Effects of IL-1β, IL-20, and BMP-2 on Intervertebral Disc Inflammation under Hypoxia
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Antibody, and Protein Preparation
2.2. Immunohistochemical (IHC) Staining
2.3. Primary Culture of Human IVD Cells
2.4. Experimental Culture Conditions for Hypoxia Stimulation
2.5. Reverse Transcription and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
2.7. Statistical Analysis
3. Results
3.1. Hypoxia Effect On Primary Cultured Disc Cells
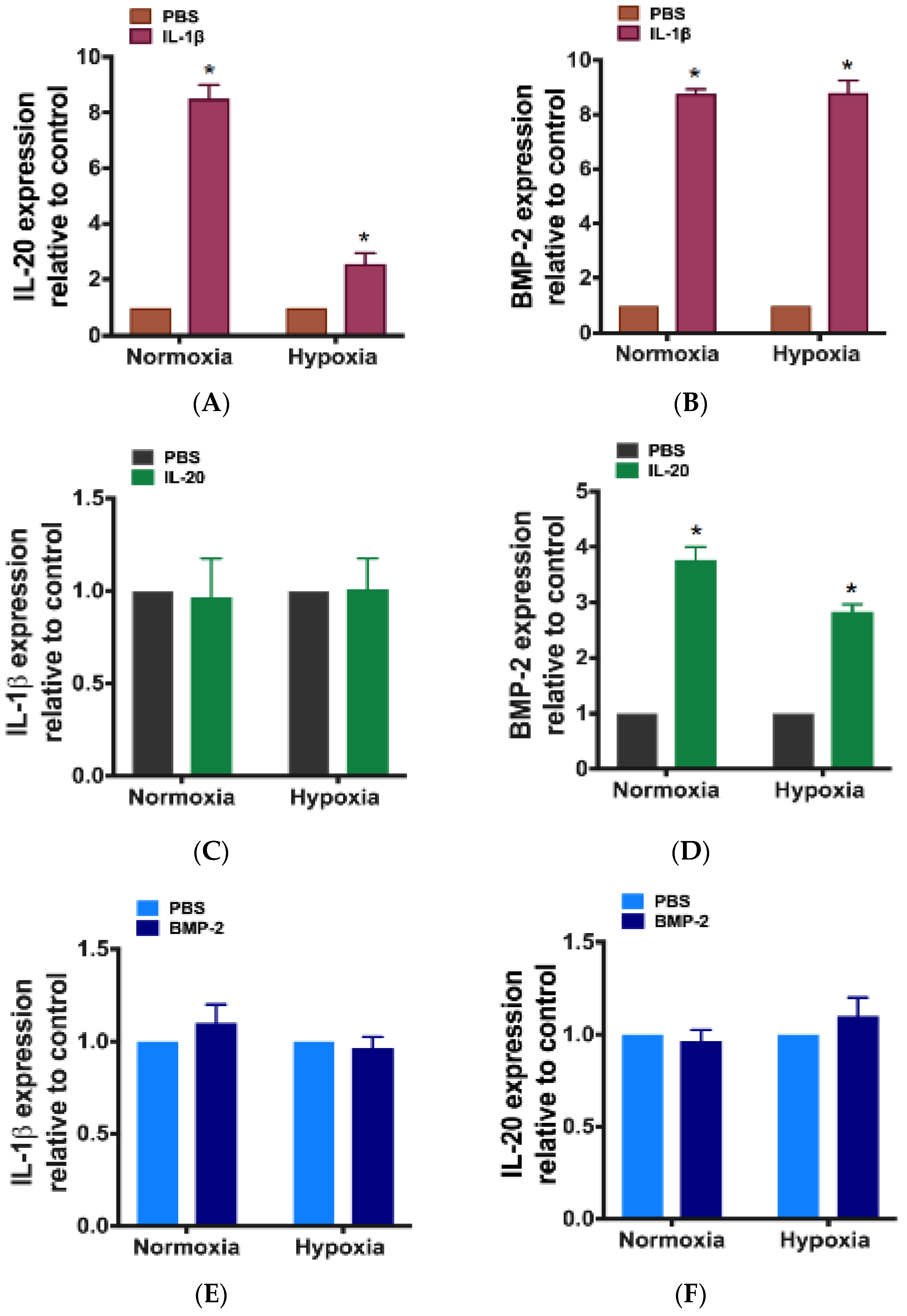
3.2. The Effects of IL-20, IL-1β, and BMP-2 in Primary Cultured Disc Cells under Hypoxia
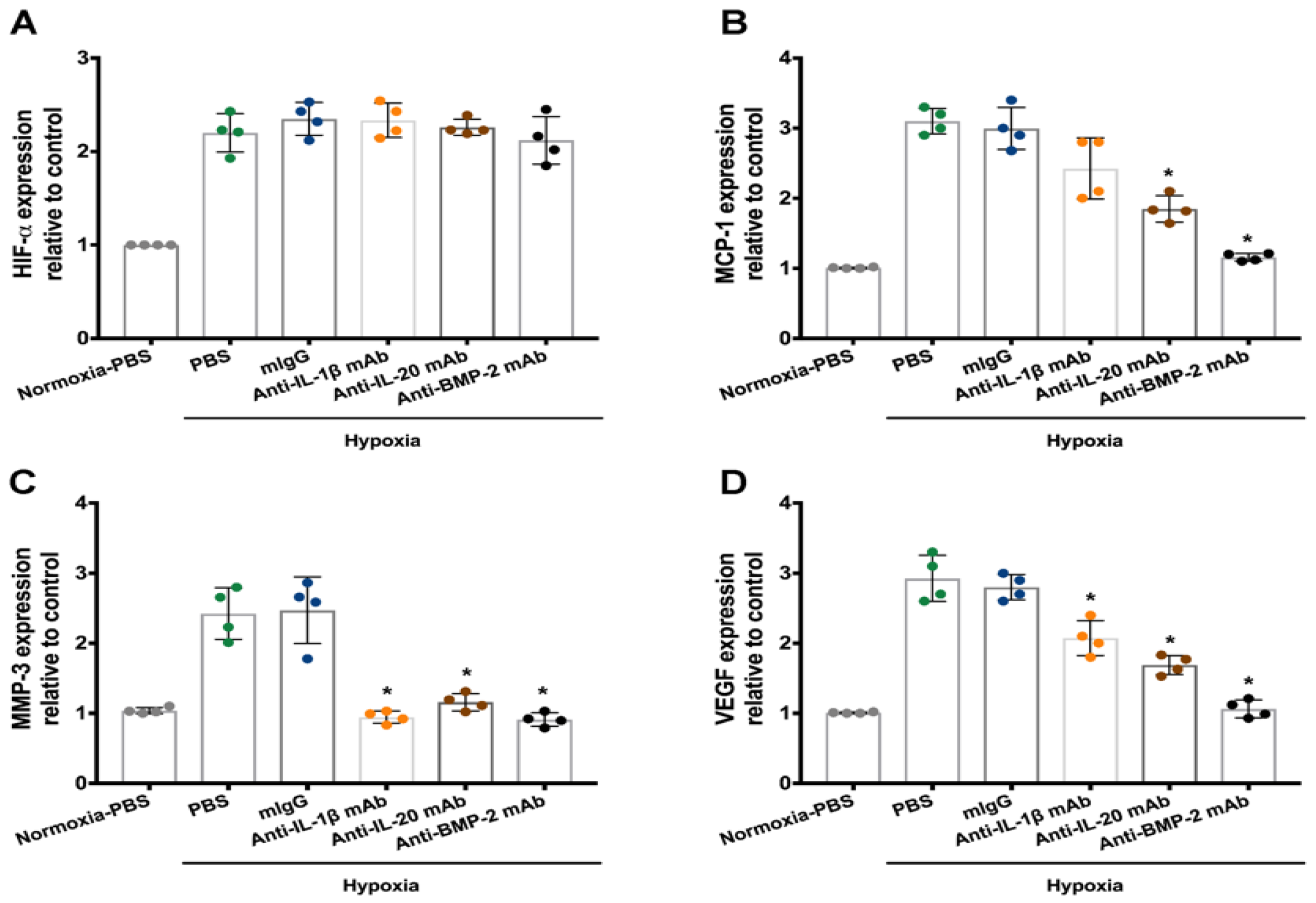
3.3. Treatment with Antibodies Against IL-1β, IL-20, and BMP-2 on Disc Cells under Hypoxia
3.4. The Relationship Between IL-1β, IL-20, and BMP-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luoma, K.; Riihimaki, H.; Luukkonen, R.; Raininko, R.; Viikari-Juntura, E.; Lamminen, A. Low back pain in relation to lumbar disc degeneration. Spine 2000, 25, 487–492. [Google Scholar] [CrossRef]
- Deyo, R.A.; Tsui-Wu, Y.J. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987, 12, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Akeson, W.H.; Woo, S.L.; Taylor, T.K.; Ghosh, P.; Bushell, G.R. Biomechanics and biochemistry of the intervertebral disks: The need for correlation studies. Clin. Orthop. Relat. Res. 1977, 129, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cs-Szabo, G.; Ragasa-San Juan, D.; Turumella, V.; Masuda, K.; Thonar, E.J.; An, H.S. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine 2002, 27, 2212–2219. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A., Jr.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Gonçalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20141191. [Google Scholar] [CrossRef]
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.W.; Chen, L.E.; Deyo, R.A.; Halabi, S.; Turner, J.A.; Avins, A.L.; James, K.; et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef]
- Cunha, C.; Silva, A.J.; Pereira, P.; Vaz, R.; Gonçalves, R.M.; Barbosa, M.A. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res. 2018, 20, 251–259. [Google Scholar] [CrossRef]
- Gronblad, M.; Virri, J.; Tolonen, J.; Seitsalo, S.; Kaapa, E.; Kankare, J.; Myllynen, P.; Karaharju, E.O. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine 1994, 19, 2744–2751. [Google Scholar] [CrossRef]
- Woertgen, C.; Rothoerl, R.D.; Brawanski, A. Influence of macrophage infiltration of herniated lumbar disc tissue on outcome after lumbar disc surgery. Spine 2000, 25, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bradley, A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 1996, 122, 2977–2986. [Google Scholar] [PubMed]
- Fukui, N.; Ikeda, Y.; Ohnuki, T.; Hikita, A.; Tanaka, S.; Yamane, S.; Suzuki, R.; Sandell, L.J.; Ochi, T. Pro-inflammatory cytokine tumor necrosis factor-alpha induces bone morphogenetic protein-2 in chondrocytes via mRNA stabilization and transcriptional up-regulation. J. Biol. Chem. 2006, 281, 27229–27241. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes Dev. 1996, 10, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Pizette, S.; Niswander, L. BMPs are required at two steps of limb chondrogenesis: Formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev. Biol. 2000, 219, 237–249. [Google Scholar] [CrossRef]
- Bostrom, M.P.; Lane, J.M.; Berberian, W.S.; Missri, A.A.; Tomin, E.; Weiland, A.; Doty, S.B.; Glaser, D.; Rosen, V.M. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1995, 13, 357–367. [Google Scholar] [CrossRef]
- Si, X.; Jin, Y.; Yang, L.; Tipoe, G.L.; White, F.H. Expression of BMP-2 and TGF-beta 1 mRNA during healing of the rabbit mandible. Eur. J. Oral Sci. 1997, 105, 325–330. [Google Scholar] [CrossRef]
- Huang, K.Y.; Yan, J.J.; Hsieh, C.C.; Chang, M.S.; Lin, R.M. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: An animal experiment. Spine 2007, 32, 1174–1180. [Google Scholar] [CrossRef]
- Chen, W.Y.; Cheng, B.C.; Jiang, M.J.; Hsieh, M.Y.; Chang, M.S. IL-20 is expressed in atherosclerosis plaques and promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2090–2095. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Li, H.H.; Hsieh, M.Y.; Liu, M.F.; Huang, K.Y.; Chin, L.S.; Chen, P.C.; Cheng, H.H.; Chang, M.S. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 2006, 54, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Hsu, Y.H.; Li, H.H.; Wang, Y.C.; Hsieh, M.Y.; Chen, W.Y.; Hsing, C.H.; Chang, M.S. IL-20: Biological functions and clinical implications. J. Biomed. Sci. 2006, 13, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Hsing, C.H.; Ho, C.L.; Chang, L.Y.; Lee, Y.L.; Chuang, S.S.; Chang, M.S. Tissue microarray analysis of interleukin-20 expression. Cytokine 2006, 35, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Parrish-Novak, J.; Xu, W.; Brender, T.; Yao, L.; Jones, C.; West, J.; Brandt, C.; Jelinek, L.; Madden, K.; McKernan, P.A.; et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J. Biol. Chem. 2002, 277, 47517–47523. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Y.; Lin, R.M.; Chen, W.Y.; Lee, C.L.; Yan, J.J.; Chang, M.S. IL-20 may contribute to the pathogenesis of human intervertebral disc herniation. Spine 2008, 33, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 2005, 7, R732–R745. [Google Scholar] [CrossRef] [PubMed]
- Franscini, N.; Wuertz, K.; Patocchi-Tenzer, I.; Durner, R.; Boos, N.; Graf-Hausner, U. Development of a novel automated cell isolation, expansion, and characterization platform. J. Lab. Autom. 2011, 16, 204–213. [Google Scholar] [CrossRef]
- Agrawal, A.; Guttapalli, A.; Narayan, S.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am. J. Physiol. Cell Physiol. 2007, 293, C621–C631. [Google Scholar] [CrossRef]
- Agrawal, A.; Gajghate, S.; Smith, H.; Anderson, D.G.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Cited2 modulates hypoxia-inducible factor-dependent expression of vascular endothelial growth factor in nucleus pulposus cells of the rat intervertebral disc. Arthritis Rheum. 2008, 58, 3798–3808. [Google Scholar] [CrossRef]
- Schipani, E.; Ryan, H.E.; Didrickson, S.; Kobayashi, T.; Knight, M.; Johnson, R.S. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001, 15, 2865–2876. [Google Scholar] [CrossRef]
- Meng, X.C.; Zhuang, L.L.; Wang, J.; Liu, Z.C.; Wang, Y.; Xiao, D.C.; Zhang, X.K. Hypoxia-inducible factor (HIF)-1alpha knockout accelerates intervertebral disc degeneration in mice. Int. J. Clin. Exp. Pathol. 2018, 11, 548–557. [Google Scholar]
- Minamide, A.; Hashizume, H.; Yoshida, M.; Kawakami, M.; Hayashi, N.; Tamaki, T. Effects of basic fibroblast growth factor on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine 1999, 24, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Haro, H.; Komori, H.; Shinomiya, K. Sequential dynamics of inflammatory cytokine, angiogenesis inducing factor and matrix degrading enzymes during spontaneous resorption of the herniated disc. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2004, 22, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways. Int. J. Oncol. 2016, 48, 5–12. [Google Scholar] [CrossRef]
- Hsieh, M.Y.; Chen, W.Y.; Jiang, M.J.; Cheng, B.C.; Huang, T.Y.; Chang, M.S. Interleukin-20 promotes angiogenesis in a direct and indirect manner. Genes Immun. 2006, 7, 234–242. [Google Scholar] [CrossRef]
- Murai, K.; Sakai, D.; Nakamura, Y.; Nakai, T.; Igarashi, T.; Seo, N.; Murakami, T.; Kobayashi, E.; Mochida, J. Primary immune system responders to nucleus pulposus cells: Evidence for immune response in disc herniation. Eur. Cells Mater. 2010, 19, 13–21. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-H.; Lin, R.-M.; Chiu, Y.-S.; Liu, W.-L.; Huang, K.-Y. Effects of IL-1β, IL-20, and BMP-2 on Intervertebral Disc Inflammation under Hypoxia. J. Clin. Med. 2020, 9, 140. https://doi.org/10.3390/jcm9010140
Hsu Y-H, Lin R-M, Chiu Y-S, Liu W-L, Huang K-Y. Effects of IL-1β, IL-20, and BMP-2 on Intervertebral Disc Inflammation under Hypoxia. Journal of Clinical Medicine. 2020; 9(1):140. https://doi.org/10.3390/jcm9010140
Chicago/Turabian StyleHsu, Yu-Hsiang, Ruey-Mo Lin, Yi-Shu Chiu, Wen-Lung Liu, and Kuo-Yuan Huang. 2020. "Effects of IL-1β, IL-20, and BMP-2 on Intervertebral Disc Inflammation under Hypoxia" Journal of Clinical Medicine 9, no. 1: 140. https://doi.org/10.3390/jcm9010140
APA StyleHsu, Y.-H., Lin, R.-M., Chiu, Y.-S., Liu, W.-L., & Huang, K.-Y. (2020). Effects of IL-1β, IL-20, and BMP-2 on Intervertebral Disc Inflammation under Hypoxia. Journal of Clinical Medicine, 9(1), 140. https://doi.org/10.3390/jcm9010140




