Evolution of Airway Inflammation in Preschoolers with Asthma—Results of a Two-Year Longitudinal Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Study Design
2.3. FeNO Measurements
2.4. Predictors of FeNO Measurements
2.5. Statistical Analysis
3. Results
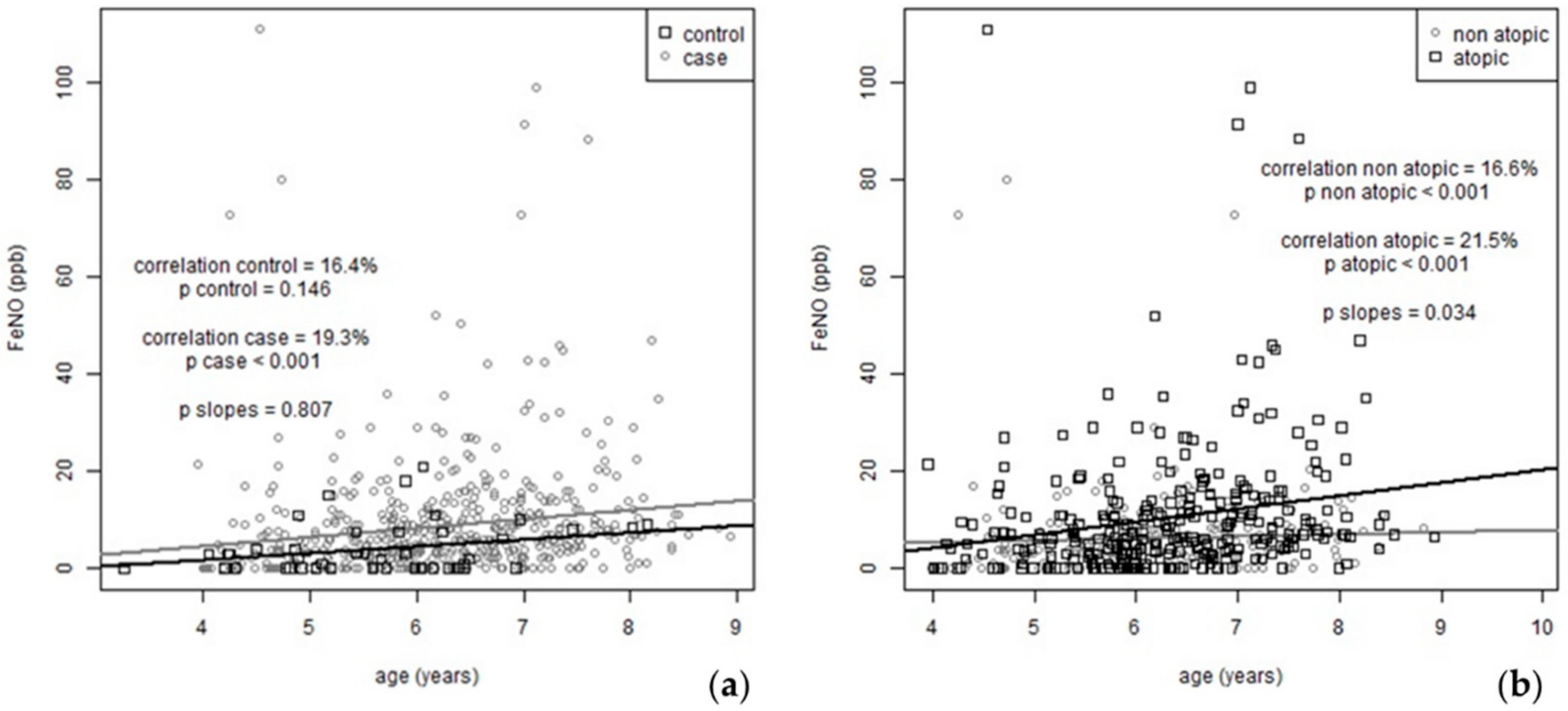
3.1. FeNO Evolution during the Two-Year Follow-Up
3.2. FeNO and Seasonality
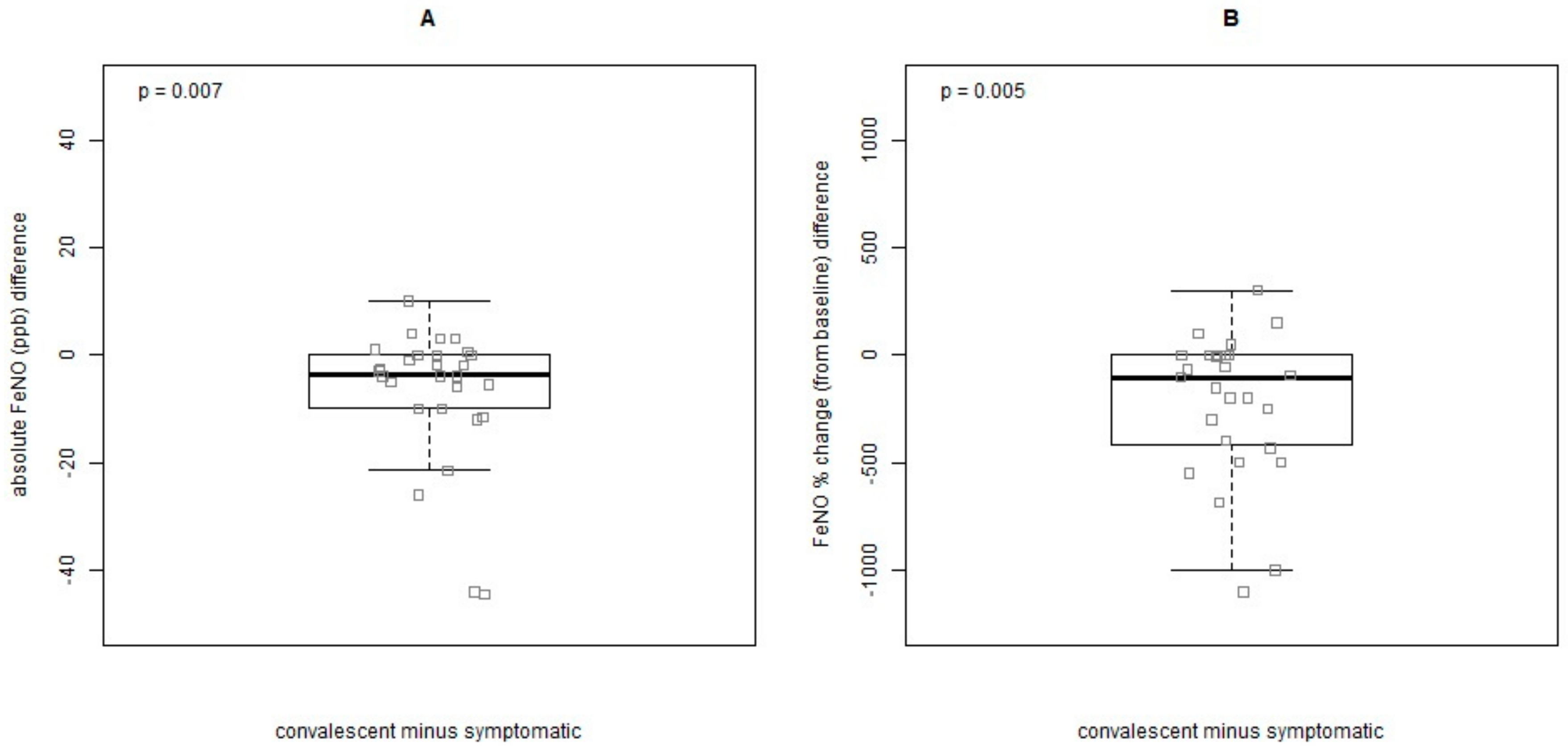
3.3. Variation in FeNO Values from Healthy State (Asymptomatic) to Exacerbation and Convalescent Visit
3.4. Predictors of FeNO Measurements at Baseline and during the Two-Year Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Debley, J.S.; Stamey, D.C.; Cochrane, E.S.; Gama, K.L.; Redding, G.J. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J. Allergy Clin. Immunol. 2010, 125, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, G.N.; Xepapadaki, P.; Manousakis, E.; Makrinioti, H.; Kouloufakou-Gratsia, K.; Saxoni-Papageorgiou, P.; Papadopoulos, N.G. Assessment of airflow limitation, airway inflammation, and symptoms during virus-induced wheezing episodes in 4 to 6-year-old children. J. Allergy Clin. Immunol. 2013, 131, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.; Carlsen, K.H.; Sly, P.D.; Baraldi, E.; Piacentini, G.; Pavord, I.; Lex, C.; Saglani, S. Monitoring asthma in childhood: Lung function, bronchial responsiveness and inflammation. Eur. Respir. Rev. 2015, 24, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Shim, J.Y.; Kwon, J.W.; Kim, H.Y.; Seo, J.H.; Kim, B.J.; Kim, H.B.; Lee, S.Y.; Jang, G.C.; Song, D.J.; et al. Exhaled nitric oxide as a better diagnostic indicator for evaluating wheeze and airway hyperresponsiveness in preschool children. J. Asthma 2015, 52, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.E.; Kim, K.M.; Kwon, J.W.; Kim, H.Y.; Seo, J.H.; Kim, H.B.; Lee, S.Y.; Jang, G.C.; Song, D.J.; Kim, W.K.; et al. Recurrent wheeze and its relationship with lung function and airway inflammation in preschool children: A cross-sectional study in South Korea. BMJ Open 2017, 7, e018010. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, F.C.; da Silva Júnior, J.L.R.; Rabahi, M.F. The Fraction Exhaled Nitric Oxide as a Biomarker of Asthma Control. Biomark. Insights 2019, 14, 1177271919826550. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Sardón, O.; Pérez-Yarza, E.G.; Korta, J.; Aldasoro, A.; Corcuera, P.; Mintegui, J. Young infants with recurrent wheezing and positive asthma predictive index have higher levels of exhaled nitric oxide. J. Asthma 2013, 50, 162–165. [Google Scholar] [CrossRef]
- Vilmann, L.; Buchvald, F.; Green, K.; Nielsen, K.G. Fractional exhaled nitric oxide and multiple breath nitrogen washout in preschool healthy and asthmatic children. Respir. Med. 2017, 133, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Baptist, A.P.; Li, L.; Dichiaro, C.A. The importance of atopy on exhaled nitric oxide levels in African American children. Ann. Allergy Asthma Immunol. 2015, 114, 399–403. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Bachert, C.; Finotto, S.; Jartti, T.; Konstantinou, G.N.; Kiefer, A.; Kowalski, M.; Lewandowska-Polak, A.; Lukkarinen, H.; Roumpedaki, E.; et al. Contribution of repeated infections in asthma persistence from preschool to school age: Design and characteristics of the PreDicta cohort. Pediatric Allergy Immunol. 2018, 29, 383–393. [Google Scholar] [CrossRef]
- GGIfARP. Available online: https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf (accessed on 22 June 2019).
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Boner, A.; Carlsen, K.H.; Eigenmann, P.A.; Frischer, T.; Gotz, M.; Helms, P.J.; Hunt, J.; Liu, A.; Papadopoulos, N.; et al. Diagnosis and treatment of asthma in childhood: A PRACTALL consensus report. Allergy 2008, 63, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Buchvald, F.; Baraldi, E.; Carraro, S.; Gaston, B.; De Jongste, J.; Pijnenburg, M.W.; Silkoff, P.E.; Bisgaard, H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J. Allergy Clin. Immunol. 2005, 115, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Dario, C.; Ongaro, R.; Scollo, M.; Azzolin, N.M.; Panza, N.; Paganini, N.; Zacchello, F. Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am. J. Respir. Crit. Care Med. 1999, 159, 1284–1288. [Google Scholar] [CrossRef]
- Scott, M.; Raza, A.; Karmaus, W.; Mitchell, F.; Grundy, J.; Kurukulaaratchy, R.J.; Arshad, S.H.; Roberts, G. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax 2010, 65, 258–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoyte, F.C.L.; Gross, L.M.; Katial, R.K. Exhaled Nitric Oxide: An Update. Immunol. Allergy Clin. North Am. 2018, 38, 573–585. [Google Scholar] [CrossRef] [PubMed]
- De Gouw, H.W.; Grünberg, K.; Schot, R.; Kroes, A.C.; Dick, E.C.; Sterk, P.J. Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur. Respir. J. 1998, 11, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, O.; Abdelateef, H.; Pozo Beltrán, C.F.; El-Sayed, Z.A.; Gomez, R.M.; Hossny, E.; Morais-Almeida, M.; Nieto, A.; Phipatanakul, W.; Pitrez, P.; et al. Challenges and choices in the pharmacological treatment of non-severe pediatric asthma: A commentary for the practicing physician. World Allergy Organ. J. 2019, 12, 100054. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.A.; Barnes, P.J. Effects of corticosteroids on noninvasive biomarkers of inflammation in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2004, 1, 191–199. [Google Scholar] [CrossRef]
- Yune, S.; Lee, J.Y.; Choi, D.C.; Lee, B.J. Fractional exhaled nitric oxide: Comparison between portable devices and correlation with sputum eosinophils. Allergy Asthma Immunol. Res. 2015, 7, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.; Maidstone, R.; Loudon, A.; Blaikley, J.; White, I.R.; Singh, D.; Ray, D.W.; Goodacre, R.; Fowler, S.J.; Durrington, H.J. Circadian rhythm of exhaled biomarkers in health and asthma. Eur. Respir. J. 2019, 54, 1901068. [Google Scholar] [CrossRef]
- Harnan, S.E.; Tappenden, P.; Essat, M.; Gomersall, T.; Minton, J.; Wong, R.; Pavord, I.; Everard, M.; Lawson, R. Measurement of exhaled nitric oxide concentration in asthma: A systematic review and economic evaluation of NIOX MINO, NIOX VERO and NObreath. Health Technol. Assess. 2015, 19, 1–330. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Carraro, S.; Bozzetto, S.; Zanconato, S.; Baraldi, E. Exhaled biomarkers in childhood asthma: Old and new approaches. Asthma Res. Pract. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Paraskakis, E.; Brindicci, C.; Fleming, L.; Krol, R.; Kharitonov, S.A.; Wilson, N.M.; Barnes, P.J.; Bush, A. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Cameli, P.; Bargagli, E.; Bergantini, L.; Refini, R.M.; Pieroni, M.; Sestini, P.; Rottoli, P. Evaluation of multiple-flows exhaled nitric oxide in idiopathic and non-idiopathic interstitial lung disease. J. Breath Res. 2019, 13, 026008. [Google Scholar] [CrossRef]
- Chinellato, I.; Piazza, M.; Peroni, D.; Sandri, M.; Chiorazzo, F.; Boner, A.L.; Piacentini, G. Bronchial and alveolar nitric oxide in exercise-induced bronchoconstriction in asthmatic children. Clin. Exp. Allergy 2012, 42, 1190–1196. [Google Scholar] [CrossRef]

| Atopic History Disease Activity | Baseline Characteristics | n |
|---|---|---|
| Atopic history | Family history of atopic disease (yes), n (%) | 94 (91) |
| Family history of asthma/rhinitis (yes), n (%) | 89 (86) | |
| Any positive skin prick test (yes), n (%) | 70 (56) | |
| Upper Respiratory Symptoms | Rhinitis symptoms are present | |
| <4 days/week, n (%) | 61 (47) | |
| Rhinitis duration | ||
| <4 consecutive weeks, n (%) | 67 (51) | |
| Are rhinitis symptoms associated with…? | ||
| Sleep disturbance (yes), n (%) | 65 (89) | |
| School impairment (yes), n (%) | 17 (24) | |
| Leisure—sport (yes), n (%) | 35 (49) | |
| Visual analogue scale, median (CIs) | 5 (3–6) | |
| Number of medication courses for rhinitis in the last 12 months, median (CIs) | 1 (0–5) | |
| Lower Respiratory Symptoms | Days with symptoms in the last 3 months | |
| <1/week, n (%) | 83 (64) | |
| >1/week but <1/day, n (%) | 37 (28) | |
| daily, n (%) | 11 (8) | |
| Nights with symptoms in the last 3 months | ||
| ≤2 times/month, n (%) | 81 (62) | |
| >2 times/month, n (%) | 15 (12) | |
| >1/week, n (%) | 24 (18) | |
| daily, n (%) | 11 (8) | |
| Cough, wheeze or difficulty in breathing during or after exercise in the last 12 months (yes), n (%) | 84 (64) | |
| Limitation of activities limited by asthma symptoms (yes), n (%) | 46 (35) | |
| Child completely well between symptomatic periods (yes), n (%) | 92 (70) | |
| Number of episodes of wheezing/asthma/cough in the last 3 months, median (25–75 percentiles) | 1 (1–2) | |
| Number of episodes of wheezing/asthma/cough in the last 12 months, median (CIs) | 5 (3–8) | |
| Inhaled corticosteroids as prophylactic treatment (yes), n (%) | 107 (82%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xepapadaki, P.; Korovessi, P.; Bachert, C.; Finotto, S.; Jartti, T.; Lakoumentas, J.; Kowalski, M.L.; Lewandowska-Polak, A.; Lukkarinen, H.; Zhang, N.; et al. Evolution of Airway Inflammation in Preschoolers with Asthma—Results of a Two-Year Longitudinal Study. J. Clin. Med. 2020, 9, 187. https://doi.org/10.3390/jcm9010187
Xepapadaki P, Korovessi P, Bachert C, Finotto S, Jartti T, Lakoumentas J, Kowalski ML, Lewandowska-Polak A, Lukkarinen H, Zhang N, et al. Evolution of Airway Inflammation in Preschoolers with Asthma—Results of a Two-Year Longitudinal Study. Journal of Clinical Medicine. 2020; 9(1):187. https://doi.org/10.3390/jcm9010187
Chicago/Turabian StyleXepapadaki, Paraskevi, Paraskevi Korovessi, Claus Bachert, Susetta Finotto, Tuomas Jartti, John Lakoumentas, Marek L. Kowalski, Anna Lewandowska-Polak, Heikki Lukkarinen, Nan Zhang, and et al. 2020. "Evolution of Airway Inflammation in Preschoolers with Asthma—Results of a Two-Year Longitudinal Study" Journal of Clinical Medicine 9, no. 1: 187. https://doi.org/10.3390/jcm9010187
APA StyleXepapadaki, P., Korovessi, P., Bachert, C., Finotto, S., Jartti, T., Lakoumentas, J., Kowalski, M. L., Lewandowska-Polak, A., Lukkarinen, H., Zhang, N., Zimmermann, T., & Papadopoulos, N. G. (2020). Evolution of Airway Inflammation in Preschoolers with Asthma—Results of a Two-Year Longitudinal Study. Journal of Clinical Medicine, 9(1), 187. https://doi.org/10.3390/jcm9010187







