Identification of the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA Revised (PISQ-IR) Cutoff Scores for Impaired Sexual Function in Women with Pelvic Floor Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. The Applied Questionnaires
2.2. Statistical Analysis
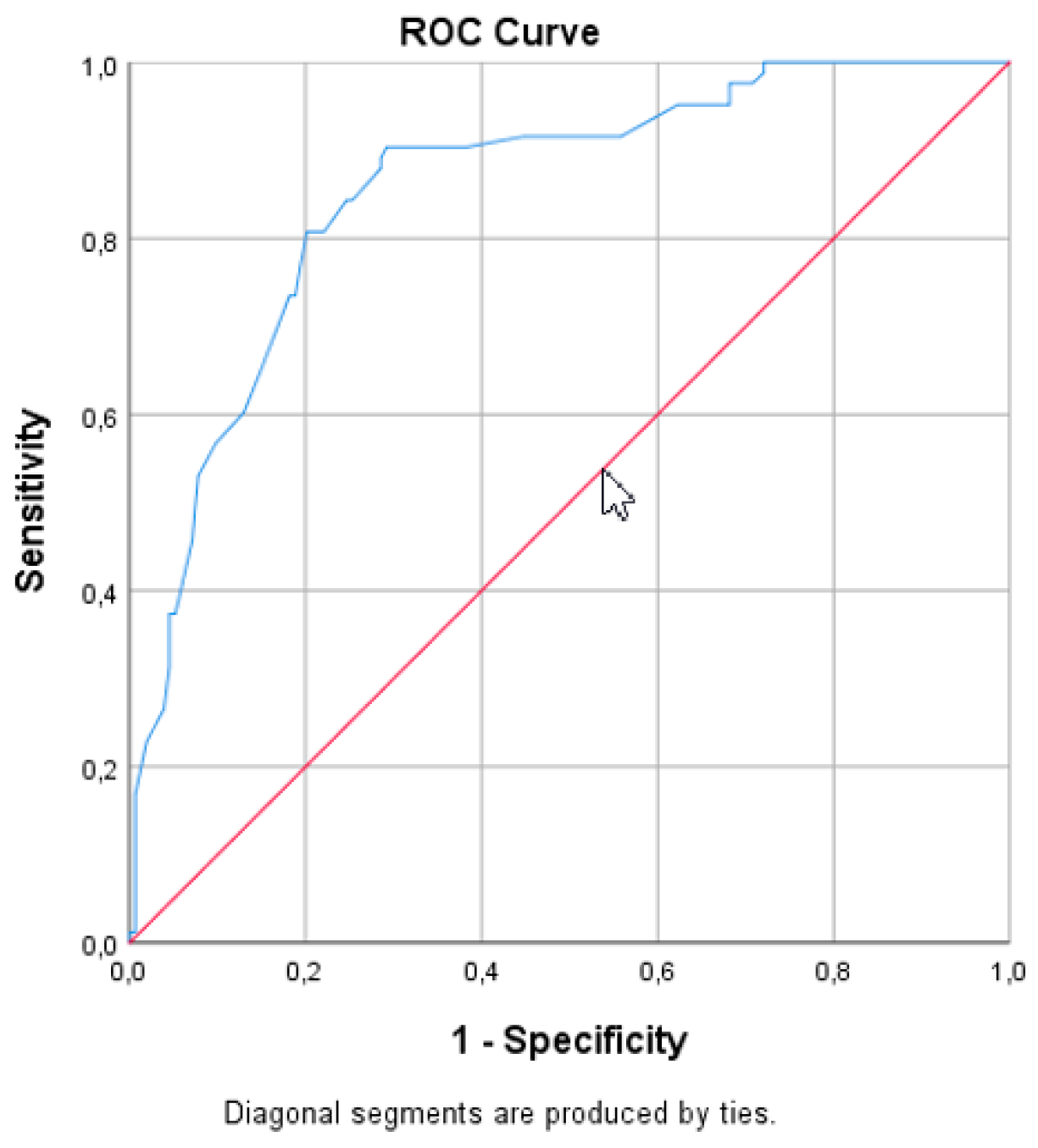
3. Results
4. Discussion
5. Concluding Message
Author Contributions
Funding
Conflicts of Interest
References
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet. Gynecol. 2014, 123, 141–148. [Google Scholar] [CrossRef]
- Salonia, A.; Zanni, G.; Nappi, R.E.; Briganti, A.; Dehò, F.; Fabbri, F.; Colombo, R.; Guazzoni, G.; Di Girolamo, V.; Rigatti, P.; et al. Sexual dysfunction is common in women with lower urinary tract symptoms and urinary incontinence: Results of a cross-sectional study. Eur. Urol. 2004, 45, 642–648. [Google Scholar] [CrossRef]
- Li-Yun-Fong, R.J.; Larouche, M.; Hyakutake, M.; Koenig, N.; Lovatt, C.; Geoffrion, R.; Brotto, L.A.; Lee, T.; Cundiff, G.W. Is Pelvic Floor Dysfunction an Independent Threat to Sexual Function? A Cross-Sectional Study in Women with Pelvic Floor Dysfunction. J. Sex. Med. 2017, 14, 226–237. [Google Scholar] [CrossRef]
- Handa, V.L.; Cundiff, G.; Chang, H.H.; Helzlsouer, K.J. Female sexual function and pelvic floor disorders. Obstet. Gynecol. 2008, 111, 1045–1052. [Google Scholar] [CrossRef]
- Bradley, S.L.; Weidner, A.C.; Siddiqui, N.Y.; Gandhi, M.P.; Wu, J.M. Shifts in national rates of inpatient prolapse surgery emphasize current coding inadequacies. Female Pelvic Med. Reconstr. Surg. 2011, 17, 204–208. [Google Scholar] [CrossRef]
- Løwenstein, E.; Ottesen, B.; Gimbel, H. Incidence and lifetime risk of pelvic organ prolapse surgery in Denmark from 1977 to 2009. Int. Urogynecol. J. 2015, 26, 49–55. [Google Scholar] [CrossRef]
- Wilkins, M.F.; Wu, J.M. Lifetime risk of surgery for stress urinary incontinence or pelvic organ prolapse. Minerva Ginecol. 2017, 69, 171–177. [Google Scholar]
- Kammerer-Doak, D. Assessment of sexual function in women with pelvic floor dysfunction. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2009, 20 (Suppl. 1), S45–S50. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, M.; Meston, C.; Rosen, R. The female sexual function index (FSFI): Cross-validation and development of clinical cutoff scores. J. Sex. Marital Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Miotla, P.; Cartwright, R.; Skorupska, K.; Bogusiewicz, M.; Markut-Miotla, E.; Futyma, K.; Rechberger, T. Impact of intravesical onabotulinumtoxinA on sexual function in women with OAB. Neurourol. Urodyn. 2017, 36, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.G.; Rockwood, T.H.; Constantine, M.L.; Thakar, R.; Kammerer-Doak, D.N.; Pauls, R.N.; Parekh, M.; Ridgeway, B.; Jha, S.; Pitkin, J.; et al. A new measure of sexual function in women with pelvic floor disorders (PFD): The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire, IUGA-Revised (PISQ-IR). Int. Urogynecol. J. 2013, 24, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Grzybowska, M.E.; Wydra, D.G. Is voluntary pelvic floor muscles contraction important for sexual function in women with pelvic floor disorders? Neurourol. Urodyn. 2019, 38, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.G.; Pauls, R.N.; Thakar, R.; Morin, M.; Kuhn, A.; Petri, E.; Fatton, B.; Whitmore, K.; Kingsberg, S.A.; Lee, J. An international Urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int. Urogynecol. J. 2018, 29, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; Maher, C.F.; Barber, M.D.; Camargo, S.; Dandolu, V.; Digesu, A.; Goldman, H.B.; Huser, M.; Milani, A.L.; Moran, P.A.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Organ Prolapse (POP). Neurourol. Urodyn. 2016, 35, 137–168. [Google Scholar] [CrossRef] [PubMed]
- Nowosielski, K.; Wróbel, B.; Sioma-Markowska, U.; Poręba, R. Development and validation of the Polish version of the Female Sexual Function Index in the Polish population of females. J. Sex. Med. 2013, 10, 386–395. [Google Scholar] [CrossRef]
- Neijenhuijs, K.I.; Hooghiemstra, N.; Holtmaat, K.; Aaronson, N.K.; Groenvold, M.; Holzner, B.; Terwee, C.B.; Cuijpers, P.; Verdonck-de Leeuw, I.M. The Female Sexual Function Index (FSFI)-A Systematic Review of Measurement Properties. J. Sex. Med. 2019, 16, 640–660. [Google Scholar] [CrossRef]
- Rockwood, T.H.; Constantine, M.L.; Adegoke, O.; Rogers, R.G.; McDermott, E.; Davila, G.W.; Domoney, C.; Jha, S.; Kammerer-Doak, D.; Lukacz, E.S.; et al. The PISQ-IR: Considerations in scale scoring and development. Int. Urogynecol. J. 2013, 24, 1105–1122. [Google Scholar] [CrossRef]
- Grzybowska, M.E.; Piaskowska-Cala, J.; Wydra, D.G. Polish translation and validation of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire, IUGA-Revised (PISQ-IR). Int. Urogynecol. J. 2019, 30, 55–64. [Google Scholar] [CrossRef]
- International Urogynecological Association (IUGA). Available online: https://www.iuga.org/publications/pisq-ir-papers (accessed on 15 December 2019).
- Constantine, M.L.; Pauls, R.N.; Rogers, R.R.; Rockwood, T.H. Validation of a single summary score for the Prolapse/Incontinence Sexual Questionnaire-IUGA revised (PISQ-IR). Int. Urogynecol. J. 2017, 28, 1901–1907. [Google Scholar] [CrossRef]
- Kelleher, C.J.; Cardozo, L.D.; Khullar, V.; Salvatore, S. A new questionnaire to assess the quality of life of urinary incontinent women. Br. J. Obstet. Gynaecol. 1997, 104, 1374–1379. [Google Scholar] [CrossRef]
- Diaz, D.C.; Robinson, D.; Bosch, R.; Costantiini, E.; Ctterill, N.; Espuña-Pons, M.; Kocjancic, E.; Lemos, N.; Tarcan, T.; Yoshida, M. Patient-reported outcome assessment. In Incontinence, 6th ed.; Abrams, P., Cardozo, L., Wagg, A., Wein, A., Eds.; ICUD ICS: Tokyo, Japan, 2017; Volume 1, pp. 541–670. [Google Scholar]
- Mamik, M.M.; Rogers, R.G.; Qualls, C.R.; Morrow, J.D. The minimum important difference for the Pelvic Organ Prolapse-Urinary Incontinence Sexual Function Questionnaire. Int. Urogynecol. J. 2014, 25, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, L.; Gamble, T.; Sanses, T.V.; van Raalte, H.; Carberry, C.; Jakus, S.; Kambiss, S.; McAchran, S.; Pham, T.; Aschkenazi, S.; et al. Sexual function is related to body image perception in women with pelvic organ prolapse. J. Sex. Med. 2009, 6, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, A.; Weir, N.; Tangyotkajohn, U.; Jacques, A.; Thompson, J.; Briffa, K. Help-seeking behaviour for pelvic floor dysfunction in women over 55: Drivers and barriers. Int. Urogynecol. J. 2018, 29, 1645–1653. [Google Scholar] [CrossRef] [PubMed]

| Domain | Questions | Minimum Score | Maximum Score |
|---|---|---|---|
| Desire | 1, 2 | 1.2 | 6.0 |
| Arousal | 3, 4, 5, 6 | 0 | 6.0 |
| Lubrication | 7, 8, 9, 10 | 0 | 6.0 |
| Orgasm | 11, 12, 13 | 0 | 6.0 |
| Satisfaction | 14, 15, 16 | 0.8 | 6.0 |
| Pain | 17, 18, 19 | 0 | 6.0 |
| Sexually Active (SA) Domains | SA–21 Items | Questions | Minimum Score | Maximum Score |
|---|---|---|---|---|
| Arousal Orgasm | SA-AO, 4 items | Q 7, 8a, 10, 11 | 1 | 5 |
| Condition-specific | SA-CS, 3 items | Q 8b, 8c, 9 | 1 | 5 |
| Partner-related | SA-PR, 3 items | Q 13, 14a,14b | 1/− * | 4/− * |
| Desire | SA-D, 3 items | Q 15, 16, 17 | 1 | 5 |
| Condition Impact | SA-CI, 4 items | Q 18, 20b, 20c, 20d | 1 | 4 |
| Global Quality | SA-GQ, 4 items | Q 19a, 19b, 19c, 20a | 1 | 4.8 |
| Summary Score | 1.545 | 3.909 | ||
| 1.722 ** | 3.888 ** | |||
| Variable | FSFI < 26.55 (n = 143) | FSFI > 26.55 (n = 83) | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 56.8 ± 10.8 | 56.3 ± 10.6 | 0.72 a |
| BMI (kg/m2), mean ± SD | 27.8 ± 4.6 | 26.7 ± 4.0 | 0.06 a |
| Postmenopausal n (%) | 107 (74.8) | 50 (60.2) | 0.02 b |
| Education n (%) | 0.09 b | ||
| Primary | 9 (6.3) | 1 (1.2) | |
| Secondary | 79 (55.2) | 41 (49.4) | |
| Higher | 55 (38.5) | 41 (49.4) | |
| Parity, mean ± SD (median) | 2.4 ± 1.0 (2) | 2.1 ± 0.8 (2) | 0.02 a |
| Vaginal delivery | 131 (91.6) | 69 (83.1) | 0.09 b |
| Cesarean delivery | 2 (1.4) | 4 (4.8) | |
| Both | 10 (7.0) | 8 (9.6) | |
| None | − | 2 (2.4) | |
| Marital status n (%) | 0.54 b | ||
| married | 131 (91.6) | 74 (89.2) | |
| partnership | 12 (8.4) | 9 (10.8) | |
| Previous surgical history | |||
| None | 93 (65.0) | 58 (69.9) | 0.47 b |
| Hysterectomy | 29 (20.3) | 9 (10.8) | 0.08 b |
| Prior prolapse surgery | 35 (24.5) | 17 (20.5) | 0.49 b |
| Prior anti-UI surgery | 7 (4.9) | 5 (6.0) | 0.71 b |
| Clinical diagnosis n (%) | |||
| Only UI | 34 (23.8) | 19 (22.9) | 0.62 b |
| Only POP | 56 (39.2) | 32 (38.5) | |
| UI and POP | 53 (37.1) | 31 (37.3) | |
| FI | 18 (12.6) | 5 (6.0) | 0.11 b |
| POP-Q n (%) | 0.08 b | ||
| 0 | 2 (1.4) | 6 (7.2) | |
| I | 7 (4.9) | 7 (8.4) | |
| II | 52 (36.4) | 21 (25.3) | |
| III | 68 (47.6) | 42 (50.6) | |
| IV | 14 (9.8) | 7 (8.4) |
| Variable | FSFI < 26.55 (n = 143) | FSFI > 26.55 (n = 83) | p-Value |
|---|---|---|---|
| PISQ-IR SA Mean ± SD | |||
| Arousal Orgasm | 2.77 ± 0.65 | 3.71 ± 0.54 | 0.000 a |
| Condition-specific | 4.11 ± 0.97 | 4.38 ± 0.70 | 0.017 a |
| Partner-related | 3.21 ± 0.50 | 3.50 ± 0.41 | 0.000 a |
| Desire | 2.70 ± 0.68 | 3.29 ± 0.56 | 0.000 a |
| Condition Impact | 2.45 ± 0.78 | 2.99 ± 0.77 | 0.000 a |
| Global Quality | 2.78 ± 0.90 | 3.64 ± 1.01 | 0.000 a |
| PISQ-IR Summary Score | 2.55 ± 0.38 | 3.06 ± 0.31 | 0.000a |
| FSFI Mean ± SD | |||
| Desire | 2.76 ± 0.91 | 3.84 ± 0.87 | 0.000 a |
| Arousal | 3.09 ± 0.96 | 4.74 ± 0.72 | 0.000 a |
| Lubrication | 3.56 ± 1.10 | 5.13 ± 0.80 | 0.000 a |
| Orgasm | 3.33 ± 1.23 | 5.13 ± 0.70 | 0.000 a |
| Satisfaction | 3.98 ± 1.06 | 5.30 ± 0.79 | 0.000 a |
| Pain | 3.94 ± 1.34 | 5.44 ± 0.69 | 0.000 a |
| Total Score | 20.65 ± 4.34 | 29.58 ± 2.01 | 0.000 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzybowska, M.E.; Futyma, K.; Wydra, D. Identification of the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA Revised (PISQ-IR) Cutoff Scores for Impaired Sexual Function in Women with Pelvic Floor Disorders. J. Clin. Med. 2020, 9, 13. https://doi.org/10.3390/jcm9010013
Grzybowska ME, Futyma K, Wydra D. Identification of the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA Revised (PISQ-IR) Cutoff Scores for Impaired Sexual Function in Women with Pelvic Floor Disorders. Journal of Clinical Medicine. 2020; 9(1):13. https://doi.org/10.3390/jcm9010013
Chicago/Turabian StyleGrzybowska, Magdalena Emilia, Konrad Futyma, and Dariusz Wydra. 2020. "Identification of the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA Revised (PISQ-IR) Cutoff Scores for Impaired Sexual Function in Women with Pelvic Floor Disorders" Journal of Clinical Medicine 9, no. 1: 13. https://doi.org/10.3390/jcm9010013
APA StyleGrzybowska, M. E., Futyma, K., & Wydra, D. (2020). Identification of the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA Revised (PISQ-IR) Cutoff Scores for Impaired Sexual Function in Women with Pelvic Floor Disorders. Journal of Clinical Medicine, 9(1), 13. https://doi.org/10.3390/jcm9010013





