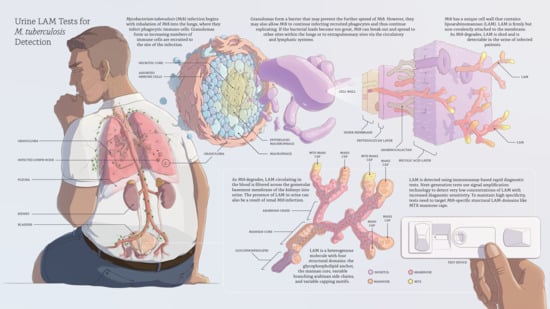
Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update
Abstract
1. Introduction
2. Lipoarabinomannan in Active TB Disease
3. First-Generation LAM Tests
4. The Need for Next-Generation, Highly Sensitive, and Specific LAM Tests
5. Considerations for Evaluating LAM Assays
6. Future Directions and Potential Impact of LAM Tests
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Huerga, H.; Ferlazzo, G.; Bevilacqua, P.; Kirubi, B.; Ardizzoni, E.; Wanjala, S.; Sitienei, J.; Bonnet, M. Incremental Yield of Including Determine-TB LAM Assay in Diagnostic Algorithms for Hospitalized and Ambulatory HIV-Positive Patients in Kenya. PLoS ONE 2017, 12, e0170976. [Google Scholar] [CrossRef] [PubMed]
- Herchline, T.; Amorosa, J. Tuberculosis (TB). Medscape, 15 August 2019. Available online: https://emedicine.medscape.com/article/230802-overview (accessed on 29 December 2019).
- World Health Organization (WHO). High-Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Robert Wood Johnson Foundation (RWJF) Microbiology Immunology & Infectious Diseases; Stanford Medicine; Duke University; UW Medicine; UCSF. Tuberculosis: Mycobacterial Cell Envelope. Available online: https://www.youtube.com/watch?v=yuHUikQy2vk (accessed on 29 December 2019).
- Correia-Neves, M.; Fröberg, G.; Korshun, L.; Viegas, S.; Vaz, P.; Ramanlal, N.; Bruchfeld, J.; Hamasur, B.; Brennan, P.; Källenius, G. Biomarkers for tuberculosis: the case for lipoarabinomannan. ERJ Open Res. 2019, 5, 115–2018. [Google Scholar] [CrossRef] [PubMed]
- Besra, G.S.; Brennan, P.J. The Mycobacterial Cell Envelope. J. Pharm. Pharmacol. 1997, 49, 25–30. [Google Scholar] [CrossRef]
- Briken, V.; Porcelli, S.; Besra, G.S.; Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol. Microbiol. 2004, 53, 391–403. [Google Scholar] [CrossRef]
- Chatterjee, D.; Khoo, K.H. Mycobacterial lipoarabinomannan: An extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 1998, 8, 113–120. [Google Scholar] [CrossRef]
- Venisse, A.; Berjeaud, J.M.; Chaurand, P.; Gilleron, M.; Puzo, G. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J. Biol. Chem. 1993, 268, 12401–12411. [Google Scholar]
- Lawn, S.D. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: A state of the art review. BMC Infect. Dis. 2012, 12, 103. [Google Scholar] [CrossRef]
- Treumann, A.; Xidong, F.; McDonnell, L.; Derrick, P.J.; Ashcroft, A.E.; Chatterjee, D.; Homans, S.W. 5-methylthiopentose: A new substituent on lipoarabinomannan in Mycobacterium tuberculosis. J. Mol. Biol. 2002, 316, 89–100. [Google Scholar] [CrossRef]
- Joe, M.; Sun, D.; Taha, H.; Completo, G.C.; Croudace, J.E.; Lammas, D.A.; Besra, G.S.; Lowary, T.L. The 5-Deoxy-5-methylthio-xylofuranose Residue in Mycobacterial Lipoarabinomannan. Absolute Stereochemistry, Linkage Position, Conformation, and Immunomodulatory Activity. J. Am. Chem. Soc. 2006, 128, 5059–5072. [Google Scholar] [CrossRef]
- De, P.; Shi, L.; Boot, C.; Ordway, D.; McNeil, M.; Chatterjee, D. Comparative Structural Study of Terminal Ends of Lipoarabinomannan from Mice Infected Lung Tissues and Urine of a Tuberculosis Positive Person. ACS Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Lukande, R.L.; Kalungi, S.; Van Marck, E.; Van de Vijver, K.; Kambugu, A.; Nelson, A.M.; Colebunders, R.; Manabe, Y.C. Is Urinary Lipoarabinomannan the Result of Renal Tuberculosis? Assessment of the Renal Histology in an Autopsy Cohort of Ugandan HIV-Infected Adults. PLoS ONE 2015, 10, e0123323. [Google Scholar] [CrossRef] [PubMed]
- Treatment Action Group (TAG). The LAM Test: Vital for Diagnosing TB in People with Advanced HIV. Available online: https://www.treatmentactiongroup.org/wp-content/uploads/2017/09/LAM-Guide-V3-1.pdf (accessed on 29 December 2019).
- Reddy, K.; Denkinger, C.M.; Broger, T.; McCann, N.; Gupta-Wright, A.; Shebl, F.; Fielding, K.; Nicol, M.P.; Wood, R.; Walensky, R. A higher-sensitivity urine LAM assay for TB testing in hospitalized patients with HIV: Cost-effectiveness analysis. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 2 November 2019. [Google Scholar]
- FIND. Accessible Pricing. Available online: https://www.finddx.org/find-negotiated-product-pricing (accessed on 29 December 2019).
- Treatment Action Group (TAG). Pipeline Report: Tuberculosis Diagnostics. Available online: http://www.treatmentactiongroup.org/sites/default/files/pipeline_tb_diagnotics_2019_db_final.pdf (accessed on 29 December 2019).
- Bjerrum, S.; Schiller, I.; Dendukuri, N.; Kohli, M.; Nathavitharana, R.R.; Zwerling, A.A.; Denkinger, C.M.; Steingart, K.R.; Shah, M. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV (Review). Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Broger, T.; Nicol, M.P.; Szekely, R.; Bjerrum, S.; Sossen, B.; Schutz, C.; Opintan, J.A.; Johansen, I.S.; Mitarai, S.; Chikamatsu, K.; et al. Diagnostic accuracy of a novel point-of-care urine lipoarabinomannan assay for people living with HIV–a meta-analysis of in-and outpatient data. PLoS Med. Submitted.
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N.; et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: A prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Use of Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis and Screening of Active Tuberculosis in People Living with HIV: Policy Guidance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Minion, J.; Leung, E.; Talbot, E.; Dheda, K.; Pai, M.; Menzies, D. Diagnosing tuberculosis with urine lipoarabinomannan: Systematic review and meta-analysis. Eur. Respir. J. 2011, 38, 1398–1405. [Google Scholar] [CrossRef]
- Shah, M.; Hanrahan, C.; Wang, Z.Y.; Dendukuri, N.; Lawn, S.D.; Denkinger, C.M.; Steingart, K.R. The lateral flow urine lipoarabinomannan (LF-LAM) test for diagnosis of tuberculosis in people living with human immunodeficiency virus (HIV). Cochrane Database Syst. Rev. 2016, 5. [Google Scholar] [CrossRef]
- Broger, T.; Sossen, B.; du Toit, E.; Kerkhoff, A.D.; Schutz, C.; Ivanova Reipold, E.; Ward, A.; Barr, D.A.; Macé, A.; Trollip, A.; et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: A diagnostic accuracy study. Lancet Infect. Dis. 2019, 19, 852–861. [Google Scholar] [CrossRef]
- Peter, J.G.; Zijenah, L.S.; Chanda, D.; Clowes, P.; Lesosky, M.; Gina, P.; Mehta, N.; Calligaro, G.; Lombard, C.J.; Kadzirange, G.; et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: A pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016, 387, 1187–1197. [Google Scholar] [CrossRef]
- Gupta-Wright, A.; Corbett, E.L.; van Oosterhout, J.J.; Wilson, D.; Grint, D.; Alufandika-Moyo, M.; Peters, J.A.; Chiume, L.; Flach, C.; Lawn, S.D.; et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): A pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018, 392, 292–301. [Google Scholar] [CrossRef]
- Grant, A.D.; Charalambous, S.; Tlali, M.; Karat, A.S.; Dorman, S.E.; Hoffmann, C.J.; Johnson, S.; Vassall, A.; Churchyard, G.J.; Fielding, K.L. Algorithm-guided empirical tuberculosis treatment for people with advanced HIV (TB Fast Track): An open-label, cluster-randomised trial. Lancet HIV 2019. [Google Scholar] [CrossRef]
- Bjerrum, S.; Broger, T.; Szekely, R.; Mitarai, S.; Opintan, J.A.; Kenu, E.; Lartey, M.; Addo, K.K.; Chikamatsu, K.; Macé, A.; et al. Diagnostic accuracy of a novel and rapid lipoarabinomannan test for diagnosing tuberculosis among people living with HIV. OFID. in press. [CrossRef]
- Sossen, B.; Broger, T.; Kerkhoff, A.D.; Schutz, C.; Trollip, A.; Moreau, E.; Schumacher, S.G.; Burton, R.; Ward, A.; Wilkinson, R.J.; et al. ‘SILVAMP TB LAM’ rapid urine tuberculosis test predicts mortality in hospitalized HIV patients in South Africa. Clin. Infect. Dis. Submitted.
- Kawasaki, M.; Echiverri, C.; Raymond, L.; Cadena, E.; Reside, E.; Gler, M.T.; Oda, T.; Ito, R.; Higashiyama, R.; Katsuragi, K.; et al. Lipoarabinomannan in sputum to detect bacterial load and treatment response in patients with pulmonary tuberculosis: Analytic validation and evaluation in two cohorts. PLoS Med. 2019, 16, e1002780. [Google Scholar] [CrossRef] [PubMed]
- Broger, T.; Tsionksy, M.; Mathew, A.; Lowary, T.L.; Pinter, A.; Plisova, T.; Bartlett, D.; Barbero, S.; Denkinger, C.M.; Moreau, E.; et al. Sensitive electrochemiluminescence (ECL) immunoassays for detecting lipoarabinomannan (LAM) and ESAT-6 in urine and serum from tuberculosis patients. PLoS ONE 2019, 14, e0215443. [Google Scholar] [CrossRef]
- Brock, M.; Hanlon, D.; Zhao, M.; Polluck, N.R. Detection of mycobacterial lipoarabinomannan in serum for diagnosis of active tuberculosis. Diagn. Microbiol. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Sigal, G.B.; Pinter, A.; Lowary, T.L.; Kawasaki, M.; Li, A.; Mathew, A.; Tsionsky, M.; Zheng, R.B.; Plisova, T.; Shen, K.; et al. A Novel Sensitive Immunoassay Targeting the 5-Methylthio-d-Xylofuranose–Lipoarabinomannan Epitope Meets the WHO’s Performance Target for Tuberculosis Diagnosis. J. Clin. Microbiol. 2018, 56, e01338-18. [Google Scholar] [CrossRef]
- Gupta-Wright, A.; Peters, J.A.; Flach, C.; Lawn, S.D. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: A systematic review and meta-analysis. BMC Med. 2016, 14, 53. [Google Scholar] [CrossRef]
- Wood, R.; Racow, K.; Bekker, L.-G.; Middelkoop, K.; Vogt, M.; Kreiswirth, B.N.; Lawn, S.D. Lipoarabinomannan in urine during tuberculosis treatment: Association with host and pathogen factors and mycobacteriuria. BMC Infect. Dis. 2012, 12, 47. [Google Scholar] [CrossRef]
- Hamasur, B.; Bruchfeld, J.; Haile, M.; Pawlowski, A.; Bjorvatn, B.; Källenius, G.; Svenson, S.B. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J. Microbiol. Methods 2001, 45, 41–52. [Google Scholar] [CrossRef]
- Lawn, S.D.; Kerkhoff, A.D.; Vogt, M.; Wood, R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: A descriptive study. Lancet Infect. Dis. 2012, 12, 201–209. [Google Scholar] [CrossRef]
- Nicol, M.P.; Allen, V.; Workman, L.; Isaacs, W.; Munro, J.; Pienaar, S.; Black, F.; Adonis, L.; Zemanay, W.; Ghebrekristos, Y. Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: A prospective study. Lancet Glob. Health 2014, 2, e278–e284. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis of Active Tuberculosis in People Living with HIV, 2019 Update; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Medecins Sans Frontieres; Stop TB Partnership. Out of Step 2017: TB Policies in 29 Countries. A Survey of Prevention, Testing and Treatment Policies and Practices; Medecins Sans Frontieres: Geneva, Switzerland, 2017. [Google Scholar]
- Mandavilli, A. Global Health: The World Needs a Urine Test for TB. But It’s Already Here. The New York Times, 17 December 2018. [Google Scholar]
- Nathavitharana, R.R.; Pai, M. New strategies for inpatients with HIV and tuberculosis. Lancet 2018, 392, 256–258. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Médecins Sans Frontiéres. Activists Call on Countries and Donors to Immediately Scale up Use of Life-Saving TB LAM Test. Available online: https://www.msfaccess.org/activists-call-countries-and-donors-immediately-scale-use-life-saving-tb-lam-test (accessed on 29 December 2019).
- World Health Organization (WHO). World Health Organization Model List of Essential In Vitro Diagnostics, 1st ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Stop TB Partnership. Global Drug Facility: Diagnostics Catalog August 2018. Available online: http://www.stoptb.org/assets/documents/gdf/Diagnostics_Catalog_2018_WEB.pdf (accessed on 29 December 2019).
- Maclean, E.; Pai, M. Urine Lipoarabinomannan for Tuberculosis Diagnosis: Evolution and Prospects. Clin. Chem. 2018, 64. [Google Scholar] [CrossRef]
- Paris, L.; Magni, R.; Zaidi, F.; Araujo, R.; Saini, N.; Harpole, M.; Coronel, J.; Kirwan, D.E.; Steinberg, H.; Gilman, R.H.; et al. Urine lipoarabinomannan glycan in HIV-negative patients with pulmonary tuberculosis correlates with disease severity. Sci. Transl. Med. 2017, 9, eaal2807. [Google Scholar] [CrossRef]
- Shapiro, A.E.; Wittwer, T.; Ngwane, N.W.; Magcaba, Z.P.; Mullins, B.; Aamotsbakken, R.; Berry, S.; Beebe, D.J.; Wilson, D.P.K.; Drain, P.K. A point-of-care test to concentrate and detect urine LAM for TB diagnosis: results from the first-in-human study of FLOW-TB. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 2 November 2019. [Google Scholar]
- Connelly, J.T.; Andama, A.; Grant, B.; Ball, L.; Lopez, B.B.; Hunt, V.; Ignatowicz, L.; Hamasur, B.; Cattamanchi, A.; Bell, D.; et al. Clinical Performance of Prototype Point-of-Care TB Lipoarabinomannan (LAM) Test in Uganda. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 2 November 2019. [Google Scholar]
- Hamasur, B.; Ignatowicz, L.; Ramachandraiah, H. SpeClean: Urine sample treatment method for ultra-sensitive LAM diagnostics. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 2 November 2019. [Google Scholar]
- Broger, T.; Sossen, B.; du Toit, E.; Kerkhoff, A.D.; Schutz, C.; Ivanova Reipold, E.; Ward, A.; Barr, D.A.; Macé, A.; Trollip, A.; et al. YouTube Video. Fujifilm SILVAMP TB LAM Test Procedure. Available online: https://www.youtube.com/watch?v=aK-QtzkLBug (accessed on 24 April 2019).
- Kerkhoff, A.D.; Sossen, B.; Schutz, C.; Reipold, E.I.; Trollip, A.; Moreau, E.; Schumacher, S.G.; Burton, R.; Ward, A.; Nicol, M.P.; et al. Diagnostic sensitivity of SILVAMP TB-LAM (FujiLAM) point-of-care urine assay for extra-pulmonary tuberculosis in people living with HIV. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Broger, T.; Muyoyeta, M.; Kerkhoff, A.D.; Denkinger, C.M.; Moreau, E. Tuberculosis test results using fresh versus biobanked urine samples with FujiLAM. Lancet Infect. Dis. 2020, 20, 22–23. [Google Scholar] [CrossRef]
- Lawn, S.D.; Kerkhoff, A.D.; Nicol, M.P.; Meintjes, G. Underestimation of the True Specificity of the Urine Lipoarabinomannan Point-of-Care Diagnostic Assay for HIV-Associated Tuberculosis. J. Acquir. Immune Defic. Syndr. 2015, 69, 144–146. [Google Scholar] [CrossRef]
- Denkinger, C.M.; Schumacher, S.G.; Gilpin, C.; Korobitsyn, A.; Wells, W.A.; Pai, M.; Leeflang, M.; Steingart, K.R.; Bulterys, M.; Schünemann, H.; et al. Guidance for the Evaluation of Tuberculosis Diagnostics That Meet the World Health Organization (WHO) Target Product Profiles: An Introduction to WHO Process and Study Design Principles. J. Infect. Dis. 2019, 220, S91–S98. [Google Scholar] [CrossRef]
- Drain, P.K.; Gardiner, J.L.; Hannah, H.; Broger, T.; Dheda, K.; Fielding, K.; Walzl, G.; Kaforou, M.; Kranzer, K.; Joosten, S.A.; et al. Guidance for Studies Evaluating the Accuracy of Biomarker-Based Nonsputum Tests to Diagnose Tuberculosis. J. Infect. Dis. 2019, 220, S108–S115. [Google Scholar] [CrossRef]
- Naidoo, P.; Theron, G.; Rangaka, M.X.; Chihota, V.N.; Vaughan, L.; Brey, Z.O.; Pillay, Y. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. J. Infect. Dis. 2017, 216, S702–S713. [Google Scholar] [CrossRef] [PubMed]
- Padayatchi, N.; Daftary, A.; Naidu, N.; Naidoo, K.; Pai, M. Tuberculosis: Treatment failure, or failure to treat? Lessons from India and South Africa. BMJ Glob. Health 2019, 4, e001097. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.G.; Chang, S.T.; Hannah, H. Estimating the impact of a combined C-reactive protein and LAM-based diagnostic algorithm for TB disease in HIV clinics in South Africa: A mathematical modeling-based analysis. In Proceedings of the 49th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease (the Union), The Hague, The Netherlands, 24–27 October 2018. [Google Scholar]
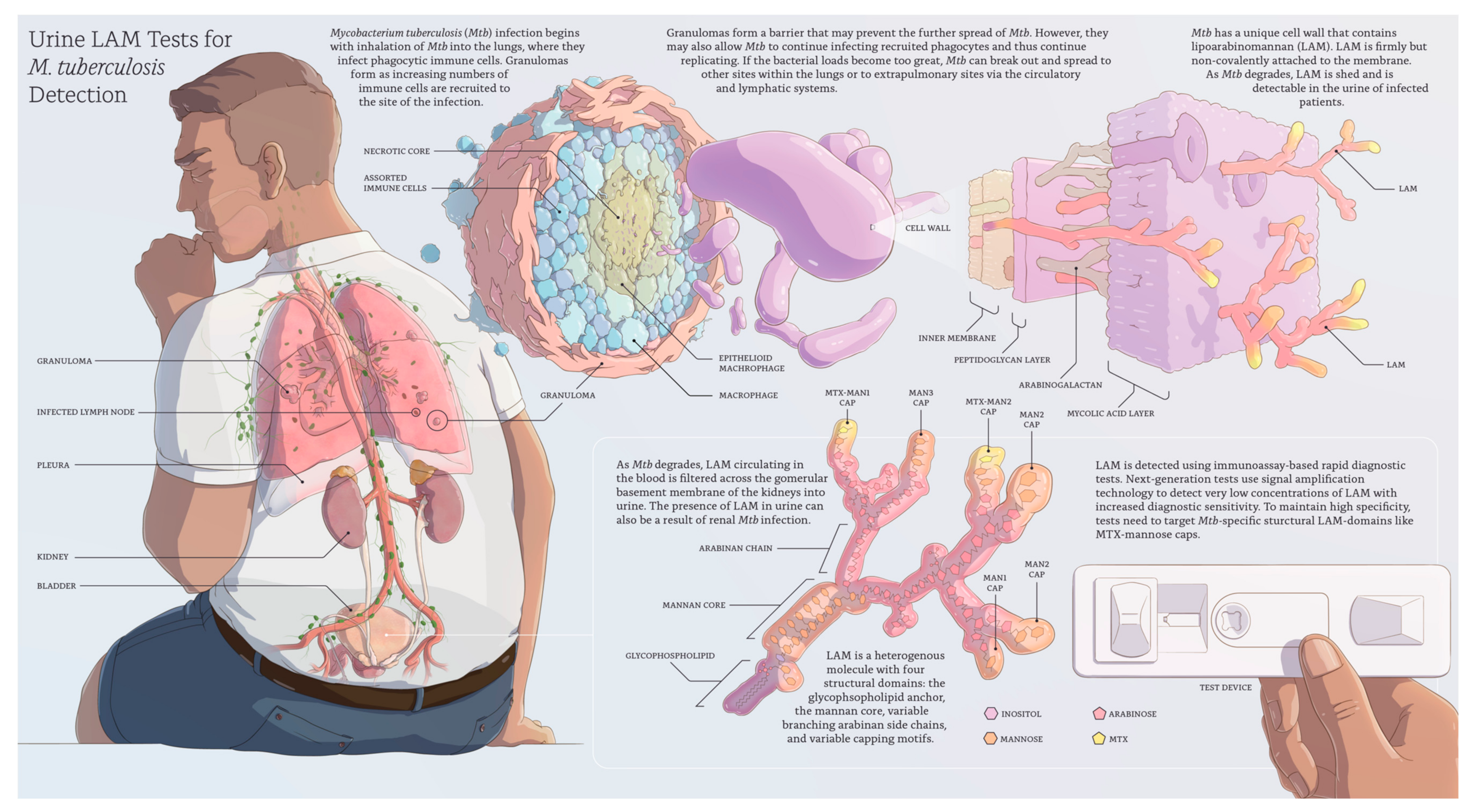
| WHO Target Product Profiles (TPPs) Minimal Criteria: “Biomarker Test” | Abbott Determine TB LAM Ag (AlereLAM) | Fujifilm SILVAMP TB LAM Assay (FujiLAM) | Sputum-Based TB Diagnosis (Smear Microscopy; Xpert MTB/RIF; Xpert MTB/RIF Ultra) | ||||
|---|---|---|---|---|---|---|---|
| Price per test (ex-works) | <US$6.00 | ✓ | US$3.00–3.50 [17] (likely cost-effective among hospitalized HIV-postive patients) | ? | Fujifilm has not yet released information on the price (initial cost-effectiveness modelling data presented [18]) | x | Xpert MTB/RIF US$9.98 [19] |
| Regulatory requirements and availability | Registered for in vitro diagnostic (IVD) use | ✓ | CE-IVD marked IVD WHO recommendation (Table 2) On the market | ? | CE-IVD marked IVD WHO evaluation expected Q4/2020 Market entry expected Q1/2021 [20] | ✓ | CE-IVD marked IVD Several WHO recommendations On the market |
| Equipment | Ideally instrument free and small, portable, or handheld, <1 kg, <US$500 instrument acceptable | ✓ | Instrument free | ✓ | Instrument free | x | US$17,500.00 [19] (GeneXpert platform plus laptop) |
| Sensitivity in HIV positive patients (independent of CD4 count) | ≥65% (not specifically defined for HIV-positives in the TPP) | x | 42% [21] | ✓ | 70.7% [22] | ✓ | 90% (Xpert MTB/RIF Ultra) [23] 77% (Xpert MTB/RIF) [23] 47% (Microscopy) [24] |
| Sensitivity in HIV negative patients | ≥65% | x | 18% [25] | ? | No information published. Studies ongoing. | ✓ | 91% (Xpert MTB/RIF Ultra) [23] 90% (Xpert MTB/RIF) [23] |
| Specificity | ≥98% | ? | 96–98% against CRS [26] (likely meeting the target as specificity might be underestimated due to limitations of the reference standard) | ? | 95.7% against CRS [27] (likely meeting the target as specificity might be underestimated due to limitations of the reference standard) | ✓ | 96% (Xpert MTB/RIF Ultra) [23] 98% (Xpert MTB/RIF) [23] 98% (Microscopy) [24] |
| Day 1 diagnostic yield in HIV-positive inpatients (TB patients diagnosed on the first day they present) | No target defined in TPP | 43.3% [27] | 64.5% [27] | 26.2% (Xpert MTB/RIF) [27] 19.1% (Microscopy) [27] | |||
| Outcome/ / mortality impact | No target defined in TPP | Mortality impact shown in hospitalized PLHIV but not in more general populations. A positive result is associated with increased risk of mortality [28,29,30] | No impact studies available. A positive result is associated with increased risk of mortality [31,32] | Unclear | |||
| Sample type | Non-sputum | ✓ | Urine | ✓ | Urine | x | Sputum |
| Time-to-result | <60 min | ✓ | 25 min | ✓ | 50–60 min | x | 100 min (Xpert MTB/RIF and Xpert MTB/RIF Ultra) |
| Number of steps | Limited number of steps, no precise measuring | ✓ | 2 steps | ✓ | 5 steps | x | Xpert MTB/RIF and Xpert MTB/RIF Ultra: 11 steps Microscopy: >10 steps |
| Setting and infrastructure needs | Primary health-care clinics with lab or microscopy center or higher levels | ✓ | Simple to use lateral flow assay | ✓ | Simple to use lateral flow assay | x | Laboratory required Electricity required Equipment susceptible to dust and shock |
| In inpatient settings, WHO strongly recommends using lateral flow urine lipoarabinomannan assay (LF-LAM) to assist in the diagnosis of active TB in HIV-positive adults, adolescents, and children:
|
In outpatient settings, WHO suggests using LF-LAM to assist in the diagnosis of active TB in HIV-positive adults, adolescents, and children:
|
In outpatient settings, WHO recommends against using LF-LAM to assist in the diagnosis of active TB in HIV-positive adults, adolescents, and children:
|
| Research and development questions | |
| 1 | What concentrations of LAM need to be detected to meet TPP sensitivity in HIV-negative populations? |
| 2 | Can sensitive, specific, low-cost, simple, and rapid platform alternatives be developed that substantially improve POC detection compared to conventional lateral-flow assays? |
| 3 | Which of the currently available antibodies yield the best performance in immunoassays? |
| 4 | What simple and POC-amenable specimen processing steps would improve the availability for detection or increase the concentration of LAM in clinical samples? |
| 5 | Are multiple molecular species of LAM present in clinical specimens, implying the need for polyclonal antibodies or multiple sets of monoclonal antibodies? |
| 6 | What purified LAM antigen preparations best mimic what is found in patient samples? |
| 7 | What is the molecular structure of LAM released from M. tuberculosis in vivo? |
| Implementation and public health impact questions | |
| Patient-related questions | |
| 8 | What is the target population and distribution? Which populations will benefit from this tool? Which populations could eventually benefit? |
| 9 | How much more impact will a next-generation test have on mortality risk reduction? |
| 10 | Can LAM tests be used to monitor treatment adherence and/or completion? |
| 11 | What are the diagnostic yields of LAM tests alone and in combination with smear microscopy or Xpert? |
| Operational and health systems questions | |
| 13 | What is the intended level of healthcare facility and user level of training required? |
| 14 | How much time is saved by using this tool versus other standard-of-care tools? |
| Policy and access questions | |
| 15 | What global institutions, technical experts, and financing organizations are considered key influencers in the global market for this product? How are we engaging with them? |
| 16 | What are the early-adopter countries that may drive product uptake and expansion? Which countries are potential new markets for next-generation LAM tests? |
| 17 | How will next-generation LAM tests integrate into current WHO guidelines and TPPs for TB detection? |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulterys, M.A.; Wagner, B.; Redard-Jacot, M.; Suresh, A.; Pollock, N.R.; Moreau, E.; Denkinger, C.M.; Drain, P.K.; Broger, T. Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update. J. Clin. Med. 2020, 9, 111. https://doi.org/10.3390/jcm9010111
Bulterys MA, Wagner B, Redard-Jacot M, Suresh A, Pollock NR, Moreau E, Denkinger CM, Drain PK, Broger T. Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update. Journal of Clinical Medicine. 2020; 9(1):111. https://doi.org/10.3390/jcm9010111
Chicago/Turabian StyleBulterys, Michelle A., Bradley Wagner, Maël Redard-Jacot, Anita Suresh, Nira R. Pollock, Emmanuel Moreau, Claudia M. Denkinger, Paul K. Drain, and Tobias Broger. 2020. "Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update" Journal of Clinical Medicine 9, no. 1: 111. https://doi.org/10.3390/jcm9010111
APA StyleBulterys, M. A., Wagner, B., Redard-Jacot, M., Suresh, A., Pollock, N. R., Moreau, E., Denkinger, C. M., Drain, P. K., & Broger, T. (2020). Point-Of-Care Urine LAM Tests for Tuberculosis Diagnosis: A Status Update. Journal of Clinical Medicine, 9(1), 111. https://doi.org/10.3390/jcm9010111