Definitive Cefazolin Therapy for Stabilized Adults with Community-Onset Escherichia coli, Klebsiella Species, and Proteus mirabilis Bacteremia: MIC Matters
Abstract
1. Introduction
2. Methods
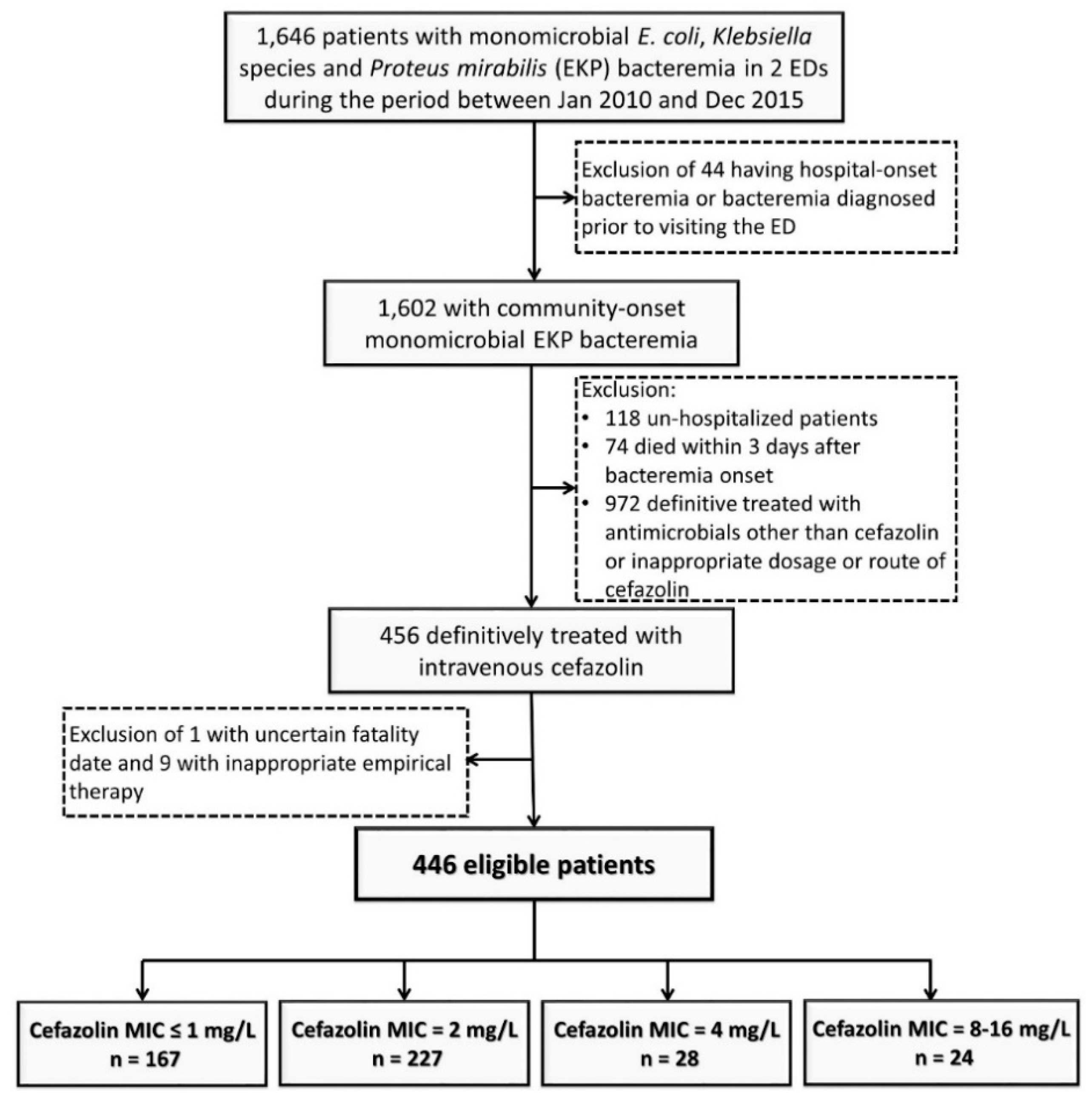
2.1. Study Design and Population
2.2. Data Collection
2.3. Patient Outcomes
2.4. Microbiological Methods
2.5. Definitions
2.6. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
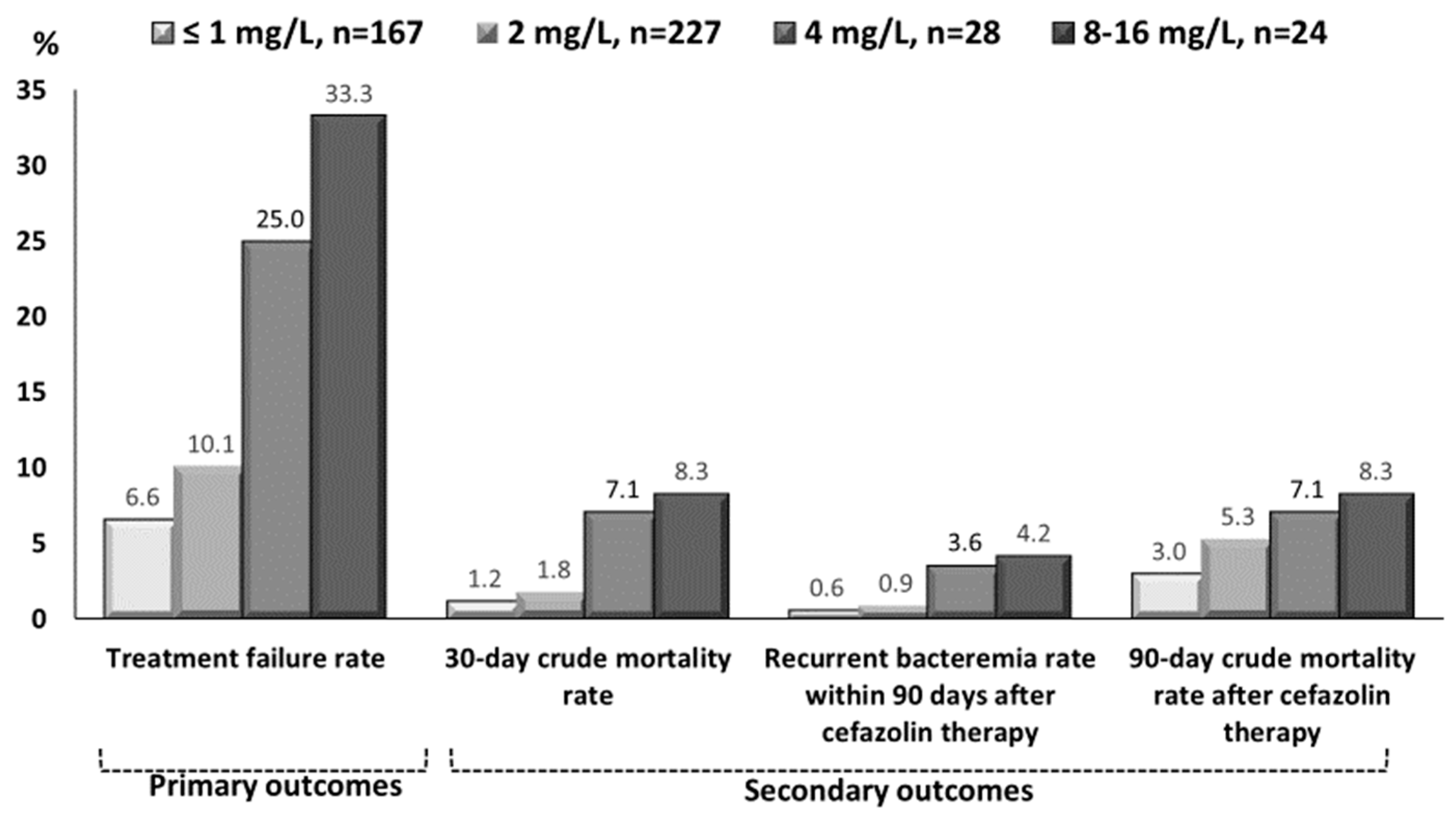
3.2. Clinical Characteristics and Outcomes in Varied Cefazolin MIC Groups
3.3. Risk Factors of Treatment Failure of Definitive Cefazolin Therapy
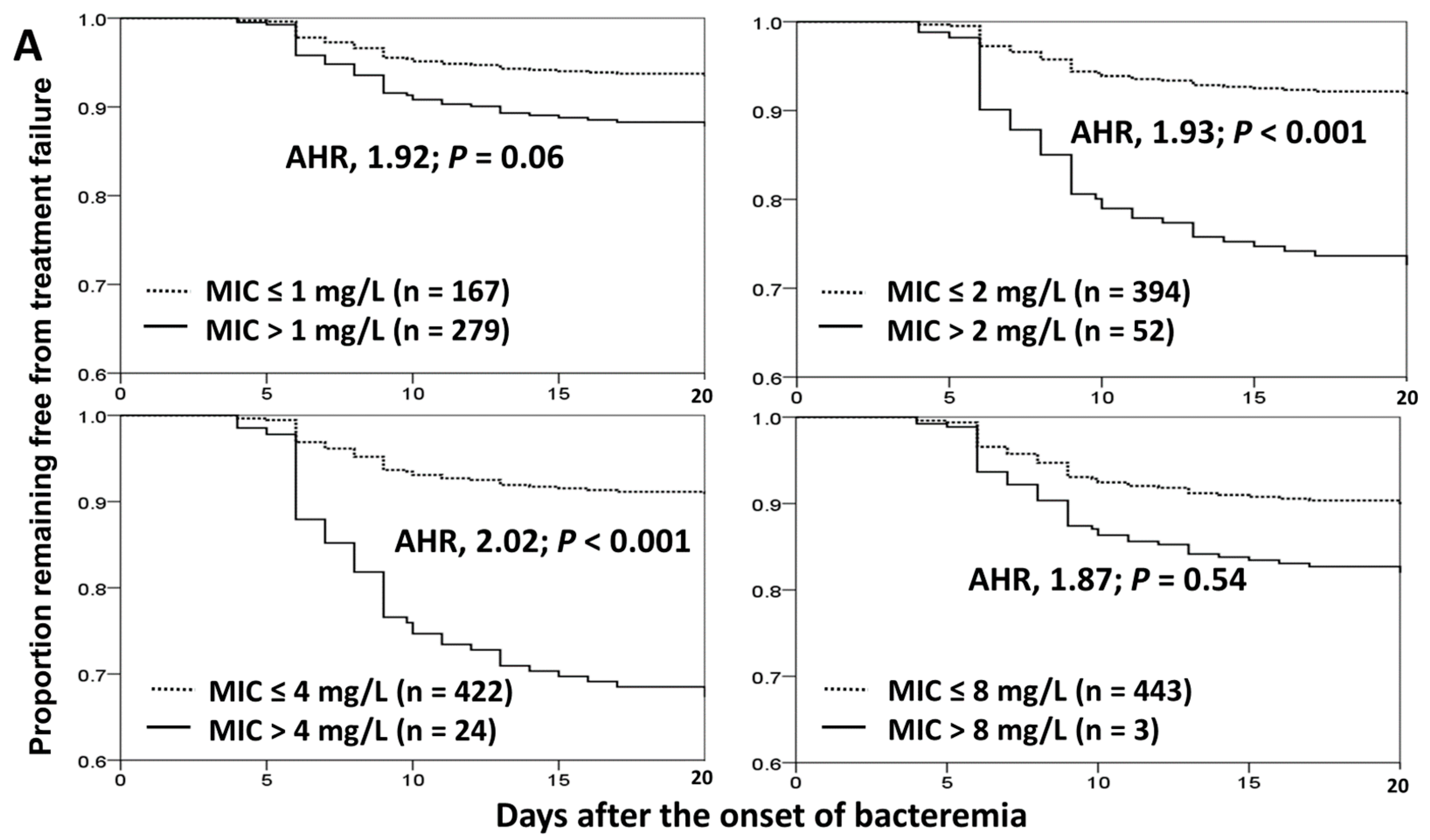
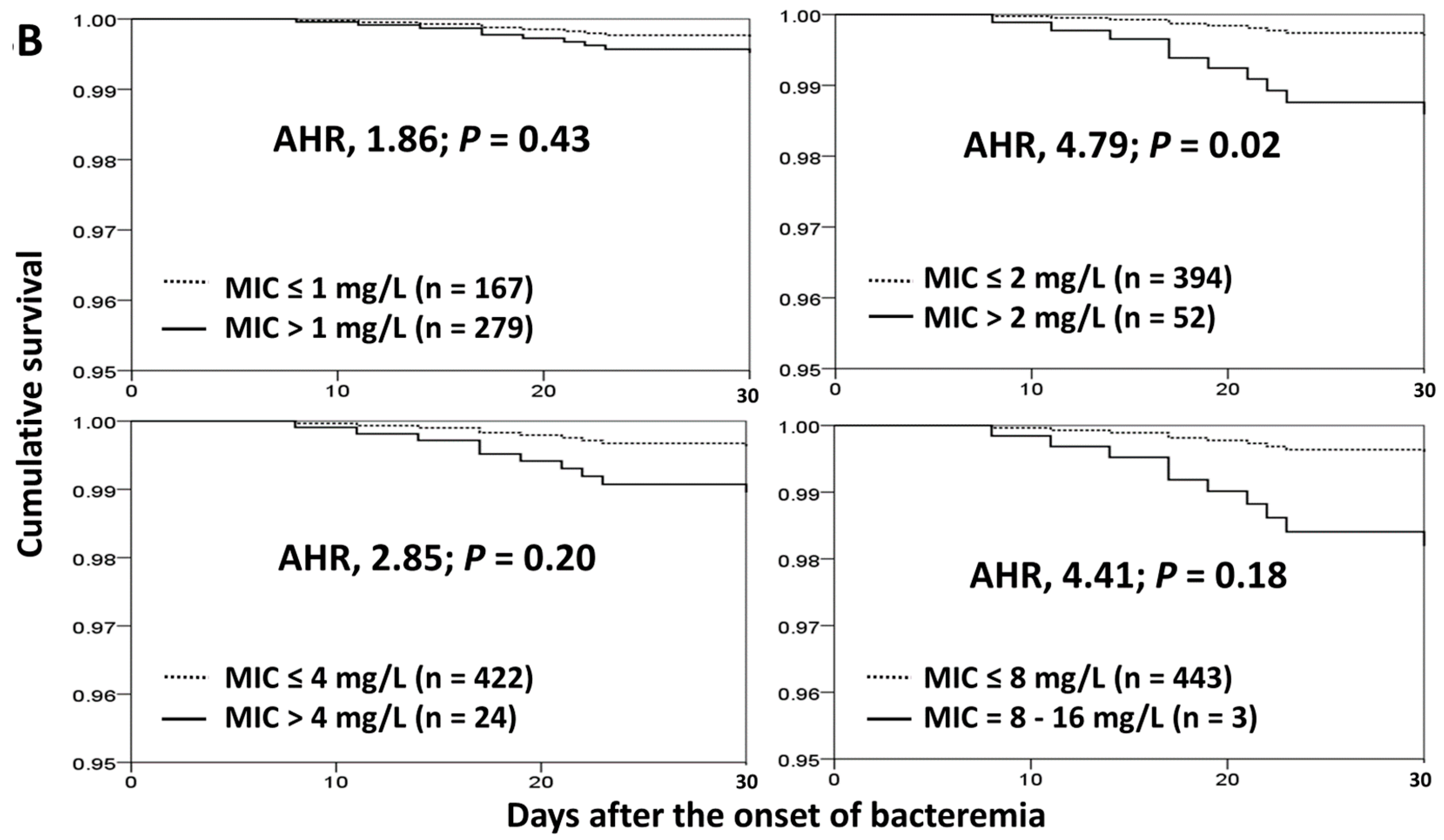
3.4. Impact of Cefazolin MICs on Treatment Failure and 30-Day Crude Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reller, L.B.; Karney, W.W.; Beaty, H.N.; Holmes, K.K.; Turck, M. Evaluation of cefazolin, a new cephalosporin antibiotic. Antimicrob. Agents Chemother. 1973, 3, 488–497. [Google Scholar] [CrossRef]
- Sader, H.S.; Flamm, R.K.; Jones, R.N. Frequency of occurrence and antimicrobial susceptibility of Gram-negative bacteremia isolates in patients with urinary tract infection: Results from United States and European hospitals (2009–2011). J. Chemother. 2014, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Melzer, M.; Toner, R.; Lacey, S.; Bettany, E.; Rait, G. Biliary tract infection and bacteraemia: Presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad. Med. J. 2007, 83, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lee, C.H.; Hong, M.Y.; Tang, H.J.; Ko, W.C. Timing of appropriate empirical antimicrobial administration and outcome of adults with community-onset bacteremia. Crit. Care 2017, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Page, C.P.; Bohnen, J.M.; Fletcher, J.R.; McManus, A.T.; Solomkin, J.S.; Wittmann, D.H. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch. Surg. 1993, 128, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choe, P.G.; Song, K.H.; Park, S.W.; Kim, H.B.; Kim, N.J.; Kim, E.C.; Park, W.B.; Oh, M.D. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob. Agents Chemother. 2011, 55, 5122–5126. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Everett, E.D.; Dellinger, P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, E.L.; Montoya, J.G.; et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 2005, 41, 1373–1406. [Google Scholar] [CrossRef]
- Peterson, P.K.; Matzke, G.; Keane, W.F. Current concepts in the management of peritonitis in patients undergoing continuous ambulatory peritoneal dialysis. Rev. Infect. Dis. 1987, 9, 604–612. [Google Scholar] [CrossRef]
- Bates, D.W.; Pruess, K.E.; Lee, T.H. How bad are bacteremia and sepsis? Outcomes in a cohort with suspected bacteremia. Arch. Intern. Med. 1995, 155, 593–598. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Approved Standard. Twenty Informational Supplement; CLSI Document M100-S20; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Approved Standard. Twenty-Ninth Informational Supplement; CLSI document M100-S29; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoint Table v. 10.0, European Committee on Antimicrobial Susceptibility Testing, London, UK. 2020. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 7 January 2020).
- Chuang, C.H.; Liu, M.F.; Lin, C.F.; Shi, Z.Y. Impact of revised susceptibility breakpoints on bacteremia of Klebsiella pneumoniae: Minimum inhibitory concentration of cefazolin and clinical outcomes. J. Microbiol. Immunol. Infect. 2016, 49, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Liu, M.F.; Lin, C.F.; Shi, Z.Y. The impact of revised CLSI cefazolin breakpoints on the clinical outcomes of Escherichia coli bacteremia. J. Microbiol. Immunol. Infect. 2016, 49, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Lee, C.C.; Lee, C.H.; Yang, C.Y.; Hsieh, C.C.; Tang, H.J.; Ko, W.C. Beneficial effects of early empirical administration of appropriate antimicrobials on survival and defervescence in adults with community-onset bacteremia. Crit. Care 2019, 23, 363. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Yang, C.Y.; Hsieh, C.C.; Hong, M.Y.; Lee, C.H.; Tang, H.J.; Ko, W.C. Timing of follow-up blood cultures for community-onset bacteremia. Sci. Rep. 2019, 9, 14500. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Wang, J.L.; Lee, C.H.; Hung, Y.P.; Hong, M.Y.; Chang, C.M.; Ko, W.C. Age-Related Trends in Adults with Community-Onset Bacteremia. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- David, N.; Gilbert, R.C.M., Jr.; George, M.; Eliopoulos, G.M.; Chambers, H.F.; Saag, M.S. Selected pharmacologic faetures of antimicrobial agents. Sanford Guide Antimicrob. Ther. 2009, 1, 78–82. [Google Scholar]
- Leibovici, L.; Shraga, I.; Drucker, M.; Konigsberger, H.; Samra, Z.; Pitlik, S.D. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 1998, 244, 379–386. [Google Scholar] [CrossRef]
- Paterson, D.L.; Ko, W.C.; Von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G.; Klugman, K.P.; Bonomo, R.A.; et al. International prospective study of Klebsiella pneumoniae bacteremia: Implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Intern. Med. 2004, 140, 26–32. [Google Scholar] [CrossRef]
- Schellevis, F.G.; Van der Velden, J.; Van de Lisdonk, E.; Van Eijk, J.T.; Van Weel, C. Comorbidity of chronic diseases in general practice. J. Clin. Epidemiol. 1993, 46, 469–473. [Google Scholar] [CrossRef]
- McCabe, W.R. Gram-negative bacteremia. Adv. Intern. Med. 1974, 19, 135–158. [Google Scholar] [PubMed]
- Lee, C.C.; Lee, N.Y.; Yan, J.J.; Lee, H.C.; Chen, P.L.; Chang, C.M.; Wu, C.J.; Ko, N.Y.; Wang, L.R.; Chi, C.H.; et al. Bacteremia due to extended-spectrum-beta-lactamase-producing Enterobacter cloacae: Role of carbapenem therapy. Antimicrob. Agents Chemother. 2010, 54, 3551–3556. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.B.; Morton-Kute, L.; Berger, S.R.; Weber, J.; Siegal, F.P.; Lopez, C.; Robbins, W.; Landesman, S.H. Recurrent Salmonella typhimurium bacteremia associated with the acquired immunodeficiency syndrome. Ann. Intern. Med. 1985, 102, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Chotiprasitsakul, D.; Han, J.H.; Cosgrove, S.E.; Harris, A.D.; Lautenbach, E.; Conley, A.T.; Tolomeo, P.; Wise, J.; Tamma, P.D.; Antibacterial Resistance Leadership Group. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score-Matched Cohort. Clin. Infect. Dis. 2018, 66, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Tansarli, G.S.; Rafailidis, P.I.; Kapaskelis, A.; Vardakas, K.Z. Impact of antibiotic MIC on infection outcome in patients with susceptible Gram-negative bacteria: A systematic review and meta-analysis. Antimicrob. Agents Chemother. 2012, 56, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Sklansky, D.J.; Palazzi, D.L.; Swami, S.K.; Milstone, A.M. Antibiotic susceptibility of common pediatric uropathogens in the United States. Clin. Infect. Dis. 2014, 59, 750–752. [Google Scholar] [CrossRef]
- Lea, A.S.; Sudan, A.W.; Wood, B.A.; Gentry, L.O. Randomized comparative study of moxalactam and cefazolin in the treatment of acute urinary tract infections in adults. Antimicrob. Agents Chemother. 1982, 22, 32–35. [Google Scholar] [CrossRef]
- Wing, D.A.; Hendershott, C.M.; Debuque, L.; Millar, L.K. A randomized trial of three antibiotic regimens for the treatment of pyelonephritis in pregnancy. Obstet. Gynecol. 1998, 92, 249–253. [Google Scholar]
- Sanchez-Ramos, L.; McAlpine, K.J.; Adair, C.D.; Kaunitz, A.M.; Delke, I.; Briones, D.K. Pyelonephritis in pregnancy: Once-a-day ceftriaxone versus multiple doses of cefazolin. A randomized, double-blind trial. Am. J. Obstet. Gynecol. 1995, 172, 129–133. [Google Scholar] [CrossRef]
- Turnidge, J.D. Subcommittee on Antimicrobial Susceptibility Testing of the C, Laboratory Standards I. Cefazolin and enterobacteriaceae: Rationale for revised susceptibility testing breakpoints. Clin. Infect. Dis. 2011, 52, 917–924. [Google Scholar] [CrossRef]
- Lee, C.C.; Lee, C.H.; Chen, P.L.; Hsieh, C.C.; Tang, H.J.; Ko, W.C. Definitive Cefazolin Treatment for Community-Onset Enterobacteriaceae Bacteremia Based on the Contemporary CLSI Breakpoint: Clinical Experience of a Medical Center in Southern Taiwan. Antibiotics 2019, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Lee, C.H.; Li, M.C.; Hong, M.Y.; Chi, C.H.; Lee, C.C. Empirical third-generation cephalosporin therapy for adults with community-onset Enterobacteriaceae bacteraemia: Impact of revised CLSI breakpoints. Int. J. Antimicrob. Agents 2016, 47, 297–303. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patient Number (%) | γ | p Values | |||
|---|---|---|---|---|---|---|
| ≤1 mg/L, n = 167 | 2 mg/L, n = 227 | 4 mg/L, n = 28 | 8–16 mg/L, n = 24 | |||
| Gender, female | 116 (69.5) | 158 (69.6) | 18 (64.3) | 13 (54.2) | −0.80 | 0.20 |
| Old age, ≥65 years | 110 (65.9) | 131 (57.7) | 21 (75.0) | 18 (75.0) | 0.74 | 0.26 |
| Nursing-home residents | 1 (0.6) | 2 (0.9) | 0 (0) | 0 (0) | −0.74 | 0.26 |
| Major comorbidities | ||||||
| Hypertension | 92 (55.1) | 110 (48.5) | 19 (67.9) | 16 (66.7) | 0.60 | 0.40 |
| Diabetes mellitus | 72 (43.1) | 101 (44.5) | 13 (46.4) | 6 (25.0) | −0.20 | 0.80 |
| Malignancies | 37 (22.2) | 48 (21.1) | 9 (32.1) | 5 (20.8) | −0.40 | 0.60 |
| Neurological diseases | 35 (21.0) | 44 (19.4) | 4 (14.3) | 2 (8.3) | −1.00 | 0.01 |
| Chronic kidney diseases | 21 (12.6) | 21 (9.3) | 6 (21.4) | 6 (25.0) | 0.80 | 0.20 |
| Liver cirrhosis | 19 (11.4) | 31 (13.7) | 2 (7.1) | 4 (16.7) | 0.40 | 0.60 |
| Major source of bacteremia | ||||||
| Urinary tract | 109 (65.3) | 147 (64.8) | 21 (75.0) | 16 (66.7) | 0.60 | 0.40 |
| Biliary tract | 20 (12.0) | 12 (5.3) | 6 (21.4) | 2 (8.3) | 0 | 1.00 |
| Intra-abdominal | 19 (11.4) | 16 (7.0) | 0 (0) | 1 (4.2) | −0.80 | 0.20 |
| Primary bacteremia | 13 (7.8) | 14 (6.2) | 1 (3.6) | 3 (12.5) | 0.20 | 0.80 |
| Pneumonia | 3 (1.8) | 7 (3.1) | 0 (0) | 2 (8.3) | 0.40 | 0.60 |
| Liver abscess | 1 (0.6) | 23 (10.1) | 0 (0) | 0 (0) | −0.74 | 0.26 |
| Rapidly or ultimately fatal comorbidities (McCabe classification) | 24 (14.4) | 36 (15.9) | 4 (14.3) | 11 (45.8) | 0.40 | 0.60 |
| Inadequate source control during antibiotic therapy | 3 (1.8) | 9 (4.0) | 0 (0) | 1 (4.2) | 0.40 | 0.60 |
| Pitt bacteremia score | ||||||
| Onset | ||||||
| 0 | 57 (34.1) | 70 (30.8) | 7 (25.0) | 5 (20.8) | −1.00 | 0.01 |
| ≥4 | 19 (11.4) | 21 (9.3) | 0 (0) | 1 (4.2) | −0.80 | 0.20 |
| Day 3 after onset | ||||||
| 0 | 139(83.2) | 168 (74.0) | 23 (82.1) | 19 (79.2) | −0.40 | 0.60 |
| ≥4 | 13 (7.8) | 13 (5.7) | 0 (0) | 1 (4.2) | −0.80 | 0.20 |
| Type of empirical antibiotics | ||||||
| Third-generation cephalosporins | 85 (50.9) | 121 (53.3) | 11 (39.3) | 13 (54.2) | 0.40 | 0.60 |
| First-generation cephalosporins | 31 (18.6) | 45 (19.8) | 3 (10.7) | 3 (12.5) | −0.60 | 0.40 |
| Second-generation cephalosporins | 26 (15.6) | 34 (15.0) | 7 (25.0) | 4 (16.7) | 0.60 | 0.40 |
| Fluoroquinolones | 9 (5.4) | 7 (3.1) | 1 (3.6) | 2 (8.3) | 0.40 | 0.60 |
| Ampicillin/sulbactam | 8 (4.8) | 3 (1.3) | 3 (10.7) | 0 (0) | −0.40 | 0.60 |
| Fourth-generation cephalosporins | 4 (2.4) | 10 (4.4) | 2 (7.1) | 1 (4.2) | 0.40 | 0.60 |
| Carbapenems | 4 (2.4) | 4 (1.8) | 1 (3.6) | 0 (0) | −0.40 | 0.60 |
| Others | 0 (0) | 3 (1.3) | 0 (0) | 1 (4.2) | 0.63 | 0.37 |
| Duration (mean ± standard deviation) | ||||||
| Time-to-appropriate antibiotic, hour * | 3.2 ± 5.6 | 2.4 ± 3.0 | 4.8 ± 8.1 | 4.5 ± 5.6 | 0.60 | 0.40 |
| Time-to-cefazolin therapy, day ** | 2.8 ± 1.9 | 2.7 ± 2.1 | 3.3 ± 2.2 | 3.0 ± 1.4 | 0.60 | 0.40 |
| Intravenous cefazolin therapy, day | 5.0 ± 3.5 | 5.4 ± 3.9 | 6.0 ± 3.6 | 5.7 ± 3.4 | 0.80 | 0.20 |
| Intravenous antimicrobial therapy, day | 8.7 ± 5.4 | 9.0 ± 6.5 | 11.7± 7.9 | 12.0 ± 5.3 | 1.00 | 0.01 |
| Total antibiotic administration, day | 12.4 ± 4.8 | 13.1 ± 5.5 | 15.9 ± 6.9 | 16.7 ± 5.2 | 1.00 | 0.01 |
| Length of hospitalization among the survivors, day | 10.7 ± 6.6 | 11.3 ± 10.2 | 13.2 ± 8.1 | 14.1 ± 5.3 | 1.00 | 0.01 |
| Primary outcomes (i.e., Treatment failure) | ||||||
| Overall | 11 (6.6) | 23 (10.1) | 7 (25.0) | 8 (33.3) | 1.00 | 0.01 |
| Escalation to broad-spectrum during cefazolin therapy | 11 (6.6) | 20 (8.8) | 7 (25.0) | 7 (29.2) | 1.00 | 0.01 |
| Breakthrough bacteremia during cefazolin therapy | 0 (0) | 1 (0.4) | 1 (3.6) | 0 (0) | 0.11 | 0.90 |
| Transfer to intensive care unit during cefazolin therapy | 0 (0) | 2 (0.8) | 0 (0) | 0 (0) | −0.26 | 0.74 |
| 15-day crude mortality after bacteremia onset | 0 (0) | 1 (0.4) | 1 (3.6) | 1 (4.2) | 1.00 | 0.01 |
| Secondary outcomes | ||||||
| 30-day crude mortality rate after bacteremia onset | 2 (1.2) | 4 (1.8) | 2 (7.1) | 2 (8.3) | 1.00 | 0.01 |
| Recurrent bacteremia within 90 days after cefazolin therapy | 1 (0.6) | 2 (0.9) | 1 (3.6) | 1 (4.2) | 1.00 | 0.01 |
| 90-day crude mortality after cefazolin therapy | 5 (3.0) | 12 (5.3) | 2 (7.1) | 2 (8.3) | 1.00 | 0.01 |
| Variable | Patient Number (%) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Failure, n = 49 | Success, n = 396 | OR (95% CI) | p Value | Adjusted OR (95%CI) | p Value | |
| Pitt bacteremia score = 0 at Day 3 | 31 (63.3) | 318 (80.1) | 0.43 (0.23–0.80) | 0.007 | 0.43 (0.23–0.81) | 0.009 |
| Rapidly or ultimately fatal comorbidities (McCabe classification) | 14 (28.6) | 61 (15.4) | 2.20 (1.12–4.34) | 0.02 | 2.21 (1.12–4.39) | 0.02 |
| Comorbid malignancies | 17 (34.7) | 82 (20.7) | 2.04 (1.08–3.86) | 0.04 | NS | NS |
| Bacteremia due to urinary tract infections | 27 (55.1) | 266 (67.0) | 0.60 (0.33–1.10) | 0.098 | NS | NS |
| Causative microorganisms | ||||||
| Escherichia coli | 29 (59.2) | 311 (78.3) | 0.40 (0.22–0.74) | 0.003 | NS | NS |
| Proteus mirabilis | 5 (10.2) | 11 (2.8) | 3.99 (1.32–12.01) | 0.02 | NS | NS |
| Variable | Patient Number (%) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Death, n = 10 | Survival, n = 436 | OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Pitt bacteremia score | ||||||
| 0 at onset | 0 (0) | 139 (31.9) | - | 0.04 | NS | NS |
| 0 at Day 3 | 1 (10.0) | 348 (79.8) | 0.03 (0.004–0.23) | <0.001 | 0.03 (0.004–0.25) | 0.001 |
| Sources of bacteremia | ||||||
| Pneumonia | 2 (20.0) | 10 (2.3) | 10.65 (2.00–56.66) | 0.001 | NS | NS |
| Urinary tract infections | 4 (40.0) | 289 (66.3) | 0.34 (0.09–1.22) | 0.099 | NS | NS |
| Causative microorganisms | ||||||
| Escherichia coli | 4 (40.0) | 336 (77.1) | 0.20 (0.06–0.72) | 0.01 | NS | NS |
| Proteus mirabilis | 2 (20.0) | 14 (3.2) | 7.54 (1.46–38.79) | 0.046 | NS | NS |
| Rapidly or ultimately fatal comorbidities (McCabe classification) | 6 (60.0) | 69 (15.8) | 7.98 (2.19–29.01) | 0.002 | 7.55 (1.82–31.31) | 0.005 |
| Comorbidity types | ||||||
| Diabetes mellitus | 0 (0) | 192 (44.0) | - | 0.006 | NS | NS |
| Malignancies | 7 (70.0) | 92 (21.1) | 8.73 (2.21–34.4) | 0.001 | NS | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-C.; Chen, P.-L.; Lee, C.-H.; Yang, C.-Y.; Lee, C.-C.; Ko, W.-C. Definitive Cefazolin Therapy for Stabilized Adults with Community-Onset Escherichia coli, Klebsiella Species, and Proteus mirabilis Bacteremia: MIC Matters. J. Clin. Med. 2020, 9, 157. https://doi.org/10.3390/jcm9010157
Hsieh C-C, Chen P-L, Lee C-H, Yang C-Y, Lee C-C, Ko W-C. Definitive Cefazolin Therapy for Stabilized Adults with Community-Onset Escherichia coli, Klebsiella Species, and Proteus mirabilis Bacteremia: MIC Matters. Journal of Clinical Medicine. 2020; 9(1):157. https://doi.org/10.3390/jcm9010157
Chicago/Turabian StyleHsieh, Chih-Chia, Po-Lin Chen, Chung-Hsun Lee, Chao-Yung Yang, Ching-Chi Lee, and Wen-Chien Ko. 2020. "Definitive Cefazolin Therapy for Stabilized Adults with Community-Onset Escherichia coli, Klebsiella Species, and Proteus mirabilis Bacteremia: MIC Matters" Journal of Clinical Medicine 9, no. 1: 157. https://doi.org/10.3390/jcm9010157
APA StyleHsieh, C.-C., Chen, P.-L., Lee, C.-H., Yang, C.-Y., Lee, C.-C., & Ko, W.-C. (2020). Definitive Cefazolin Therapy for Stabilized Adults with Community-Onset Escherichia coli, Klebsiella Species, and Proteus mirabilis Bacteremia: MIC Matters. Journal of Clinical Medicine, 9(1), 157. https://doi.org/10.3390/jcm9010157






