Relationship between the Renal Function and Adverse Clinical Events in Patients with Atrial Fibrillation: A Japanese Multicenter Registry Substudy
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Correction and Outcome Measures
2.3. Statistical Analysis
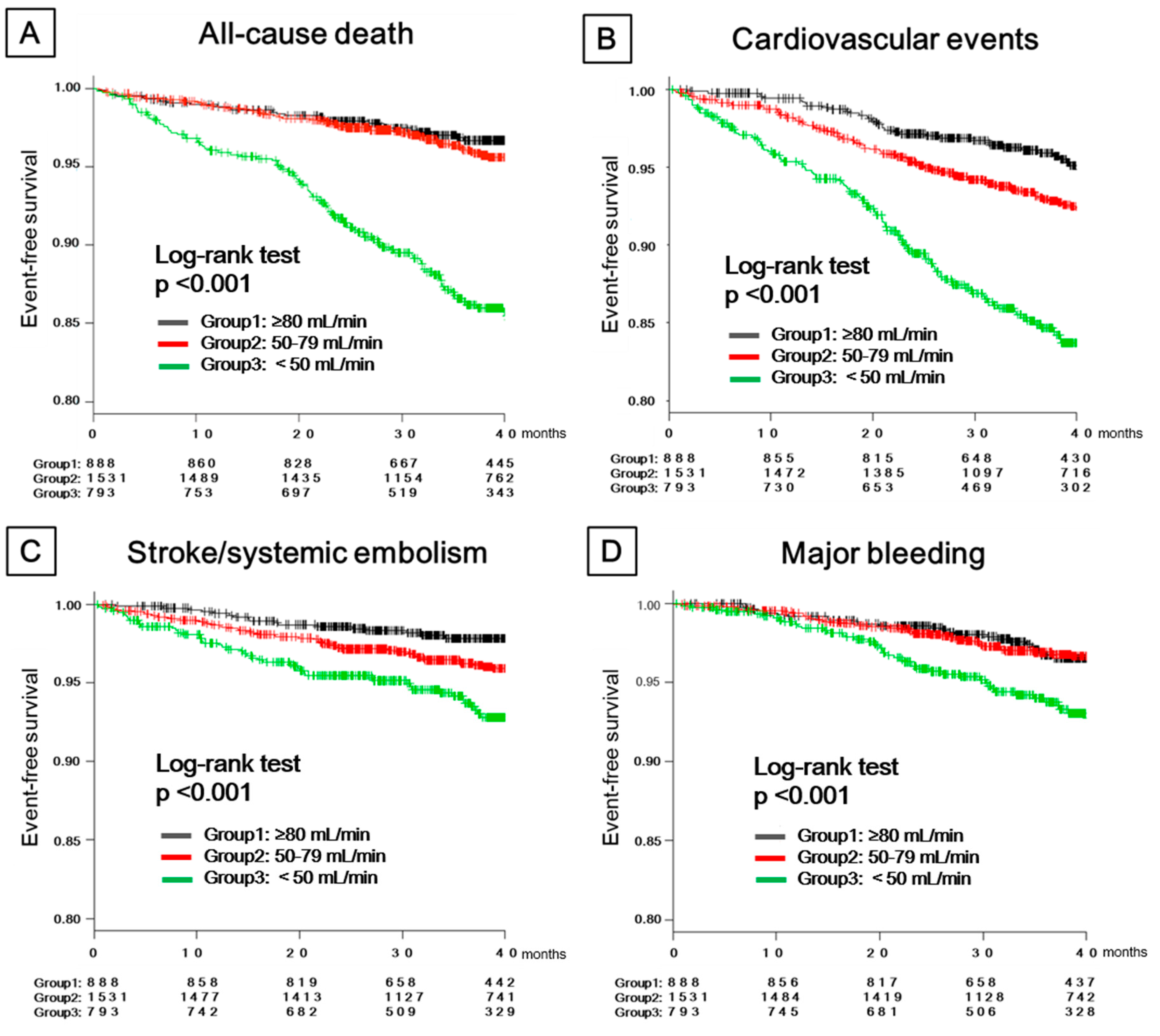
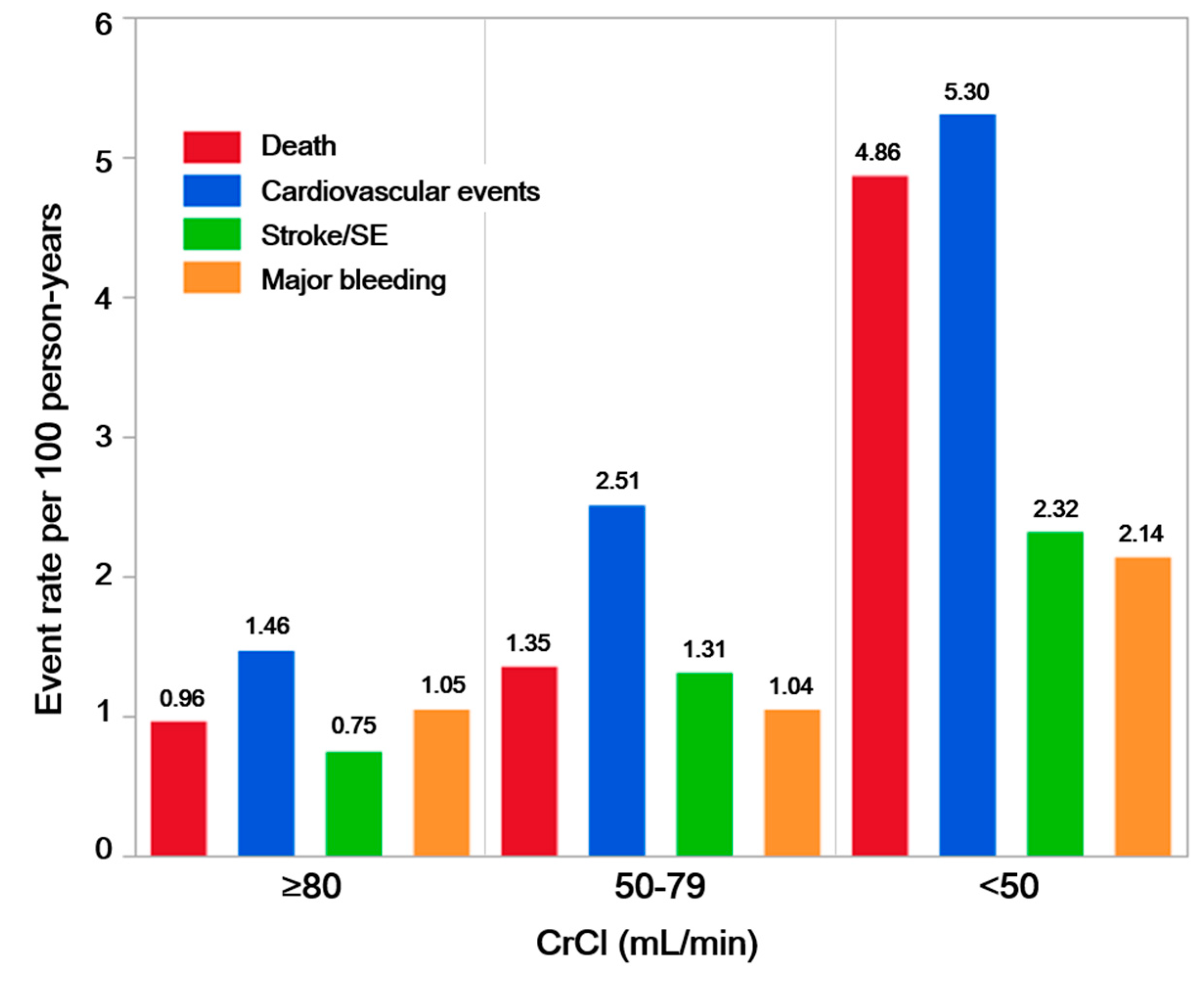
3. Results
3.1. Baseline Characteristics
3.2. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alonso, A.; Lopez, F.L.; Matsushita, K.; Loehr, L.R.; Agarwal, S.K.; Chen, L.Y.; Soliman, E.Z.; Astor, B.C.; Coresh, J. Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011, 123, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Watanabe, T.; Sasaki, S.; Nagai, K.; Roden, D.M.; Aizawa, Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: The Niigata preventive medicine study. Am. Heart J. 2009, 158, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Fujiki, A.; Origasa, H.; Ogawa, S.; Okumura, K.; Kubota, I.; Aizawa, Y.; Yamashita, T.; Atarashi, H.; Horie, M.; et al. Prevalence of atrial fibrillation in the general population of Japan: An analysis based on periodic health examination. Int. J. Cardiol. 2009, 137, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Laroche, C.; Diemberger, I.; Popescu, M.I.; Rasmussen, L.H.; Petrescu, L.; Crijns, H.J.G.M.; Tavazzi, L.; Maggioni, A.P.; Lip, G.Y.H. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes. Sci. Rep. 2016, 6, 30271. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Spinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Huiart, L.; Ferdynus, C.; Renoux, C.; Beaugrand, A.; Lafarge, S.; Bruneau, L.; Suissa, S.; Maillard, O.; Ranouil, X. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open 2018, 8, e018180. [Google Scholar] [CrossRef]
- Go, A.S.; Fang, M.C.; Udaltsova, N.; Chang, Y.; Pomernacki, N.K.; Borowsky, L.; Singer, D.E. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009, 119, 1363–1369. [Google Scholar] [CrossRef]
- Kodani, E.; Atarashi, H.; Inoue, H.; Okumura, K.; Yamashita, T.; Origasa, H. Impact of creatinine clearance on outcomes in patients with non-valvular atrial fibrillation: A subanalysis of the J-RHYTHM Registry. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Ogawa, H.; Ishii, M.; Masunaga, N.; Esato, M.; Chun, Y.H.; Tsuji, H.; Wada, H.; Hasegawa, K.; Lip, G.Y.; et al. Relation of stroke and major bleeding to creatinine clearance in patients with atrial fibrillation (from the Fushimi AF Registry). Am. J. Cardiol. 2017, 119, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Yokoyama, K.; Matsumoto, N.; Tachibana, E.; Kuronuma, K.; Oiwa, K.; Matsumoto, M.; Kojima, T.; Hanada, S.; Nomoto, K.; et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: Findings from the SAKURA AF Registry. J. Arrhythm. 2017, 33, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Yokoyama, K.; Matsumoto, N.; Tachibana, E.; Kuronuma, K.; Oiwa, K.; Matsumoto, M.; Kojima, T.; Hanada, S.; Nomoto, K.; et al. Three-year clinical outcomes associated with warfarin vs. direct oral anticoagulant use among Japanese patients with atrial fibrillation- Findings from the SAKURA AF Registry. Circ. J. 2018, 82, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Rosendaal, F.R.; Cannegieter, S.C.; van der Meer, F.J.; Briet, E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb. Haemost. 1993, 69, 236–239. [Google Scholar] [CrossRef] [PubMed]
- JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013). Circ. J. 2014, 78, 1997–2021. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Hijazi, Z.; Thomas, L.; Alexander, J.H.; Amerena, J.; Hanna, M.; Keltai, M.; Lanas, F.; Lopes, R.D.; Lopez-Sendon, J.; et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Eur. Heart J. 2012, 33, 2821–2830. [Google Scholar] [CrossRef]
- Hijazi, Z.; Hohnloser, S.H.; Oldgren, J.; Andersson, U.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Reilly, P.A.; Siegbahn, A.; Yusuf, S.; et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: A RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014, 129, 961–970. [Google Scholar] [CrossRef]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke results from the national registry of atrial fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, H.; Inoue, H.; Okumura, K.; Yamashita, T.; Kumagai, N.; Origasa, H. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: A report from the J-RHYTHM Registry. Circ. J. 2011, 75, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Uozumi, R.; Hamatani, Y.; Esato, M.; Chun, Y.H.; Tsuji, H.; Wada, H.; Hasegawa, K.; Ogawa, H.; Abe, M.; et al. Current status and outcomes of direct oral anticoagulant use in real-world atrial fibrillation patients- Fushimi AF Registry. Circ. J. 2017, 81, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef]
- Inoue, H.; Okumura, K.; Atarashi, H.; Yamashita, T.; Origasa, H.; Kumagai, N.; Sakurai, M.; Kawamura, Y.; Kubota, I.; Matsumoto, K.; et al. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: Results of the J-RHYTHM Registry. Circ. J. 2013, 77, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Ruff, C.T.; Kuder, J.F.; Murphy, S.A.; Antman, E.M.; Braunwald, E. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 Trial. Circulation 2016, 134, 24–36. [Google Scholar] [CrossRef]
- Goto, S.; Angchaisuksiri, P.; Bassand, J.P.; Camm, A.J.; Dominguez, H.; Illingworth, L.; Gibbs, H.; Goldhaber, S.Z.; Goto, S.; Jing, Z.C.; et al. Management and 1-year outcomes of patients with newly diagnosed atrial fibrillation and chronic kidney disease: Results from the prospective GARFIELD—AF Registry. J. Am. Heart Assoc. 2019, 8, e010510. [Google Scholar] [CrossRef]
| Number of Patients (%) | Overall | Normal Renal Function (CrCl ≥ 80 mL/min) | Mild CKD (CrCl 50–79 mL/min) | Moderate–Severe CKD (CrCl < 50 mL/min) | p-Value * | p-Value for Trend |
|---|---|---|---|---|---|---|
| 3242 | 893 (27.5) | 1550 (47.8) | 799 (24.6) | |||
| Age (years) | 72.00 ± 9.38 | 63.54 ± 8.70 | 72.82 ± 6.61 | 79.87 ± 6.69 | <0.001 | <0.001 |
| <65 | 614 (18.9) | 443 (49.6) | 159 (10.3) | 12 (1.5) | <0.001 | <0.001 |
| 65–74 | 1286 (39.7) | 376 (42.1) | 759 (49.0) | 151 (18.9) | ||
| ≥75 | 1342 (41.4) | 74 (8.3) | 632 (40.8) | 636 (79.6) | ||
| Female sex | 848 (26.2) | 115 (12.9) | 410 (26.5) | 323 (40.4) | <0.001 | <0.001 |
| Body height (cm) | 162.48 ± 9.49 | 168.01 ± 7.96 | 162.31 ± 8.41 | 156.62 ± 9.44 | <0.001 | <0.001 |
| Body weight (kg) | 63.85 ± 12.96 | 74.16 ± 12.47 | 62.67 ± 10.09 | 54.61 ± 10.17 | <0.001 | <0.001 |
| BMI (kg/m2) | 24.05 ± 3.73 | 26.25 ± 4.01 | 23.74 ± 3.16 | 22.19 ± 3.19 | <0.001 | <0.001 |
| Paroxysmal AF | 1195 (36.9) | 351 (39.3) | 593 (38.3) | 251 (31.4) | 0.001 | 0.001 |
| Medical history | ||||||
| Hypertension | 2312 (71.3) | 616 (69.0) | 1106 (71.4) | 590 (73.8) | 0.087 | 0.027 |
| Dyslipidemia | 1256 (38.7) | 371 (41.5) | 635 (41.0) | 250 (31.3) | <0.001 | <0.001 |
| Diabetes | 741 (22.9) | 221 (24.7) | 338 (21.8) | 182 (22.8) | 0.249 | 0.310 |
| Heart failure | 719 (22.2) | 165 (18.5) | 292 (18.8) | 262 (32.8) | <0.001 | <0.001 |
| Stroke/TIA | 364 (11.2) | 54 (6.0) | 193 (12.5) | 117 (14.6) | <0.001 | <0.001 |
| Ischemic heart disease | 312 (9.6) | 57 (6.4) | 159 (10.3) | 96 (12.0) | <0.001 | <0.001 |
| AF ablation | 299 (9.2) | 147 (16.5) | 123 (7.9) | 29 (3.6) | <0.001 | <0.001 |
| DOAC use | 1679 (51.8) | 493 (55.2) | 818 (52.8) | 368 (46.1) | <0.001 | <0.001 |
| Warfarin use | 1563 (48.2) | 400 (44.8) | 732 (47.2) | 431 (53.9) | <0.001 | <0.001 |
| TTR (%) | 71.50 (43.20, 93.40) | 64.60 (33.80, 87.67) | 74.60 (46.18, 94.30) | 74.20 (49.70, 94.90) | <0.001 | <0.001 |
| TTR ≥ 65% | 805 (57.6) | 176 (49.7) | 394 (60.1) | 235 (60.7) | 0.002 | 0.003 |
| Antiplatelet use | 517 (15.9) | 92 (10.3) | 248 (16.0) | 177 (22.2) | <0.001 | <0.001 |
| Antiarrhythmic drug class Ⅰ | 423 (13.0) | 141 (15.8) | 196 (12.6) | 86 (10.8) | 0.007 | 0.002 |
| Beta-blocker use | 1471 (45.4) | 403 (45.1) | 689 (44.5) | 379 (47.4) | 0.382 | 0.362 |
| Amiodarone use | 32 (1.0) | 9 (1.0) | 11 (0.7) | 12 (1.5) | 0.184 | 0.334 |
| Bepridil use | 322 (9.9) | 113 (12.7) | 168 (10.8) | 41 (5.1) | <0.001 | <0.001 |
| CHADS2 score | 2 (1, 2) | 1 (1, 2) | 2 (1, 2) | 2 (2, 3) | <0.001 | <0.001 |
| CHA2DS2-VASc score | 3 (2, 4) | 2 (1, 3) | 3 (2, 4) | 4 (3, 5) | <0.001 | <0.001 |
| New use (OAC therapy duration <3 months) | 637 (19.6) | 186 (20.8) | 301 (19.4) | 150 (18.8) | 0.541 | 0.283 |
| SCr (mg/dL) | 0.87 (0.75, 1.04) | 0.78 (0.69, 0.86) | 0.88 (0.75, 1.00) | 1.09 (0.88, 1.34) | <0.001 | <0.001 |
| CrCl (mL/min) | 64.65 (50.16, 82.03) | 94.26 (86.11, 108.90) | 63.74 (57.01, 70.88) | 40.05 (32.57, 45.93) | <0.001 | <0.001 |
| Outcome | Number of Patients | Number of Events | Hazard Ratio | |||
|---|---|---|---|---|---|---|
| CrCl (mL/min) | Crude (95% CI) | p-Value | Adjusted (95% CI) | p-Value | ||
| Death | ||||||
| ≥80 (reference) | 893 | 26 | 1.00 | 1.00 | ||
| 50–79 | 1550 | 63 | 1.41 (0.89–2.23) | 0.138 | 0.99 (0.60–1.62) | 0.9635 |
| <50 | 799 | 109 | 5.14 (3.35–7.89) | <0.0001 | 2.40 (1.41–4.07) | 0.0012 |
| CV events | ||||||
| ≥80 (reference) | 893 | 39 | 1.00 | 1.00 | ||
| 50–79 | 1550 | 113 | 1.72 (1.19–2.47) | 0.0036 | 1.51 (1.03–2.22) | 0.0358 |
| <50 | 799 | 112 | 3.67 (2.55–5.28) | <0.0001 | 2.53 (1.62–3.94) | <0.0001 |
| Stroke/SE | ||||||
| ≥80 (reference) | 893 | 20 | 1.00 | 1.00 | ||
| 50–79 | 1550 | 60 | 1.77 (1.07–2.93) | 0.0273 | 1.45 (0.84–2.47) | 0.1787 |
| <50 | 799 | 51 | 3.15 (1.88–5.28) | <0.0001 | 2.13 (1.34–4.00) | 0.0182 |
| Major bleeding | ||||||
| ≥80 (reference) | 893 | 28 | 1.00 | 1.00 | ||
| 50–79 | 1550 | 48 | 1.00 (0.63–1.59) | 0.9981 | 0.92 (0.56–1.51) | 0.7389 |
| <50 | 799 | 47 | 2.08 (1.30–3.33) | 0.0021 | 1.83 (1.02–3.29) | 0.0434 |
| Clinical Outcome | Normal Renal Function (CrCl ≥ 80 mL/min) | Mild CKD (CrCl 50–79 mL/min) | Moderate-Severe CKD (CrCl < 50 mL/min) | p-Value for Interaction | |||
|---|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | ||
| Death | 0.97 (0.44–2.12) | 0.9112 | 1.09 (0.66–1.81) | 0.7322 | 0.99 (0.67–1.47) | 0.9671 | 0.9369 |
| Cardiovascular events | 1.26 (0.66–2.41) | 0.4889 | 1.19 (0.81–1.73) | 0.3721 | 1.02 (0.69–1.50) | 0.9272 | 0.6535 |
| Stroke/SE | 0.77 (0.31–1.89) | 0.5705 | 1.43 (0.85–2.41) | 0.1774 | 1.30 (0.74–2.27) | 0.3664 | 0.4442 |
| Major bleeding | 0.90 (0.42–1.91) | 0.7825 | 0.98 (0.55–1.74) | 0.9443 | 0.95 (0.53–1.73) | 0.8775 | 0.9946 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuzawa, Y.; Kuronuma, K.; Okumura, Y.; Yokoyama, K.; Matsumoto, N.; Tachibana, E.; Oiwa, K.; Matsumoto, M.; Kojima, T.; Haruta, H.; et al. Relationship between the Renal Function and Adverse Clinical Events in Patients with Atrial Fibrillation: A Japanese Multicenter Registry Substudy. J. Clin. Med. 2020, 9, 167. https://doi.org/10.3390/jcm9010167
Yuzawa Y, Kuronuma K, Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Oiwa K, Matsumoto M, Kojima T, Haruta H, et al. Relationship between the Renal Function and Adverse Clinical Events in Patients with Atrial Fibrillation: A Japanese Multicenter Registry Substudy. Journal of Clinical Medicine. 2020; 9(1):167. https://doi.org/10.3390/jcm9010167
Chicago/Turabian StyleYuzawa, Yasuhumi, Keiichiro Kuronuma, Yasuo Okumura, Katsuaki Yokoyama, Naoya Matsumoto, Eizo Tachibana, Koji Oiwa, Michiaki Matsumoto, Toshiaki Kojima, Hironori Haruta, and et al. 2020. "Relationship between the Renal Function and Adverse Clinical Events in Patients with Atrial Fibrillation: A Japanese Multicenter Registry Substudy" Journal of Clinical Medicine 9, no. 1: 167. https://doi.org/10.3390/jcm9010167
APA StyleYuzawa, Y., Kuronuma, K., Okumura, Y., Yokoyama, K., Matsumoto, N., Tachibana, E., Oiwa, K., Matsumoto, M., Kojima, T., Haruta, H., Nomoto, K., Sonoda, K., Arima, K., Kogawa, R., Takahashi, F., Kotani, T., Okubo, K., Fukushima, S., Itou, S., ... on behalf of the SAKURA AF Registry Investigators. (2020). Relationship between the Renal Function and Adverse Clinical Events in Patients with Atrial Fibrillation: A Japanese Multicenter Registry Substudy. Journal of Clinical Medicine, 9(1), 167. https://doi.org/10.3390/jcm9010167





