations of angiopoietin-2 and soluble fms-like tyrosine kinase 1 (sFlt-1) are associated with coagulopathy among patients with acute pancreatitis. Int. J. Mol. Sci. 2017, 18, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, J.A.; Hajian-Tilaki, K.O. Sampling variability of nonparametric estimates of the areas under receiver operating characteristic curves: An update. Acad. Radiol. 1997, 4, 49–58. [Google Scholar] [CrossRef]
- Chai, X.; Huang, H.-B.; Feng, G.; Cao, Y.-H.; Cheng, Q.-S.; Li, S.-H.; He, C.-Y.; Lu, W.-H.; Qin, M.-M. Baseline Serum Cystatin C Is a Potential Predictor for Acute Kidney Injury in Patients with Acute Pancreatitis. Dis. Markers 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.K.; Wong, H.R.; Krawczeski, C.D.; Wheeler, D.S.; Manning, P.B.; Chawla, L.S.; Devarajan, P.; Goldstein, S.L. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J. Am. Coll. Cardiol. 2014, 64, 2753–2762. [Google Scholar] [CrossRef] [Green Version]
- Juraschek, S.P.; Coresh, J.; Inker, L.A.; Levey, A.S.; Köttgen, A.; Foster, M.C.; Astor, B.C.; Eckfeldt, J.H.; Selvin, E. Comparison of serum concentrations of β-trace protein, β2-microglobulin, cystatin C, and creatinine in the US population. Clin. J. Am. Soc. Nephrol. 2013, 8, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Foster, M.C.; Tighiouart, H.; Anderson, A.H.; Beck, G.J.; Contreras, G.; Coresh, J.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; et al. Non-GFR Determinants of Low-Molecular-Weight Serum Protein Filtration Markers in CKD. Am. J. Kidney Dis. 2016, 68, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, D.; Akbari, A.; Knoll, G.A.; Flemming, J.A.; Lowe, C.; Akbari, S.; White, C.A. Serum BTP concentrations are not affected by hepatic dysfunction. BMC Nephrol. 2018, 19, 87. [Google Scholar] [CrossRef] [Green Version]
- Werner, K.; Pihlsgård, M.; Elmståhl, S.; Legrand, H.; Nyman, U.; Christensson, A. Combining Cystatin C and Creatinine Yields a Reliable Glomerular Filtration Rate Estimation in Older Adults in Contrast to β-Trace Protein and β2-Microglobulin. Nephron 2017, 137, 29–37. [Google Scholar] [CrossRef]
- Saydam, O.; Türkmen, E.; Portakal, O.; Arici, M.; Doğan, R.; Demircin, M.; Paşaoğlu, İ.; Yilmaz, M. Emerging biomarker for predicting acute kidney injury after cardiac surgery: Cystatin C. Turkish J. Med. Sci. 2018, 48, 1096–1103. [Google Scholar] [CrossRef]
- Dai, X.; Zeng, Z.; Fu, C.; Zhang, S.; Cai, Y.; Chen, Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit. Care 2015, 19, 223. [Google Scholar] [CrossRef] [Green Version]
- Leem, A.Y.; Park, M.S.; Park, B.H.; Jung, W.J.; Chung, K.S.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Kim, Y.S.; et al. Value of Serum Cystatin C Measurement in the Diagnosis of Sepsis-Induced Kidney Injury and Prediction of Renal Function Recovery. Yonsei Med. J. 2017, 58, 604. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, P.K.; Kochhar, R.; Sarotra, P.; Medhi, B.; Jha, V.; Gupta, V. Neutrophil gelatinase-associated lipocalin: An early biomarker for predicting acute kidney injury and severity in patients with acute pancreatitis. JGH Open 2018, 3, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ebert, N.; Delanaye, P.; Shlipak, M.; Jakob, O.; Martus, P.; Bartel, J.; Gaedeke, J.; van der Giet, M.; Schuchardt, M.; Cavalier, E.; et al. Cystatin C standardization decreases assay variation and improves assessment of glomerular filtration rate. Clin. Chim. Acta. 2016, 456, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydoğdu, M.; Gürsel, G.; Sancak, B.; Yeni, S.; Sari, G.; Taşyürek, S.; Türk, M.; Yüksel, S.; Senes, M.; Özis, T.N. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and Cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis. Markers 2013, 34, 237–246. [Google Scholar] [CrossRef]
- Buddingh, K.T.; Koudstaal, L.G.; van Santvoort, H.C.; Besselink, M.G.; Timmer, R.; Rosman, C.; van Goor, H.; Nijmeijer, R.M.; Gooszen, H.; Leuvenink, H.G.D.; et al. Early angiopoietin-2 levels after onset predict the advent of severe pancreatitis, multiple organ failure, and infectious complications in patients with acute pancreatitis. J. Am. Coll. Surg. 2014, 218, 26–32. [Google Scholar] [CrossRef]
- Dumnicka, P.; Sporek, M.; Mazur-Laskowska, M.; Ceranowicz, P.; Kuźniewski, M.; Drożdż, R.; Ambroży, T.; Olszanecki, R.; Kuśnierz-Cabala, B. Serum soluble fms-Like tyrosine kinase 1 (sFlt-1) predicts the severity of acute pancreatitis. Int. J. Mol. Sci. 2016, 17, 2038. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.L.; Lee, K.T.; Chang, C.H.; Chen, Y.C.; Lin, S.M.; Chu, P.H. Elevated plasma thrombomodulin and angiopoietin-2 predict the development of acute kidney injury in patients with acute myocardial infarction. Crit. Care 2014, 18, R100. [Google Scholar] [CrossRef] [Green Version]
- Jongman, R.M.; Van Klarenbosch, J.; Molema, G.; Zijlstra, J.G.; De Vries, A.J.; Van Meurs, M.; Long, D. Angiopoietin/Tie2 dysbalance is associated with acute kidney injury after cardiac surgery assisted by cardiopulmonary bypass. PLoS ONE 2015, 10, e0136205. [Google Scholar] [CrossRef] [Green Version]
- Araújo, C.B.; de Oliveira Neves, F.M.; de Freitas, D.F.; Arruda, B.F.T.; de Macêdo Filho, L.J.M.; Salles, V.B.; Meneses, G.C.; Martins, A.M.C.; Libório, A.B. Angiopoietin-2 as a predictor of acute kidney injury in critically ill patients and association with ARDS. Respirology 2019, 24, 345–351. [Google Scholar] [CrossRef]
- Robinson-Cohen, C.; Katz, R.; Price, B.L.; Harju-Baker, S.; Mikacenic, C.; Himmelfarb, J.; Liles, W.C.; Wurfel, M.M. Association of markers of endothelial dysregulation Ang1 and Ang2 with acute kidney injury in critically ill patients. Crit. Care 2016, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dumnicka, P.; Maduzia, D.; Ceranowicz, P.; Olszanecki, R.; Drożdż, R.; Kuśnierz-Cabala, B. The interplay between inflammation, coagulation and endothelial injury in the early phase of acute pancreatitis: Clinical implications. Int. J. Mol. Sci. 2017, 18, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.L.; Ricciotti, E.; Liang, X.; Grosser, T.; Grant, G.R.; FitzGerald, G.A. Lipocalin-like prostaglandin D synthase but not hemopoietic prostaglandin D synthase deletion causes hypertension and accelerates thrombogenesis in mice. J. Pharmacol. Exp. Ther. 2018, 367, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Coresh, J.; Inker, L.A.; Rynders, G.P.; Eckfeldt, J.H.; Selvin, E. The effects of freeze–thaw on β-trace protein and β2-microglobulin assays after long-term sample storage. Clin. Biochem. 2012, 45, 694–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
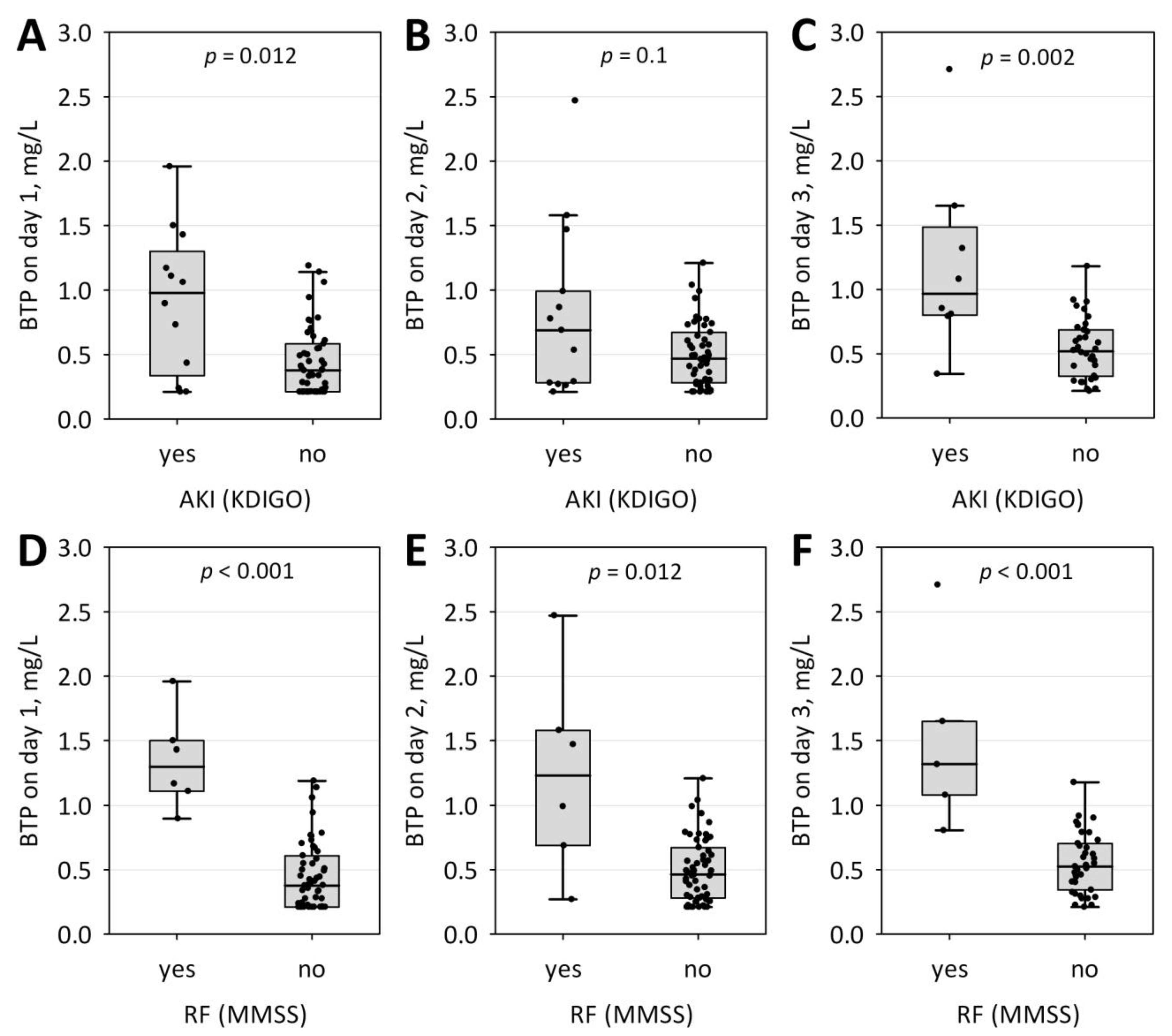
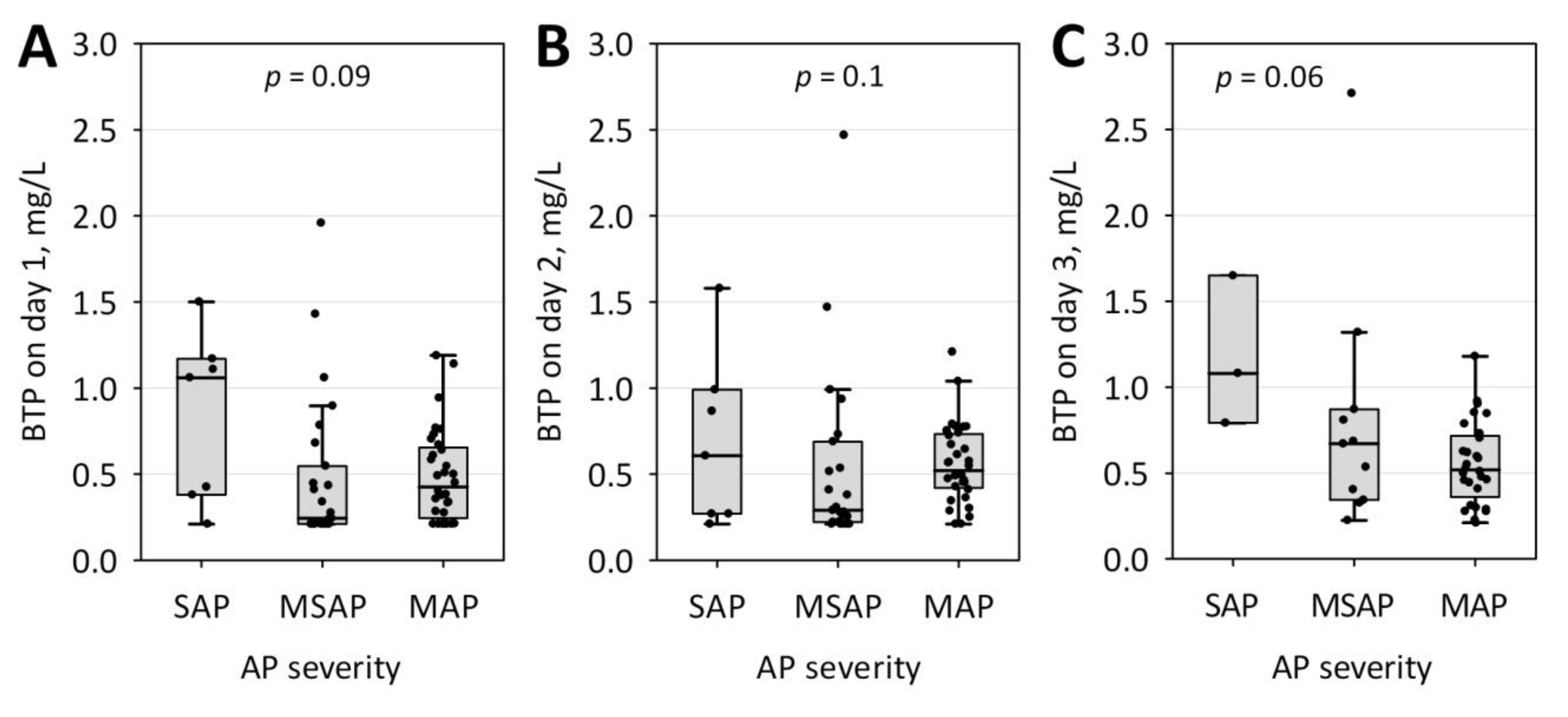
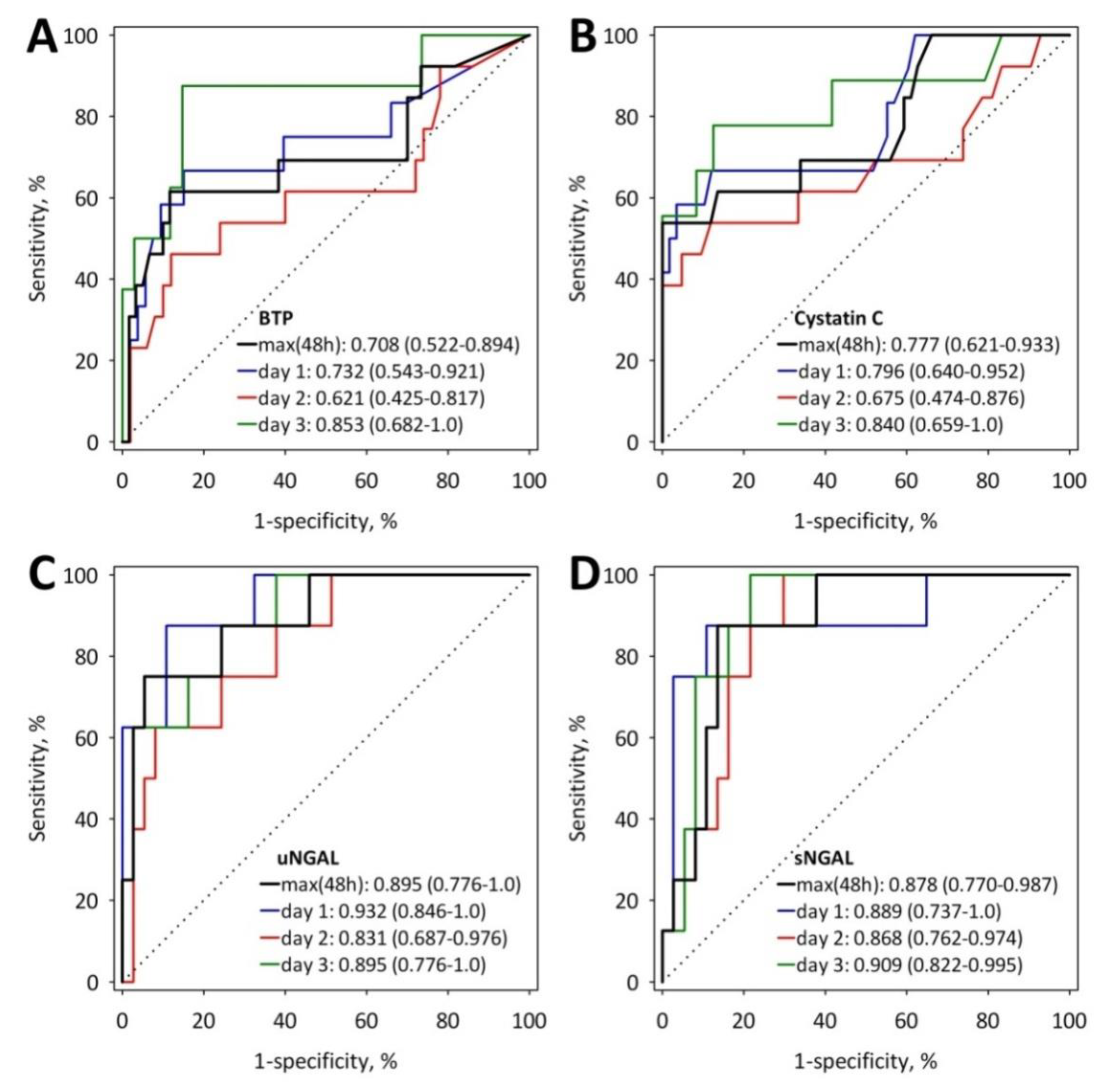
| Characteristic | AKI (n = 13) | No AKI (n = 60) | p |
|---|---|---|---|
| Age, years | 75 (67; 81) | 56 (40; 72) | 0.003 |
| Male sex, n (%) | 7 (54) | 29 (48) | 0.7 |
| Preexisting comorbidities, n (%) | 11 (85) | 38 (63) | 0.1 |
| Ischemic heart disease, n (%) | 6 (46) | 16 (27) | 0.2 |
| Diabetes, n (%) | 3 (23) | 6 (10) | 0.2 |
| Pulmonary diseases, n (%) | 1 (8) | 5 (8) | 0.9 |
| Renal diseases, n (%) | 3 (23) | 1 (2) | 0.002 |
| BMI >30 kg/m2, n (%) | 0 | 9 (15) | 0.2 |
| AP etiology | |||
| Billiary, n (%) | 9 (69) | 28 (47) | 0.3 |
| Alcohol, n (%) | 1 (8) | 14 (23) | |
| Hyperlipemia, n (%) | 0 | 6 (10) | |
| Other or idiopathic, n (%) | 3 (23) | 12 (20) | |
| Pancreatic necrosis, n (%) | 1 (8) | 6 (10) | 0.8 |
| Pleural effusion, n (%) | 8 (62) | 27 (45) | 0.3 |
| SIRS, n (%) | 8 (62) | 23 (38) | 0.1 |
| BISAP score at 24 h, points | 3 (2; 3) | 1 (0; 2) | 0.002 |
| BISAP ≥3 points, n (%) | 8 (62) | 7 (12) | <0.001 |
| Organ failure according to MMSS | |||
| Transient, n (%) | 8 (62) | 15 (25) | 0.010 |
| Persistent, n (%) | 4 (31) | 3 (5) | 0.004 |
| AP severity | |||
| MAP, n (%) | 1 (8) | 37 (62) | <0.001 |
| MSAP, n (%) | 8 (62) | 20 (33) | |
| SAP, n (%) | 4 (31) | 3 (5) | |
| Surgery, n (%) | 0 | 5 (8) | 0.3 |
| Parenteral nutrition, n (%) | 2 (15) | 3 (5) | 0.2 |
| Length of hospital stay, days | 11 (8; 25) | 7 (5; 11) | 0.012 |
| Mortality, n (%) | 3 (23) | 1 (2) | 0.002 |
| Amylase, U/L | 829 (619; 1526) | 1027 (537; 1897) | 0.5 |
| Hematocrit, % | 37.4 (33.5; 45.2) | 43.4 (41.0; 46.9) | 0.011 |
| Leukocyte count, ×103/µL | 14.8 (12.3; 22.9) | 12.1 (9.9; 16.2) | 0.1 |
| Neutrophil count, ×103/µL | 11.4 (8.6; 19.6) | 9.4 (7.5; 12.9) | 0.2 |
| CRP, mg/L | 258 (182; 313) | 104 (49; 229) | 0.018 |
| Glucose, mmol/L | 8.93 (8.33; 12.25) | 7.78 (6.56; 10.11) | 0.06 |
| Bilirubin, µmol/L | 48.7 (36.4; 81.0) | 35.5 (17.8; 65.1) | 0.07 |
| Urea, mmol/L | 11.68 (6.72; 15.80) | 5.83 (4.21; 6.57) | <0.001 |
| Creatinine, µmol/L | 120 (95; 207) | 71 (61; 85) | <0.001 |
| Cystatin C, mg/L | 2.05 (0.84; 2.70) | 0.86 (0.69; 1.13) | 0.002 |
| BTP, mg/L | 0.897 (0.291; 1.470) | 0.459 (0.254; 0.631) | 0.019 |
| Serum NGAL, ng/mL | 313 (275; 489) | 142 (101; 232) | <0.001 |
| Urine NGAL, ng/mL | 837 (551; 1252) | 38 (20; 68) | 0.002 |
| Uromodulin, ng/mL | 105 (90; 152) | 146 (95; 205) | 0.2 |
| D-dimer, µg/mL | 6.32 (3.82; 15.69) | 2.90 (1.33; 4.20) | 0.003 |
| Angiopoietin 2, ng/mL | 14.48 (5.77; 23.69) | 3.25 (2.37; 5.33) | <0.001 |
| sFlt-1, pg/mL | 215 (192; 250) | 140 (114; 173) | <0.001 |
| Variable | Serum BTP Concentrations | |||
|---|---|---|---|---|
| Maximum of Day 1 and 2* (n = 73) | Day 1 (n = 65) | Day 2 (n = 63) | Day 3 (n = 45) | |
| Urea | R = 0.52; p < 0.001 | R = 0.50; p < 0.001 | R = 0.41; p < 0.001 | R = 0.73; p < 0.001 |
| Creatinine | R = 0.56; p < 0.001 | R = 0.63; p < 0.001 | R = 0.52; p < 0.001 | R = 0.71; p < 0.001 |
| Cystatin C | R = 0.60; p < 0.001 | R = 0.68; p < 0.001 | R = 0.63; p < 0.001 | R = 0.91; p < 0.001 |
| Serum NGAL | R = 0.17; p = 0.3 | R = 0.43; p = 0.007 | R = 0.22; p = 0.2 | R = 0.35; p = 0.023 |
| Urine NGAL | R = 0.23; p = 0.3 | R = 0.17; p = 0.38 | R = 0.30; p = 0.1 | R = 0.37; p = 0.07 |
| Uromodulin | R = −0.42; p = 0.004 | R = −0.43; p = 0.007 | R = −0.44; p = 0.003 | R = −0.33; p = 0.037 |
| D-dimer | R = 0.05; p = 0.7 | R = 0.31; p = 0.012 | R = −0.07; p = 0.6 | R = −0.04; p = 0.8 |
| Angiopoietin-2 | R = 0.26; p = 0.045 | R = 0.37; p = 0.007 | R = 0.11; p = 0.4 | R = 0.22; p = 0.2 |
| sFlt-1 | R = 0.25; p = 0.044 | R = 0.34; p = 0.011 | R = −0.03; p = 0.8 | no data |
| Leukocytes | R = 0.11; p = 0.4 | R = 0.07; p = 0.6 | R = −0.02; p = 0.9 | R = 0.05; p = 0.8 |
| Neutrophils | R = 0.10; p = 0.4 | R = −0.01; p = 0.9 | R = −0.03; p = 0.8 | R = 0.09; p = 0.6 |
| CRP | R = −0.10; p = 0.4 | R = 0.15; p = 0.2 | R = −0.19; p = 0.1 | R = −0.08; p = 0.6 |
| BTP, per 1 mg/L | Cystatin C, per 1 mg/L | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Maximum of day 1 and 2 | 1.29 (0.72–2.33) | 0.4 | 2.10 (30.1–698) | 0.002 |
| Day 1 | 1.31 (0.72–2.37) | 0.4 | 14.0 (2.34–83.6) | 0.003 |
| Day 2 | 1.15 (0.53–2.50) | 0.7 | 5.12 (1.24–21.2) | 0.021 |
| Day 3 | 125 (2.7–5796) | 0.001 | 33.2 (2.45–451) | 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajda, J.; Dumnicka, P.; Sporek, M.; Maziarz, B.; Kolber, W.; Ząbek-Adamska, A.; Ceranowicz, P.; Kuźniewski, M.; Kuśnierz-Cabala, B. Does Beta-Trace Protein (BTP) Outperform Cystatin C as a Diagnostic Marker of Acute Kidney Injury Complicating the Early Phase of Acute Pancreatitis? J. Clin. Med. 2020, 9, 205. https://doi.org/10.3390/jcm9010205
Wajda J, Dumnicka P, Sporek M, Maziarz B, Kolber W, Ząbek-Adamska A, Ceranowicz P, Kuźniewski M, Kuśnierz-Cabala B. Does Beta-Trace Protein (BTP) Outperform Cystatin C as a Diagnostic Marker of Acute Kidney Injury Complicating the Early Phase of Acute Pancreatitis? Journal of Clinical Medicine. 2020; 9(1):205. https://doi.org/10.3390/jcm9010205
Chicago/Turabian StyleWajda, Justyna, Paulina Dumnicka, Mateusz Sporek, Barbara Maziarz, Witold Kolber, Anna Ząbek-Adamska, Piotr Ceranowicz, Marek Kuźniewski, and Beata Kuśnierz-Cabala. 2020. "Does Beta-Trace Protein (BTP) Outperform Cystatin C as a Diagnostic Marker of Acute Kidney Injury Complicating the Early Phase of Acute Pancreatitis?" Journal of Clinical Medicine 9, no. 1: 205. https://doi.org/10.3390/jcm9010205
APA StyleWajda, J., Dumnicka, P., Sporek, M., Maziarz, B., Kolber, W., Ząbek-Adamska, A., Ceranowicz, P., Kuźniewski, M., & Kuśnierz-Cabala, B. (2020). Does Beta-Trace Protein (BTP) Outperform Cystatin C as a Diagnostic Marker of Acute Kidney Injury Complicating the Early Phase of Acute Pancreatitis? Journal of Clinical Medicine, 9(1), 205. https://doi.org/10.3390/jcm9010205








