Continuous Positive Airway Pressure Treatment in Patients with Alzheimer’s Disease: A Systematic Review
Abstract
1. Introduction
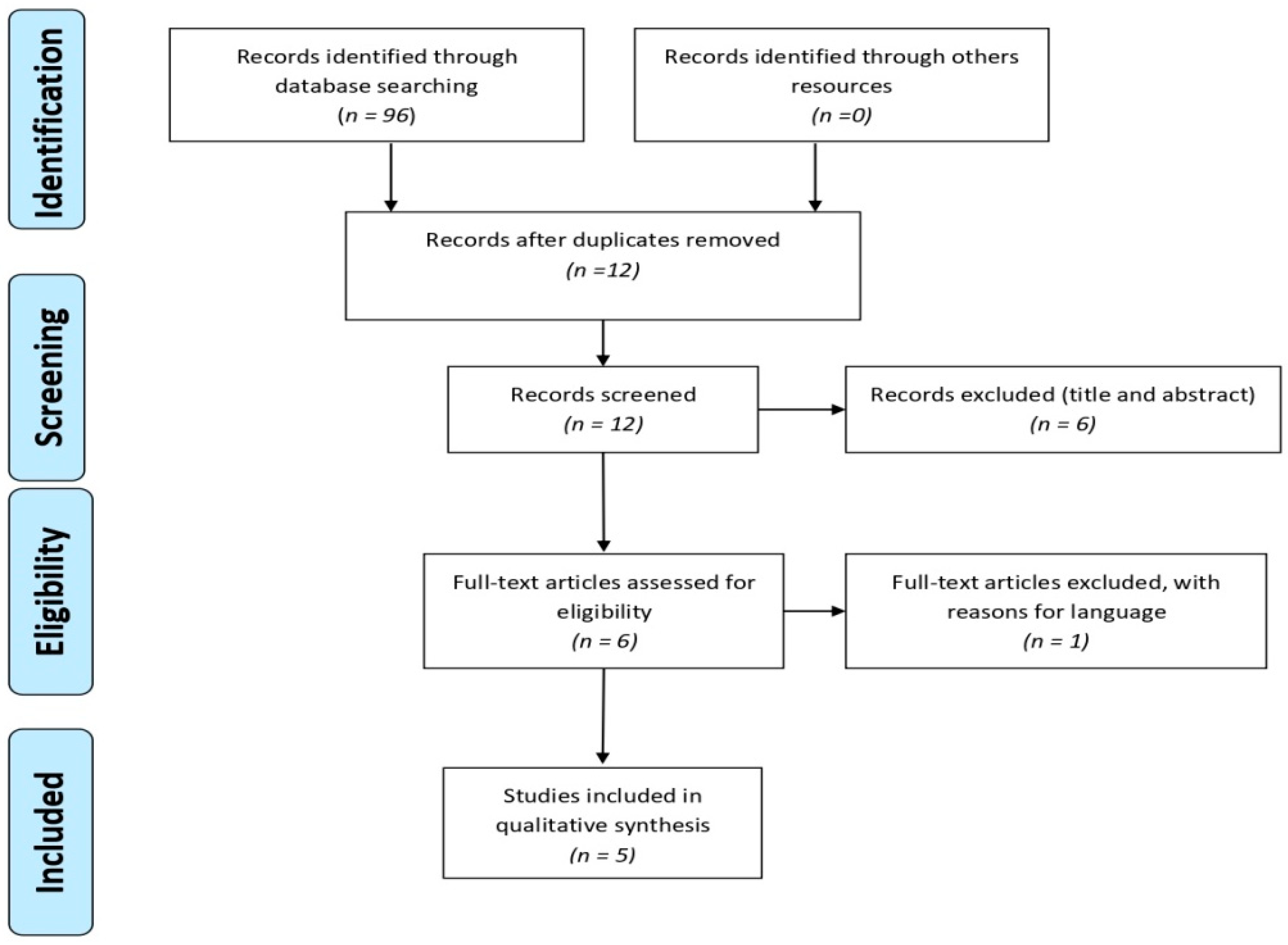
2. Material and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Evaluation of Clinical Relevance
3. Results
4. Discussion
4.1. Alzheimer’s Disease and Obstructive Sleep Apnea
4.2. Alzheimer’s Disease and Neurocognitive Impairment
4.3. Limitations of the Study and Prospective Research
- –
- The sample should be well delimited in terms of the evolution phase of AD, since the initial phase is the best time to see the CPAP impact on cognitive impairment [15].
- –
- Patients with AD that have a genetic load favorable to the disease should be excluded or considered in separate groups, due to the physiological implications of the genetic mutation in the oxidative process [31].
- –
- Neuroimaging tests should be included to assess possible brain changes [32].
- –
- It is very important to follow these patients to evaluate short- and long-term consequences.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Talih, F.R.; Ajaltouni, J.J.; Tamim, H.M.; Kobeissy, F.H. Risk of obstructive sleep apnea and excessive daytime sleepiness in hospitalized psychiatric patients. Neuropsychiatr. Dis. Treat. 2017, 13, 1193–1200. [Google Scholar] [CrossRef]
- Pan, W.; Kastina, A.J. Can sleep apnea cause Alzheimer’s disease? Neurosci. Biobehav. Rev. 2014, 47, 656–669. [Google Scholar] [CrossRef]
- Jayaraj, R.; Mohan, J.; Kanagasabai, A. A Review on Detection and Treatment Methods of Sleep Apnea. J. Clin. Diagn. Res. 2017, 11, 1–3. [Google Scholar] [CrossRef]
- Buratti, L.; Viticchi, G.; Falsetti, L.; Cagnetti, C.; Luzzi, S.; Bartolini, M.; Provinciali, L.; Silvestrini, M. Vascular impairment in Alzheimer’s disease: The role of obstructive sleep apnea. J. Alzheimers. Dis. 2014, 38, 445–453. [Google Scholar] [CrossRef]
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef]
- Kloepfer, C.; Riemann, D.; Nofzinger, E.A.; Feige, B.; Unterrainer, J.; O’Hara, R.; Sorichter, S.; Nissen, C. Memory before and after Sleep in Patients with Moderate Obstructive Sleep Apnea. J. Clin. Sleep Med. 2009, 5, 540–548. [Google Scholar]
- De Hoyos-Alonso, M.C.; Bonis, J.; Tapias-Merino, E.; Castell, M.V.; Otero, A. Estimación de la prevalencia de demencia a partir del análisis de bases de datos sobre uso de fármacos. La situación en la Comunidad de Madrid (España). Neurología 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Baietto, C.; Di Gioia, M.R.; Castaldi, P.; Castronovo, C.; Zucconi, M.; Cappa, S.F. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): Partial reversibility after continuous positive airway pressure (CPAP). Brain Res. Bull. 2003, 61, 87–92. [Google Scholar] [CrossRef]
- Hutton, B.; Catalá-López, F.; Moher, D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Mckhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimer’s Dement. 2011, 1–7. [Google Scholar] [CrossRef]
- Chong, M.S.; Ayalon, L.; Marler, M.; Loredo, J.S.; Corey-Bloom, J.; Palmer, B.W.; Liu, L.; Ancoli-Israel, S. Continuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer’s disease with sleep disordered breathing. J. Am. Geriatr. Soc. 2006, 54, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Palmer, B.W.; Cooke, J.R.; Corey-Bloom, J.; Fiorentino, L.; Natarajan, L.; Liu, L.; Ayalon, L.; He, F.; Loredo, J.S. Cognitive Effects of Treating Obstructive Sleep Apnea in Alzheimer’s Disease: A Randomized Controlled Study. J. Am. Geriatr. Soc. 2008, 56, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.R.; Ancoli-Israel, S.; Liu, L.; Loredo, J.S.; Natarajan, L.; Palmer, B.S.; He, F.; Corey-Bloom, J. Continuous positive airway pressure deepens sleep in patients with Alzheimer’s disease and obstructive sleep apnea. Sleep Med. 2009, 10, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.R.; Ayalon, L.; Palmer, B.W.; Loredo, J.S.; Corey-Bloom, J.; Natarajan, L.; Liu, L.; Ancoli-Israel, S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: A preliminary study. J. Clin. Sleep Med. 2009, 5, 305–309. [Google Scholar]
- Troussière, A.-C.; Charley, C.M.; Salleron, J.; Richard, F.; Delbeuck, X.; Derambure, P.; Pasquier, F.; Bombois, S. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1405–1408. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Burguers, J.S.; Grol, R.; Klazinga, N.S.; Mäkelä, M.; Zaat, J.; AGREE Collaboration. Towards evidence-based clinical practice: An international survey of 18 clinical guideline programs. Int. J. Qual. Heal. Care 2003, 15, 31–45. [Google Scholar] [CrossRef]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. Evid. Based. Med. 2016, 21, 125–127. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Sackett, D.L.; Sinclair, J.C.; Hayward, R.; Cook, D.J.; Cook, R.J. Users’ guides to the medical literature. IX. A method for grading health care recommendations. Evidence-Based Medicine Working Group. JAMA J. Am. Med. Assoc. 1995, 274, 1800–1804. [Google Scholar] [CrossRef]
- Verhagen, A.P.; De Vet, H.C.W.; De Bie, R.A.; Kessels, A.G.H.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Foley, N.C.; Teasell, R.W.; Bhogal, S.K.; Speechley, M.R. Stroke Rehabilitation Evidence-Based Review: Methodology. Top. Stroke Rehabil. 2003, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yin, Y.; Zhao, Z.; Huang, L.; Huang, S.; Zhuang, J.; Wu, H.; Peng, H.; Li, P. Elevation of serum TNF-α levels in mild and moderate Alzheimer patients with daytime sleepiness. J. Neuroimmunol. 2012, 244, 97–102. [Google Scholar] [CrossRef]
- Semelka, M.; Wilson, J.; Floyd, R. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. Am. Fam. Physician 2016, 94, 355–360. [Google Scholar]
- Ben-Israel, N.; Tarasiuk, A.; Zigel, Y. Nocturnal Sound Analysis for the Diagnosis of Obstructive Sleep Apnea. In Proceedings of the 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6146–6149. [Google Scholar] [CrossRef]
- Qaseem, A.; Dallas, P.; Owens, D.K.; Starkey, M.; Holty, J.-E.C.; Shekelle, P. Diagnosis of Obstructive Sleep Apnea in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med. 2014, 161, 210–221. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Lombardi, G.E.; Marelli, S.; Galbiati, A. Neurological Deficits in Obstructive Sleep Apnea. Curr. Treat. Options Neurol. 2017, 19, 1–13. [Google Scholar] [CrossRef]
- Dalmases Claries, M. Efectos del Envejecimiento en las Alteraciones Neurocognitivas en la Apnea Obstructiva del Sueño: Mecanismos y Tratamientos con CPAP; Universitat de Barcelona: Barcelona, Spain, 2016. [Google Scholar]
- Zhou, L.; Chen, P.; Peng, Y.; Ouyang, R. Role of Oxidative Stress in the Neurocognitive Dysfunction of Obstructive Sleep Apnea Syndrome. Oxid. Med. Cell. Longev. 2016, 1–15. [Google Scholar] [CrossRef]
- Spira, A.P.; Blackwell, T.; Stone, K.L.; Redline, S.; Cauley, J.A.; Ancoli-Israel, S.; Yaffe, K. Sleep-Disordered Breathing and Cognition in Older Women. J. Am. Geriatr. Soc. 2008, 56, 45–50. [Google Scholar] [CrossRef]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-israel, S. Sleep-Disordered Breathing, Hypoxia, and Risk of Mild Cognitive Impairment and Dementia in Older Women. J. Am. Med. Assoc. 2011, 306, 613–619. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J. Neurosci. Res. 2015, 93, 1778–1794. [Google Scholar] [CrossRef]
- Zafarlotfi, S.; Quadri, M.; Borodovsky, J. Understanding Brain Damage and Sleep Apnea: A Review. Health Outcomes Res. Med. 2010, 1, e103–e110. [Google Scholar] [CrossRef]
| Study Type of Evidence | Age, Mean (Standard Deviation) | Sample Size (Complete the Study) | Variables | DR | Results |
|---|---|---|---|---|---|
| Chong et al. 2006 [11] RCT (Randomized controlled trial) | t-CPAP 77.7 p-group 77.9 | 39 t-CPAP group = 19 p-CPAP group = 20 | ESS RDI | A | EES t-CPAP: 3 weeks p = 0.04 6 weeks p = 0.004 p-CPAP 3 weeks p = 0.33 6 weeks p = 0.06 RDI t-CPAP: 3 weeks p = 0.001 6 weeks p = 0.001 p-CPAP 3 weeks p > 0.05 6 weeks p = 0.001 |
| Ancoli-Israel et al. 2008 [12] RCT | t-CPAP 78.6 (6.8) p-CPAP 77.7 (7.7) | 52 (12 loss) t-CPAP group = 27 p-CPAP group = 25 | 14 Neuropsychol. tests (composite score) AHI | B | 3 weeks NO significant differences. 6 weeks: p = 0.01 composite score |
| Cooke et al. 2009 [14] Pilot study | 75.7 (5.9) | 10 patients +9 caregivers +CPAP = 5 −CPAP = 5 | PSQI ESS FISQ Cornell Scale Neuropsychology Inventory | - | —CPAP +: remained stable or improved in almost all measures —CPAP-continued to deteriorate —The caregivers of CPAP + patients improved their own quality of sleep, mood and reported patients stabilization. |
| Cooke et al. 2009 [13] RCT | t-CPAP 78.6 (6.8) p-CPAP 77.7 (7.7) | 52 39 compled the study t-CPAP = 27 p-CPAP = 25 | Polysomnography (1st night and at 3 weeks) | B | 1st night T-CPAP Step 1 (p = 0.04) Stage 2 of sleep (p = 0.02) 3-weeks t-CPAP Time awake after sleep (p = 0.005) Step 1 (p = 0.001) Step 3 (p = 0.006). Awakenings (p = 0.005) |
| Troussière et al. 2014 [15] Pilot study | t-CPAP group 73.4 no-CPAP group 77.6 | 28 (5 loss) t-CPAP = 14 no-CPAP = 9 | Mini Mental State Examination (MMSE) | - | T-CPAP = (−0.7) No-CPAP = (−2.2) MMSE p = 0.013 |
| Section Topic | Study | ||
|---|---|---|---|
| Chong et al. [11] | Ancoli-Israel et al. [12] | Cooke et al. [13] | |
| 1. Eligibility criteria were specified | Yes | Yes | Yes |
| 2. Randomly allocated to groups | Yes | Yes | Yes |
| 3. Concealed allocation | No | No | Yes |
| 4. Comparability of base | Yes | Yes | Yes |
| 5. Blinding of subjects | No | No | Yes |
| 6. Blinding of therapists | No | No | No |
| 7. Blinding of assessors | No | No | No |
| 8. Proper continuation | No | Yes | Yes |
| 9. Intention to treat | Yes | No | Yes |
| 10. Between-group statistical comparisons | Yes | Yes | Yes |
| 11. Point measure and measures of variability | No | Yes | Yes |
| Total | 4/10 | 5/10 | 8/10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Cabezas, V.; Ruiz-Molinero, C.; Jimenez-Rejano, J.J.; Gonzalez-Medina, G.; Galan-Mercant, A.; Martin-Valero, R. Continuous Positive Airway Pressure Treatment in Patients with Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020, 9, 181. https://doi.org/10.3390/jcm9010181
Perez-Cabezas V, Ruiz-Molinero C, Jimenez-Rejano JJ, Gonzalez-Medina G, Galan-Mercant A, Martin-Valero R. Continuous Positive Airway Pressure Treatment in Patients with Alzheimer’s Disease: A Systematic Review. Journal of Clinical Medicine. 2020; 9(1):181. https://doi.org/10.3390/jcm9010181
Chicago/Turabian StylePerez-Cabezas, Veronica, Carmen Ruiz-Molinero, Jose Jesus Jimenez-Rejano, Gloria Gonzalez-Medina, Alejandro Galan-Mercant, and Rocio Martin-Valero. 2020. "Continuous Positive Airway Pressure Treatment in Patients with Alzheimer’s Disease: A Systematic Review" Journal of Clinical Medicine 9, no. 1: 181. https://doi.org/10.3390/jcm9010181
APA StylePerez-Cabezas, V., Ruiz-Molinero, C., Jimenez-Rejano, J. J., Gonzalez-Medina, G., Galan-Mercant, A., & Martin-Valero, R. (2020). Continuous Positive Airway Pressure Treatment in Patients with Alzheimer’s Disease: A Systematic Review. Journal of Clinical Medicine, 9(1), 181. https://doi.org/10.3390/jcm9010181







