Comparative Effectiveness of Chuna Manipulative Therapy for Non-Acute Lower Back Pain: A Multi-Center, Pragmatic, Randomized Controlled Trial
Abstract
1. Introduction
2. Experimental Section
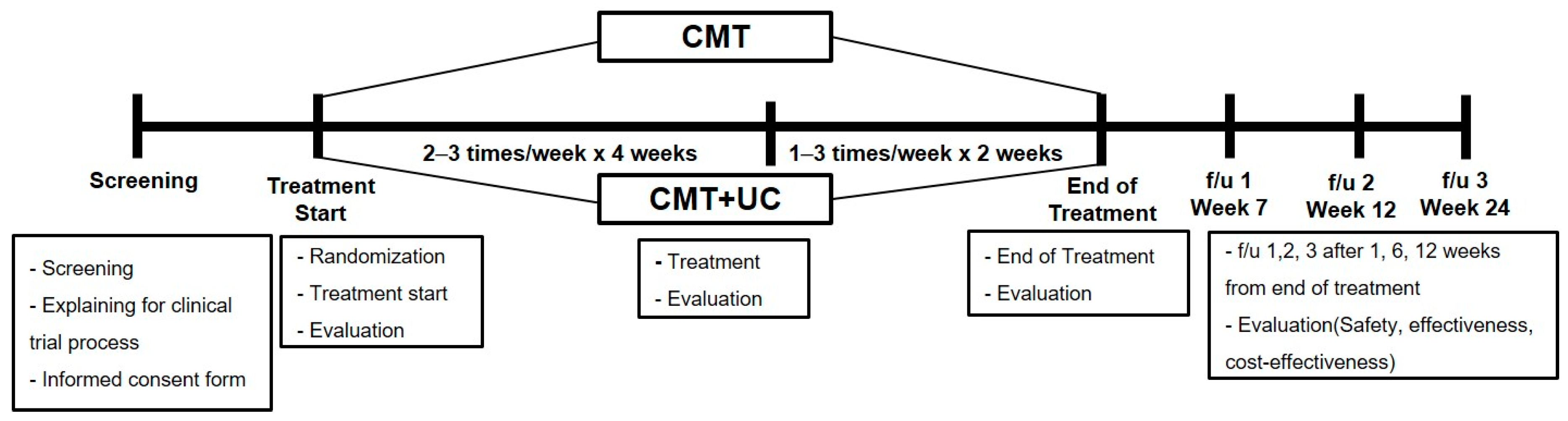
2.1. Study Design and Setting
2.2. Participants
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Randomization, Allocation Concealment
2.4. Blinding
2.5. Sample Size
2.6. Interventions
2.6.1. Chuna Manipulative Therapy
2.6.2. Usual Care
2.7. Outcomes
2.7.1. Primary Outcome Measurement
2.7.2. Secondary Outcome Measures
2.8. Statistical Analysis
2.9. Safety
2.10. Availability of Data and Material
3. Results
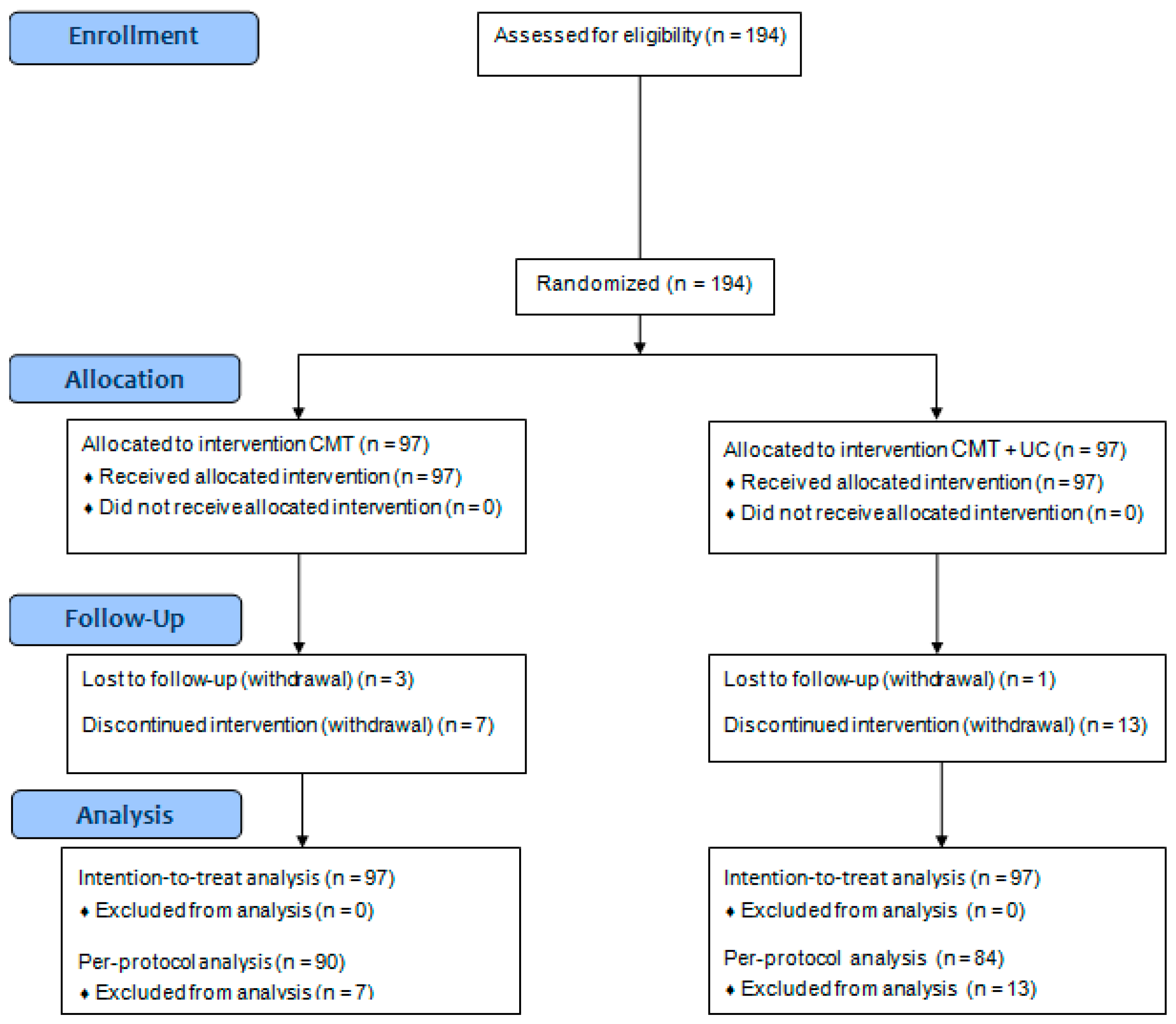
3.1. Patient Characteristics
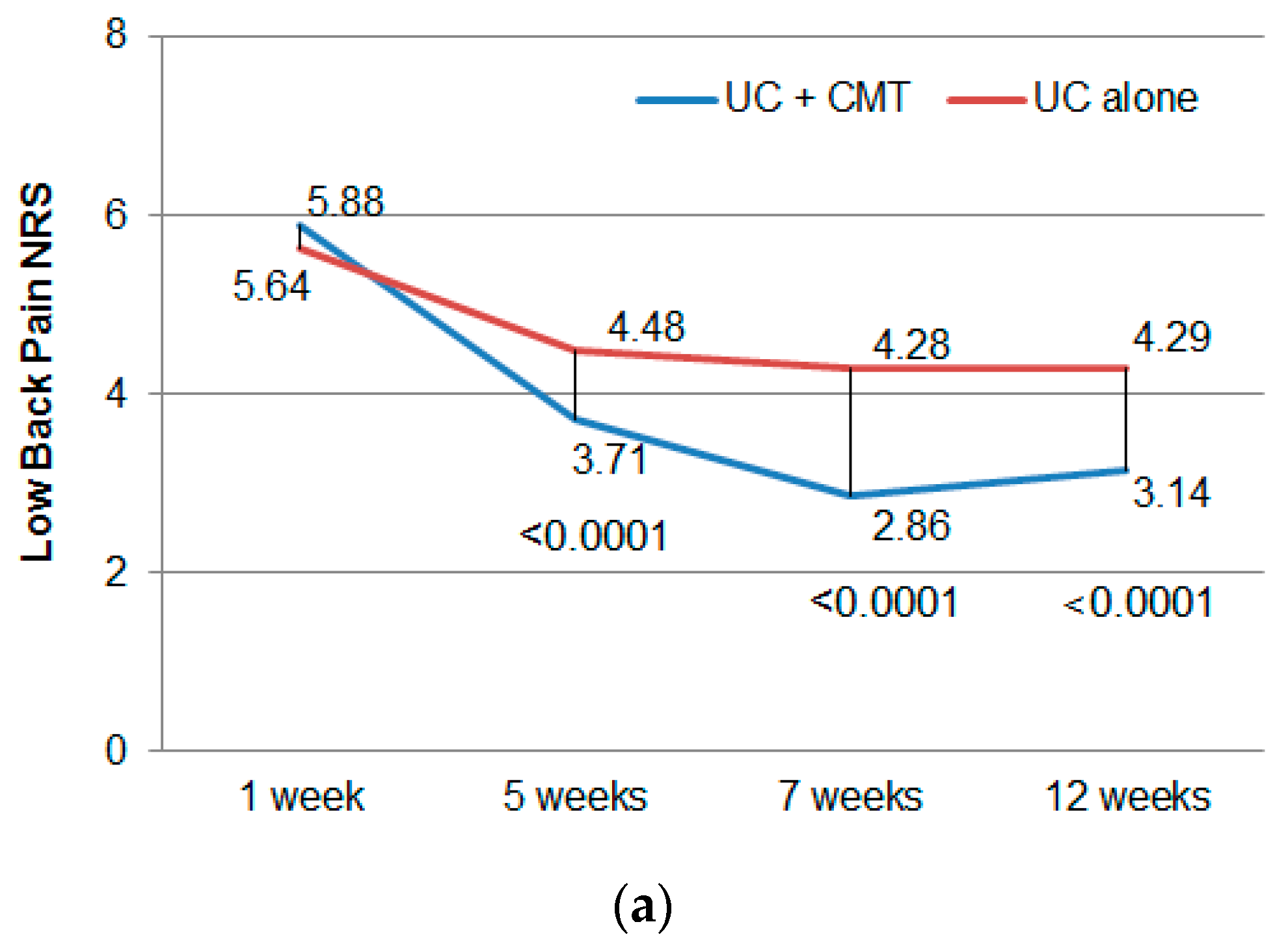
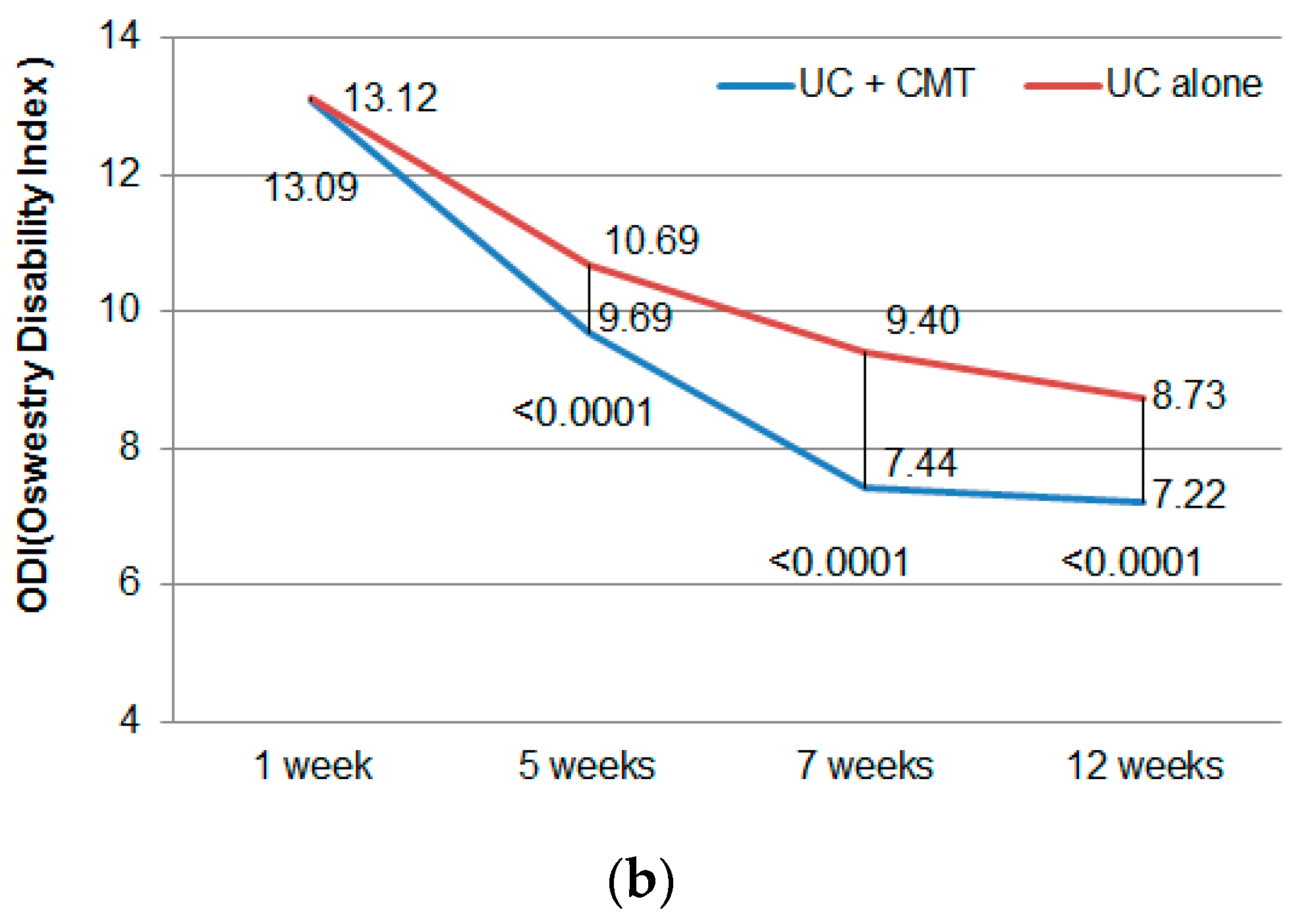
3.2. Primary Outcome
3.3. Secondary Outcome
3.4. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Engers, A.J.; Jellema, P.; Wensing, M.; Van Der Windt, D.A.; Grol, R.; Van Tulder, M.W. Individual patient education for low back pain. Cochrane Database Syst. Rev. 2008, Cd004057. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- Rubinstein, S.M.; van Middelkoop, M.; Assendelft, W.J.; de Boer, M.R.; van Tulder, M.W. Spinal manipulative therapy for chronic low-back pain. Cochrane Database Syst. Rev. 2011, Cd008112. [Google Scholar] [CrossRef]
- Kuijpers, T.; van Middelkoop, M.; Rubinstein, S.M.; Ostelo, R.; Verhagen, A.; Koes, B.W.; van Tulder, M.W. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur. Spine J. 2011, 20, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Boswell, M.V.; Singh, V.; Benyamin, R.M.; Fellows, B.; Abdi, S.; Buenaventura, R.M.; Conn, A.; Datta, S.; Derby, R.; et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician 2009, 12, 699–802. [Google Scholar] [PubMed]
- Chou, R.; Deyo, R.; Friedly, J.; Skelly, A.; Hashimoto, R.; Weimer, M.; Fu, R.; Dana, T.; Kraegel, P.; Griffin, J.; et al. Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann. Int. Med. 2017, 166, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A. Clinical Guidelines Committee of the American College of Physicians Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Shin, S.W.; Park, J.H. A comparative study on the concepts of the Chuna. J. Korean Med. Class. 2008, 21, 173–191. [Google Scholar]
- Park, T.Y.; Moon, T.W.; Cho, D.C.; Lee, J.H.; Ko, Y.S.; Hwang, E.H.; Heo, K.H.; Choi, T.Y.; Shin, B.C. An introduction to Chuna manual medicine in Korea: History, insurance coverage, education, and clinical research in Korean literature. Integr. Med. Res. 2014, 3, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.S.; Cho, H.W.; Lee, H.Y.; Heo, K.H.; Hwang, E.H.; Shin, M.S.; Shin, B.C. Research trends on Chuna treatment in Korean medicine – Focused on type of clinical trials, published year, academic journals and treatment technique for each usedparts. J. Korea Chuna Man. Med. Spine Nerves 2013, 1, 49–61. [Google Scholar]
- Lim, K.T.; Hwang, E.H.; Cho, J.H.; Jung, J.Y.; Kim, K.W.; Ha, I.H.; Kim, M.R.; Kim, N.K.; Lee, J.H.; Shin, B.C. Comparative effectiveness of Chuna manual therapy versus conventional usual care for non-acute low back pain: A pilot randomized controlled trial. Trials 2019, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Hayden, J.; Bombardier, C.; van Tulder, M. Effect sizes of non-surgical treatments of non-specific low-back pain. Eur. Spine J. 2007, 16, 1776–1788. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.C.; Kim, M.R.; Cho, J.H.; Jung, J.Y.; Kim, K.W.; Lee, J.H.; Nam, K.; Lee, M.H.; Hwang, E.H.; Heo, K.H.; et al. Comparative effectiveness and cost-effectiveness of Chuna manual therapy versus conventional usual care for nonacute low back pain: Study protocol for a pilot multicenter, pragmatic randomized controlled trial (pCRN study). Trials 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Shin, J.S.; Lee, J.; Lee, Y.J.; Kim, M.R.; Ahn, Y.J.; Park, K.B.; Shin, B.C.; Lee, M.S.; Kim, J.H.; et al. A survey among Korea Medicine doctors (KMDs) in Korea on patterns of integrative Korean Medicine practice for lumbar intervertebral disc displacement: Preliminary research for clinical practice guidelines. BMC Complement Altern. Med. 2015, 15, 432. [Google Scholar] [CrossRef]
- Health Insurance Review and Assessment Service. Available online: http://www.hira.or.kr/ (accessed on 5 January 2017).
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Turk, D.C.; Rudy, T.E.; Sorkin, B.A. Neglected topics in chronic pain treatment outcome studies: Determination of success. Pain 1993, 53, 3–16. [Google Scholar] [CrossRef]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef]
- Jensen, M.P.; Karoly, P. Self-report scales and procedures for assessing pain in adults. In Handbook of Pain Assessment; The Guilford Press: New York, NY, USA, 1992; pp. 135–151. [Google Scholar]
- Jeon, C.H.; Kim, D.J.; Kim, S.K.; Kim, D.J.; Lee, H.M.; Park, H.J. Validation in the cross-cultural adaptation of the Korean version of the Oswestry Disability Index. J. Korean Med. Sci. 2006, 21, 1092–1097. [Google Scholar] [CrossRef]
- Lee, C.; Fu, T.; Liu, C.; Hung, C. Psychometric evaluation of the Oswestry Disability Index in patients with chronic low back pain: Factor and Mokken analyses. Health Qual. Life Outcomes 2017, 15, 192. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Couper, J.; Davies, J.B.; O’Brien, J.P. The Oswestry low back pain disability questionnaire. Physiotherapy 1980, 66, 271–273. [Google Scholar]
- Scott, W.; McCracken, L.M. Patients’ impression of change following treatment for chronic pain: Global, specific, a single dimension, or many? J. Pain 2015, 16, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Cho, Y.S.; Uhm, W.S.; Kim, S.; Bae, S.C. Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual. Life Res. 2005, 14, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.P.; Dritsaki, M.; Jommi, C. Generic Preference-based Measures for Low Back Pain: Which of Them Should Be Used? Spine 2016, 41, E364–E374. [Google Scholar] [CrossRef] [PubMed]
- Klemenc-Ketis, Z. Disability in patients with chronic non-specific low back pain: Validation of the Slovene version of the Oswestry Disability Index. Slov. J. Public Heal. 2011, 50, 87–94. [Google Scholar] [CrossRef][Green Version]
- Kopec, J.A.; Willison, K.D. A comparative review of four preference-weighted measures of health-related quality of life. J. Clin. Epidemiol. 2003, 56, 317–325. [Google Scholar] [CrossRef]
- Al Zoubi, F.; Preuss, R. Reliability of a measure of total lumbar spine range of motion in individuals with low back pain. J. Appl. Biomech. 2013, 29, 670–677. [Google Scholar] [CrossRef][Green Version]
- Saur, P.M.M.; Ensink, F.-B.M.; Frese, K.; Seeger, D.; Hildebrandt, J. Lumbar Range of Motion: Reliability and Validity of the Inclinometer Technique in the Clinical Measurement of Trunk Flexibility. Spine 1996, 21, 1332–1338. [Google Scholar] [CrossRef]
- Hart, D.L.; Werneke, M.W. Re: Pengel LHM, Refshauge KM, Maher CG. Responsiveness of pain, disability, and physical impairment outcomes in patients with low back pain. Spine 2004, 29, 2475–2476. [Google Scholar]
- Pengel, L.H.; Refshauge, K.M.; Maher, C.G. Responsiveness of pain, disability, and physical impairment outcomes in patients with low back pain. Spine 2004, 29, 879–883. [Google Scholar] [CrossRef]
- Boutron, I.; Altman, D.G.; Moher, D.; Schulz, K.F.; Ravaud, P. CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann. Intern. Med. 2017, 167, 40–47. [Google Scholar] [CrossRef]
- Walker, B.F.; French, S.D.; Grant, W.; Green, S. A Cochrane review of combined chiropractic interventions for low-back pain. Spine 2011, 36, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Amie, S.; Tobias, S.; Rebecca, R.; Lesley, W.; Felicity, L.B.; Matthew, L.; Holger, C.; Jon, W.; Jon, A. Osteopathic manipulative treatment: A systematic review and critical appraisal of comparative effectiveness and health economics research. Musculoskelet. Sci. Pract. 2017, 27, 165–175. [Google Scholar]
- Andersson, G.B.; Lucente, T.; Davis, A.M.; Kappler, R.E.; Lipton, J.A.; Leurgans, S. A Comparison of Osteopathic Spinal Manipulation with Standard Care for Patients with Low Back Pain. N. Engl. J. Med. 1999, 341, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Chown, M.; Whittamore, L.; Rush, M.; Allan, S.; Stott, D.; Archer, M. A prospective study of patients with chronic back pain randomised to group exercise, physiotherapy or osteopathy. Physiotherapy 2008, 94, 21–28. [Google Scholar] [CrossRef]
- Licciardone, J.; Gamber, R.; Cardarelli, K. Patient satisfaction and clinical outcomes associated with osteopathic manipulative treatment. J. Am. Osteopat. Assoc. 2002, 102, 13–20. [Google Scholar]
- Walker, J.; Mertens, U.K.; Schmidt, C.O.; Chenot, J.F. Effect on healthcare utilization and costs of spinal manual therapy for acute low back pain in routine care: A propensity score matched cohort study. PLoS ONE 2017, 12, e0177255. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Kim, N.S.; Do, S.R.; Lee, Y.H.; Yoon, G.J.; Park, J.H.; Jang, D.H.; Chun, J.Y.; Kim, H.Y.; Lee, N.H.; et al. 2011 National Survey on the Use of Korean Medicine and Korean Herbal Medicine; Korea Ministry of Health and Welfare, Korea Institute for Health and Social Affairs: Sejong, Korea, 2011; pp. 1–554. [Google Scholar]
- Branchini, M.; Lopopolo, F.; Andreoli, E.; Loreti, I.; Marchand, A.M.; Stecco, A. Fascial Manipulation(R) for chronic aspecific low back pain: A single blinded randomized controlled trial. F1000Res 2015, 4, 1208. [Google Scholar] [CrossRef]
- Copay, A.G.; Glassman, S.D.; Subach, B.R.; Berven, S.; Schuler, T.C.; Carreon, L.Y. Minimum clinically important difference in lumbar spine surgery patients: A choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008, 8, 968–974. [Google Scholar] [CrossRef]
- Davidson, M.; Keating, J.L.; Eyres, S. A low back-specific version of the SF-36 Physical Functioning scale. Spine 2004, 29, 586–594. [Google Scholar] [CrossRef]
- Ostelo, R.W.; De Vet, H.C. Clinically important outcomes in low back pain. Best Pr. Res. Clin. Rheumatol. 2005, 19, 593–607. [Google Scholar] [CrossRef]
- Van Der Roer, N.; Ostelo, R.W.J.G.; Bekkering, G.E.; Van Tulder, M.W.; De Vet, H.C.W. Minimal Clinically Important Change for Pain Intensity, Functional Status, and General Health Status in Patients With Nonspecific Low Back Pain. Spine 2006, 31, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Senna, M.; Machaly, S. Does maintained spinal manipulation therapy for chronic nonspecific low back pain result in better long-term outcome? Spine 2011, 36, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Licciardone, J.C.; Stoll, S.T.; Fulda, K.G.; Russo, D.P.; Siu, J.; Winn, W.; Swift, J. Osteopathic manipulative treatment for chronic low back pain: A randomized controlled trial. Spine 2003, 28, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, K.E.; Zwarenstein, M.; Oxman, A.D.; Treweek, S.; Furberg, C.D.; Altman, D.G.; Tunis, S.; Bergel, E.; Harvey, I.; Magid, D.J.; et al. A pragmatic-explanatory continuum indicator summary (PRECIS): A tool to help trial designers. J. Clin. Epidemiol. 2009, 62, 464–475. [Google Scholar] [CrossRef]
- Franke, H.; Franke, J.-D.; Fryer, G. Osteopathic manipulative treatment for nonspecific low back pain: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2014, 15, 286. [Google Scholar] [CrossRef]
- Kirsch, I. The placebo effect and the cognitive-behavioral revolution. Cogn. Ther. Res. 1978, 2, 255–264. [Google Scholar] [CrossRef]
- Learman, K.E.; Myers, J.B.; Lephart, S.M.; Sell, T.C.; Kerns, G.J.; Cook, C.E. Effects of Spinal Manipulation on Trunk Proprioception in Subjects With Chronic Low Back Pain During Symptom Remission. J. Manip. Physiol. Ther. 2009, 32, 118–126. [Google Scholar] [CrossRef]
- Hancock, M.J.; Maher, C.G.; Latimer, J.; McAuley, J.H. Selecting an appropriate placebo for a trial of spinal manipulative therapy. Aust. J. Physiother. 2006, 52, 135–138. [Google Scholar] [CrossRef]
- Zaorsky, N.; Showalter, T. How will Comparative Effectiveness Research Influence Clinical Decision Making? Med. Forum 2012, 13, 22. [Google Scholar] [CrossRef][Green Version]
| Characteristic | Usual Care + CMT (n = 97) n (%) or Mean (SD) | Usual Care (n = 97) n (%) or Mean (SD) |
|---|---|---|
| Sex (male) | 17 (17.5) | 23 (23.7) |
| Age (years) | 44.5 ± 12.5 | 39.0 ± 11.6 |
| Height (cm) | 162.5 ± 7.5 | 164.6 ± 7.7 |
| Weight (kg) | 62.3 ± 11.4 | 61.5 ± 13.0 |
| BMI (kg/m) | 23.47 ± 3.24 | 22.58 ± 3.67 |
| Smoking | ||
| Non-smoker | 87 (89.7) | 82 (85.4) |
| Ex-smoker | 8 (8.3) | 10 (10.4) |
| Smoker | 2 (2.1) | 4 (4.2) |
| Alcohol consumption | ||
| No | 67 (69.1) | 53 (54.6) |
| Yes | 30 (30.9) | 44 (45.4) |
| Duration LBP (years) | 6.45 ± 6.66 | 4.85 ± 5.10 |
| Primary outcome | ||
| NRS of lower back pain | 5.88 ± 0.89 | 5.64 ± 1.00 |
| Secondary outcomes | ||
| NRS of radiating leg pain | 4.18 ± 2.38 | 3.51 ± 2.26 |
| PGIC (5 weeks) | 2.24 ± 0.84 | 2.95 ± 0.80 |
| ODI | 13.09 ± 4.41 | 13.12 ± 5.22 |
| ODI (%) | 26.19 ± 8.81 | 26.25 ± 10.45 |
| ROM (Flexion) | 85.15 ± 18.24 | 86.96 ± 16.37 |
| ROM (Extension) | 18.14 ± 5.74 | 19.69 ± 6.61 |
| ROM (Lateroflexion Rt.) | 24.18 ± 7.10 | 24.8 ± 5.93 |
| ROM (Lateroflexion Lt.) | 24.69 ± 6.80 | 25.37 ± 5.64 |
| ROM (Rotation Rt.) | 39.79 ± 11.51 | 41.44 ± 10.26 |
| ROM (Rotation Lt.) | 39.53 ± 11.68 | 41.7 ± 11.13 |
| EQ-5D | 0.84 ± 0.09 | 0.85 ± 0.09 |
| UC+CMT (n = 97) Mean ± SD | UC (n = 97) Mean ± SD | p-Value | |
|---|---|---|---|
| NRS (LBP) | |||
| Baseline | 5.88 ± 0.89 | 5.64 ± 1.00 | |
| 7th week | 2.86 ± 1.84 | 4.28 ± 1.75 | |
| Difference | −3.02 ± 1.72 | −1.36 ± 1.75 | <0.0001 (1) |
| 12th week | 3.14 ± 2.09 | 4.29 ± 1.96 | |
| Difference | −2.73 ± 2 | −1.35 ± 1.9 | <0.0001 (2) |
| 24th week | 3.44 ± 2.18 | 4.52 ± 1.92 | |
| Difference | −2.43 ± 2.09 | −1.12 ± 1.76 | <0.0001 (3) |
| Radiating pain leg NRS | |||
| Baseline | 4.38 ± 2.24 | 3.68 ± 2.18 | |
| 7th week | 2.29 ± 2.01 | 3.11 ± 2.18 | |
| Difference | −2.00 ± 2.33 | −0.44 ± 1.86 | <0.0001 (1) |
| 12th week | 2.46 ± 2.06 | 3.05 ± 2.18 | |
| Difference | −1.78 ± 2.11 | −0.44 ± 1.97 | <0.0001 (2) |
| 24th week | 2.64 ± 2.43 | 3.5 ± 2.21 | |
| Difference | −1.67 ± 2.56 | −0.07 ± 2.34 | <0.0001 (3) |
| PGIC | |||
| 5th week | 2.24 ± 0.84 | 2.95 ± 0.8 | |
| 7th week | 1.96 ± 0.82 | 2.98 ± 0.78 | |
| Difference | −0.28 ± 0.85 | 0.01 ± 0.66 | 0.0119 (4) |
| 12th week | 2.1 ± 0.87 | 2.92 ± 0.88 | |
| Difference | −0.13 ± 1.01 | −0.05 ± 0.78 | 0.5185 (5) |
| ODI | |||
| Baseline | 13.09 ± 4.41 | 13.12 ± 5.22 | |
| 7th week | 7.44 ± 5.29 | 9.4 ± 5.58 | |
| Difference | −5.65 ± 4.29 | −3.72 ± 4.63 | 0.003 (1) |
| 12th week | 7.22 ± 5.36 | 8.73 ± 5.71 | |
| Difference | −5.88 ± 4.42 | −4.39 ± 4.78 | 0.0257 (2) |
| EQ-5D | |||
| Baseline | 0.84 ± 0.09 | 0.85 ± 0.09 | |
| 7th week | 0.90 ± 0.08 | 0.89 ± 0.07 | |
| Difference | 0.05 ± 0.07 | 0.04 ± 0.09 | 0.2056 (1) |
| 12th week | 0.9 ± 0.07 | 0.89 ± 0.08 | |
| Difference | 0.06 ± 0.07 | 0.04 ± 0.1 | 0.0732 (2) |
| 24th week | 0.91 ± 0.06 | 0.9 ± 0.07 | |
| Difference | 0.07 ± 0.08 | 0.05 ± 0.09 | 0.0587 (3) |
| ROM (Rotation Right) | |||
| Baseline | 39.79 ± 11.51 | 41.44 ± 10.26 | |
| 7th week | 42.45 ± 10.34 | 41.22 ± 10 | |
| Difference | 2.42 ± 8.86 | −0.35 ± 7.78 | 0.0289 (1) |
| 12th week | 42.3 ± 10.86 | 42.13 ± 10.94 | |
| Difference | 3.26 ± 9.51 | −0.91 ± 9.53 | 0.113 (2) |
| ROM (Rotation Left) | |||
| Baseline | 39.53 ± 11.68 | 41.7 ± 11.13 | |
| 7th week | 42.88 ± 11.15 | 42.21 ± 11.02 | |
| Difference | 3.02 ± 8.56 | 0.23 ± 8.47 | 0.0307 (1) |
| 12th week | 43.16 ± 10.68 | 42.74 ± 11.39 | |
| Difference | 4.13 ± 9.8 | 1.16 ± 9.79 | 0.0512 (2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Hwang, E.-H.; Cho, J.-H.; Kim, K.-W.; Ha, I.-H.; Kim, M.-r.; Nam, K.; Lee, M.h.; Lee, J.-H.; Kim, N.; et al. Comparative Effectiveness of Chuna Manipulative Therapy for Non-Acute Lower Back Pain: A Multi-Center, Pragmatic, Randomized Controlled Trial. J. Clin. Med. 2020, 9, 144. https://doi.org/10.3390/jcm9010144
Park S-Y, Hwang E-H, Cho J-H, Kim K-W, Ha I-H, Kim M-r, Nam K, Lee Mh, Lee J-H, Kim N, et al. Comparative Effectiveness of Chuna Manipulative Therapy for Non-Acute Lower Back Pain: A Multi-Center, Pragmatic, Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(1):144. https://doi.org/10.3390/jcm9010144
Chicago/Turabian StylePark, Sun-Young, Eui-Hyoung Hwang, Jae-Heung Cho, Koh-Woon Kim, In-Hyuk Ha, Me-riong Kim, Kibong Nam, Min ho Lee, Jun-Hwan Lee, Namkwen Kim, and et al. 2020. "Comparative Effectiveness of Chuna Manipulative Therapy for Non-Acute Lower Back Pain: A Multi-Center, Pragmatic, Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 1: 144. https://doi.org/10.3390/jcm9010144
APA StylePark, S.-Y., Hwang, E.-H., Cho, J.-H., Kim, K.-W., Ha, I.-H., Kim, M.-r., Nam, K., Lee, M. h., Lee, J.-H., Kim, N., & Shin, B.-C. (2020). Comparative Effectiveness of Chuna Manipulative Therapy for Non-Acute Lower Back Pain: A Multi-Center, Pragmatic, Randomized Controlled Trial. Journal of Clinical Medicine, 9(1), 144. https://doi.org/10.3390/jcm9010144





