Management of Schneiderian Membrane Perforations during Sinus Augmentation Procedures: A Preliminary Comparison of Two Different Approaches
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Population
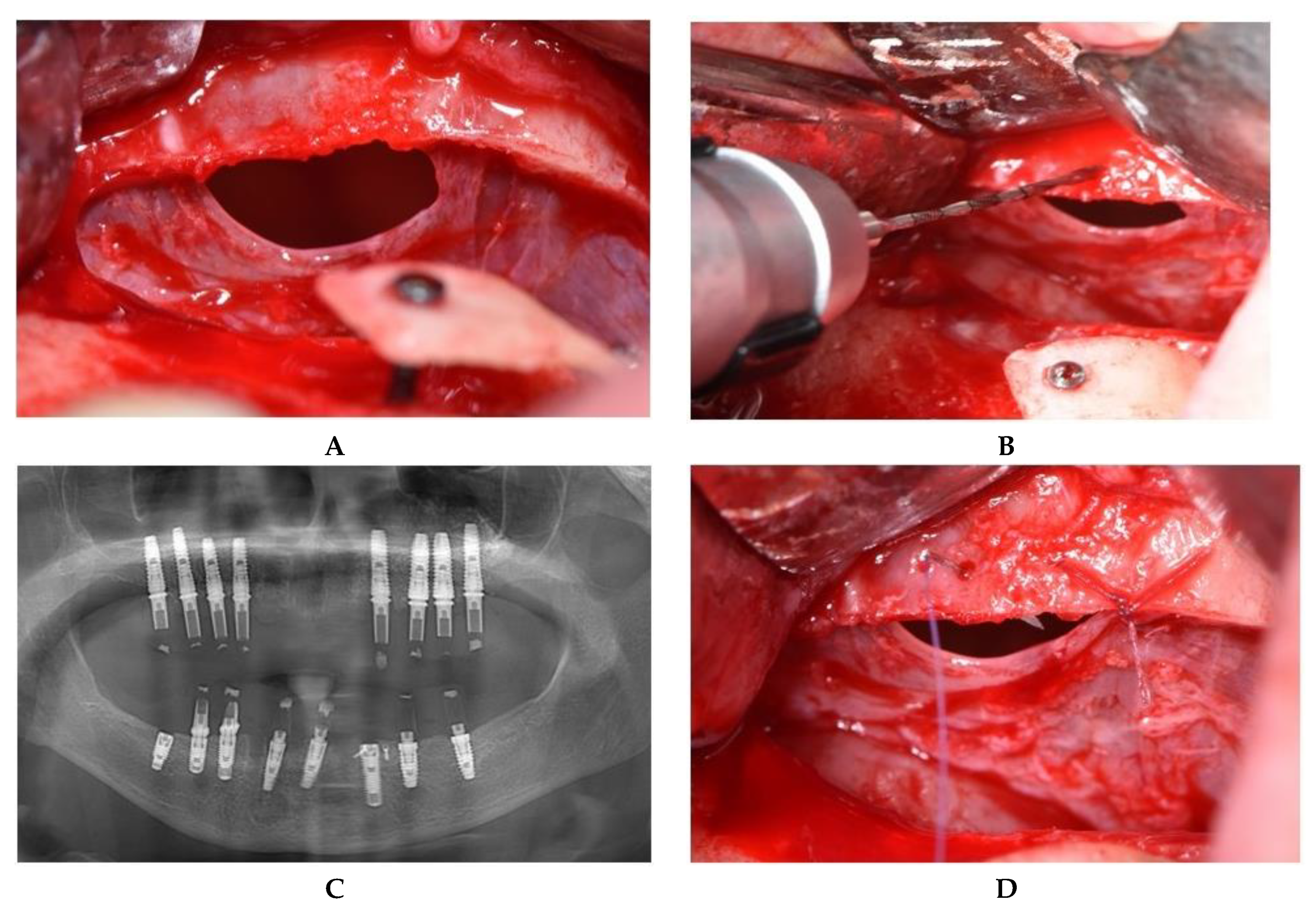
2.2. Operative Phase
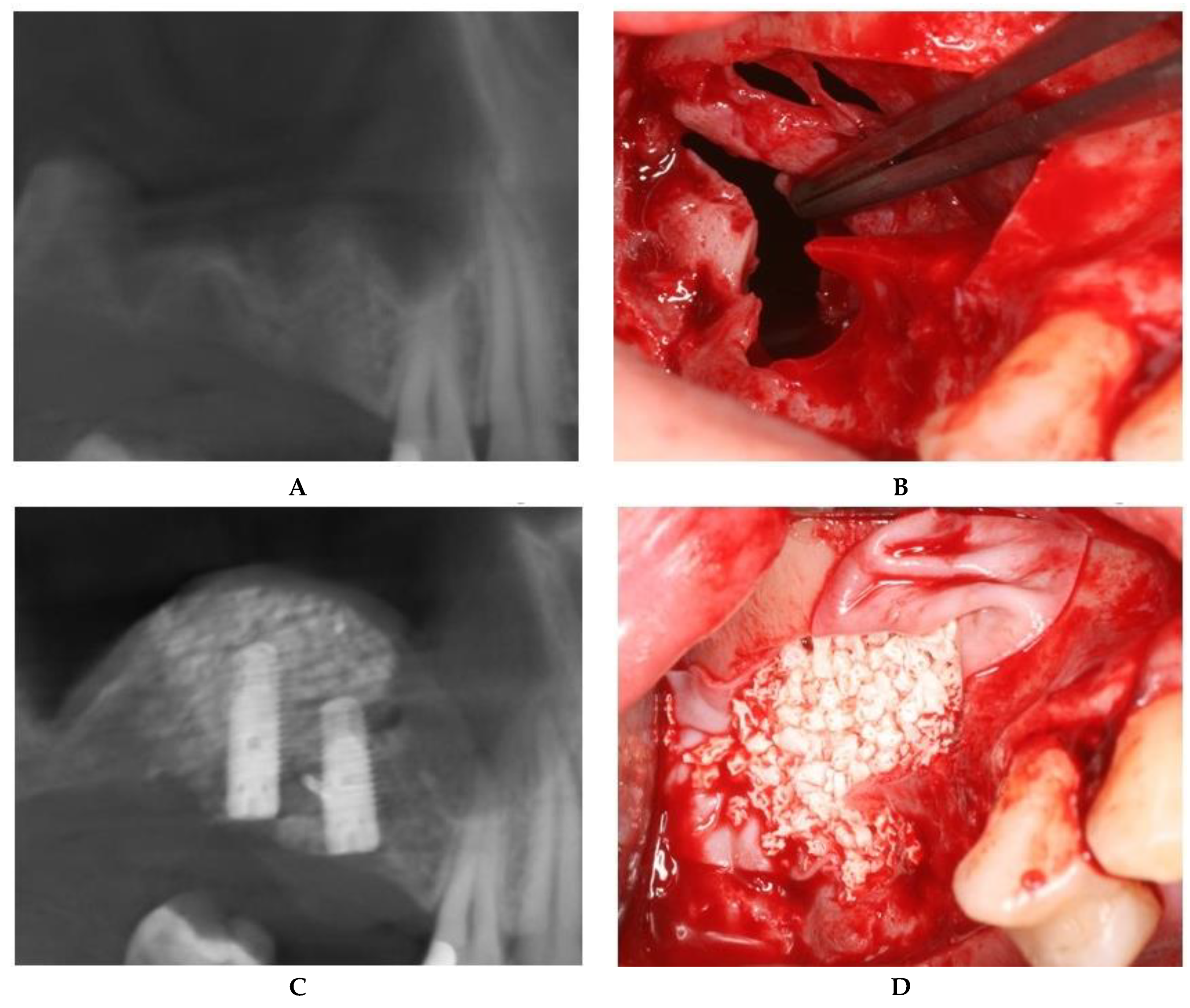
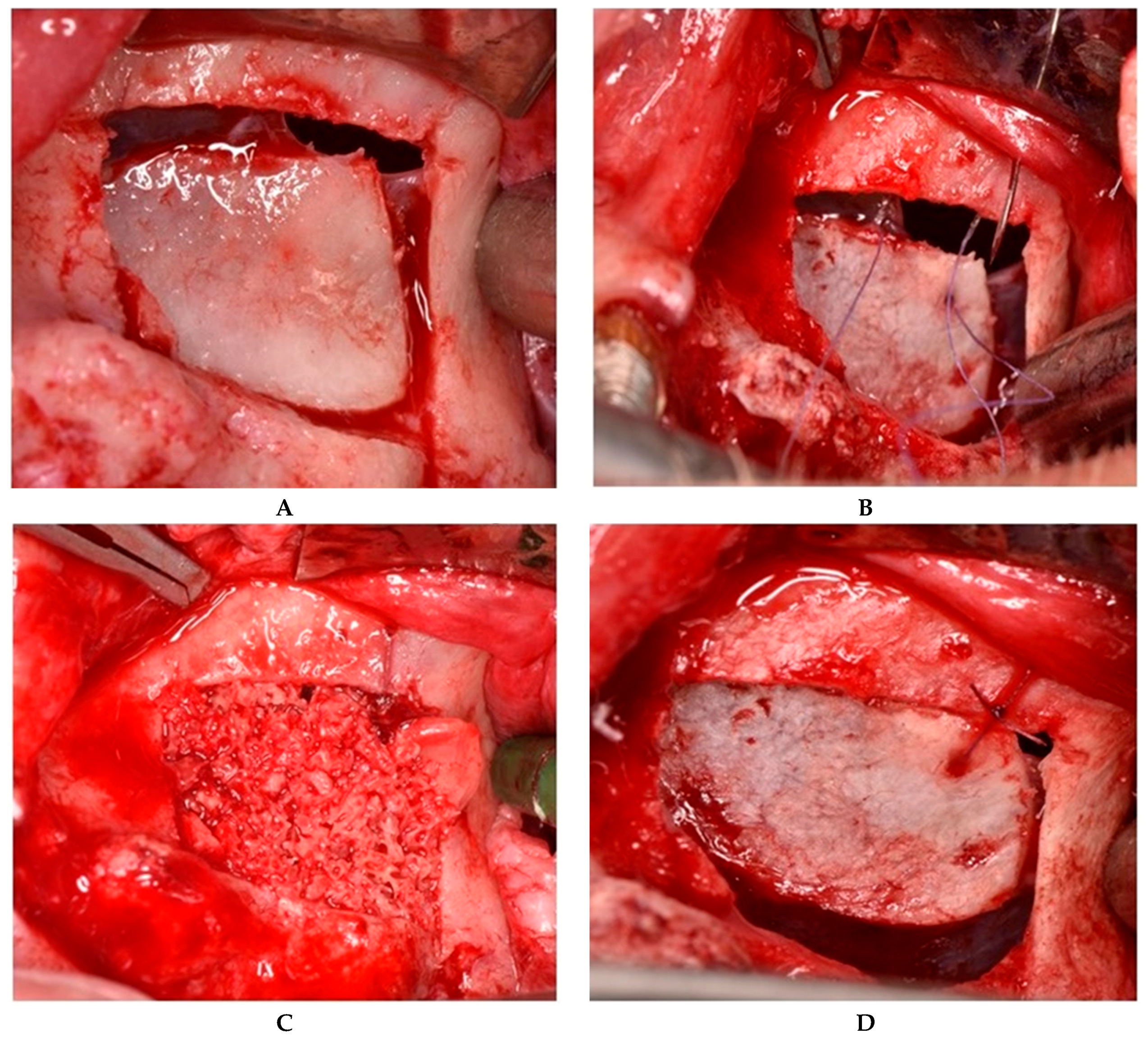
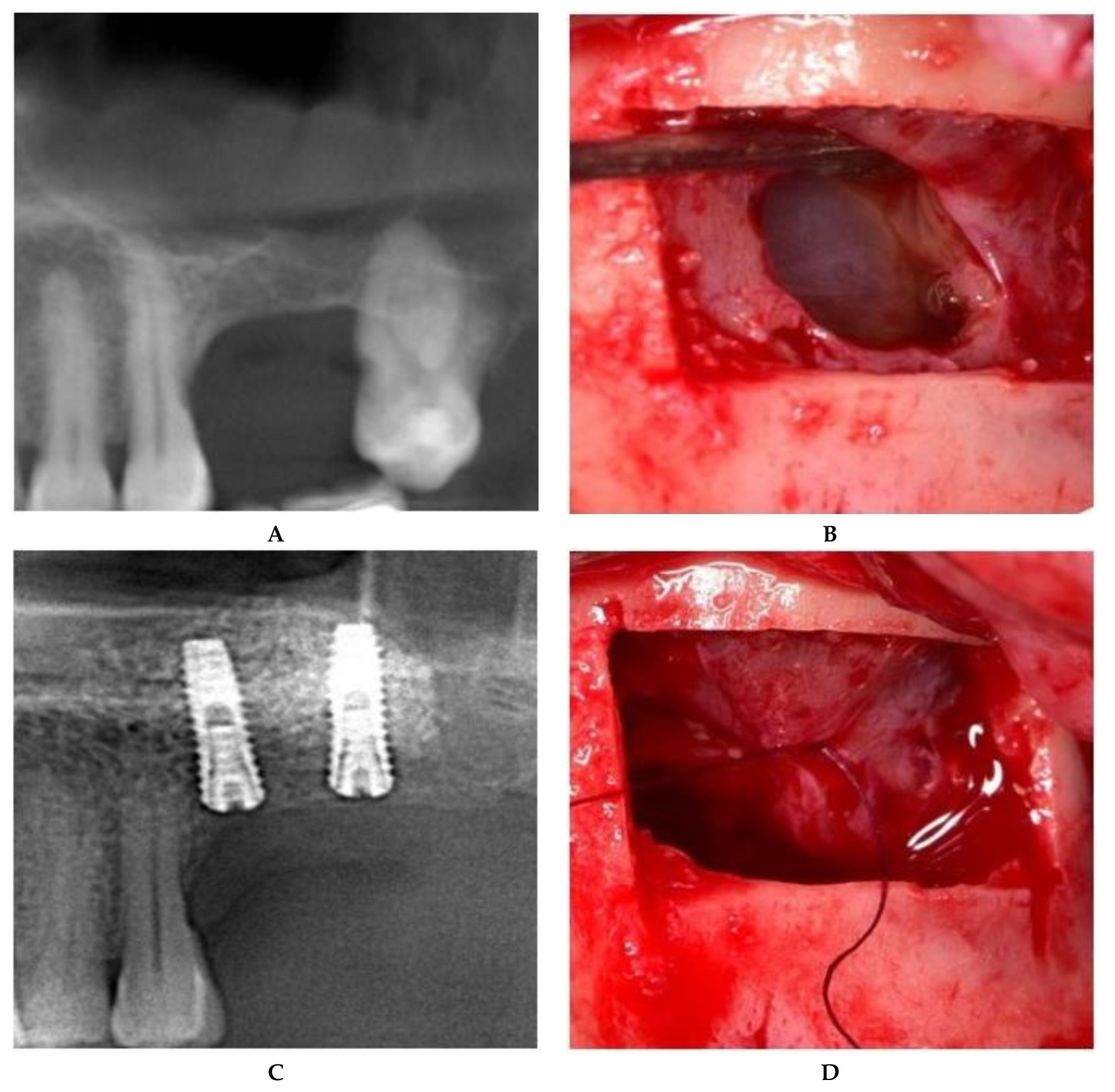
2.2.1. Sealing technique No. I—Collagen Membrane Lining
2.2.2. Sealing technique No. II—Suturing Technique
2.3. Postoperative Protocol
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Aludden, H.; Hallman, M.; Dahlin, C.; Christensen, A.E.; Mordenfeld, A. A systematic review and meta-analysis of long-term studies (five or more years) assessing maxillary sinus floor augmentation. Int. J. Oral Maxillofac. Surg. 2018, 47, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Weinstein, T.; Taschieri, S.; Wallace, S.S. Risk factors in lateral window sinus elevation surgery. Periodontol. 2000 2019, 81, 91–123. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Andolsek, F.; Berton, F.; Perinetti, G.; Navarra, C.O.; Di Lenarda, R. Intraoperative Complications during Sinus Floor Elevation with Lateral Approach: A Systematic Review. Int. J. Oral Maxillofac. Implant 2017, 32, e107–e118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gun, R.; Youssef, A.; Carrau, R.L.; Prevedello, D.M.; Otto, B.A.; Ditzel, L. Anatomical study of critical features on the posterior wall of the maxillary sinus: Clinical implications. Laryngoscope 2014, 124, 2451–2455. [Google Scholar] [CrossRef]
- Ardekian, L.; Oved-Peleg, E.; Mactei, E.E.; Peled, M. The clinical significance of sinus membrane perforation during augmentation of the maxillary sinus. J. Oral Maxillofac. Surg. 2006, 64, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Schiebel, V.; Hof, M.; Ulm, C.; Watzek, G.; Pommer, B. Risk Factors of Membrane Perforation and Postoperative Complications in Sinus Floor Elevation Surgery: Review of 407 Augmentation Procedures. J. Oral Maxillofac. Surg. 2015, 73, 1275–1282. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.; Tawse-Smith, A.; Faggion, C.M., Jr.; Duncan, W.J. Piezoelectric surgery vs rotary instruments for lateral maxillary sinus floor elevation: A systematic review and meta-analysis of intra-and postoperative complications. Int. J. Oral Maxillofac. Implant 2015, 30, 1262–1271. [Google Scholar] [CrossRef]
- Stacchi, C.; Vercellotti, T.; Toschetti, A.; Speroni, S.; Salgarello, S.; Di Lenarda, R. Intraoperative complications during sinus floor elevation using two different ultrasonic approaches: A two-center, randomized, controlled clinical trial. Clin. Implant Dent. Relat. Res. 2015, 17, e117–e125. [Google Scholar] [CrossRef]
- Wen, S.C.; Chan, H.L.; Wang, H.L. Classification and management of antral septa for maxillary sinus augmentation. Int. J. Periodontics Restor. Dent. 2013, 33, 509–517. [Google Scholar] [CrossRef]
- Kim, S.B.; Yun, P.Y.; Kim, Y.K. Clinical evaluation of sinus bone graft in patients with mucous retention cyst. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Hermes, M.; Lommen, J.; Kübler, N.R.; Lytvyniuk, I.; Singh, D.D.; Schorn, L.; Holtmann, H. Influence of Schneiderian Membrane Perforations on the Prognosis and Outcomes of Lateral Window Sinus Augmentation Operations: A Retrospective Case Series Study. J. Dent. Oral Disord. Ther. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Robiony, M.; Tenani, G.; Sbuelz, M.; Casadei, M. A simple method for repairing membrane sinus perforation. Open J. Stomatol. 2012, 2, 348–351. [Google Scholar] [CrossRef][Green Version]
- Salgado Velazquez, A.; Esteve Colomina, L. Handling Perforations of the Sinus Membrane. Inspyred: The Alternative EAO Voice. Available online: http://www.clinicaesteve.es/formacion/wp-content/uploads/2018/01/2017-Perforaciones-Membrana-sinusal-Lino-Esteve.pdf (accessed on 1 June 2019).
- Aricioglu, C.; Dolanmaz, D.; Esen, A.; Isik, K.; Avunduk, M.C. Histological evaluation of effectiveness of platelet-rich fibrin on healing of sinus membrane perforations: A preclinical animal study. J. Cranio-Maxillo-Facial Surg. 2017, 45, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Solar, P.; Geyerhofer, U.; Traxler, H.; Windisch, A.; Ulm, C.; Watzek, G. Blood supply to the maxillary sinus relevant to sinus floor elevation procedures. Clin. Oral Implant Res. 1999, 10, 34–44. [Google Scholar] [CrossRef]
- Viña-Almunia, J.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M. Influence of perforation of the sinus membrane on the survival rate of implants placed after direct sinus augmentation. Literature update. Med. Oral Patol. Oral Cir. Bucal 2009, 14, E133–E136. [Google Scholar] [PubMed]
- Proussaefs, P.; Lozada, J.; Kim, J. Effects of sealing the perforated sinus membrane with a resorbable collagen membrane: A pilot study in humans. J. Oral Implantol. 2003, 29, 235–241. [Google Scholar] [CrossRef]
- Hernández-Alfaro, F.; Torradeflot, M.M.; Marti, C. Prevalence and management of Schneiderian membrane perforations during sinus-augmentation procedures. Clin. Oral Implant Res. 2008, 19, 91–98. [Google Scholar] [CrossRef]
- Troedhan, A.; Kurrek, A.; Wainwright, M. Biological Principles and Physiology of Bone Regeneration under the Schneiderian Membrane after Sinus Augmentation Surgery: A Radiological Study in 14 Patients Treated with the Transcrestal Hydrodynamic Ultrasonic Cavitational Sinus Augmentation (Intraaugmentation). Int. J. Dent. 2012. [Google Scholar] [CrossRef]
- Scala, A.; Botticelli, D.; Faeda, R.S.; Garcia Rangel, I., Jr.; Américo de Oliveira, J.; Lang, N.P. Lack of influence of the Schneiderian membrane in forming new bone apical to implants simultaneously installed with sinus floor elevation: An experimental study in monkeys. Clin. Oral Implant Res. 2012, 23, 175–181. [Google Scholar] [CrossRef]
- Rong, Q.; Li, X.; Chen, S.L.; Zhu, S.X.; Huang, D.Y. Effect of the Schneiderian membrane on the formation of bone after lifting the floor of the maxillary sinus: An experimental study in dogs. Br. J. Oral Maxillofac. Surg. 2015, 53, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Avila, G.; Wang, H.L.; Galindo-Moreno, P.; Misch, C.E.; Bagramian, R.A.; Rudek, I.; Benavides, E.; Moreno-Riestra, I.; Braun, T.; Neiva, R. The influence of the bucco-palatal distance on sinus augmentation outcomes. J. Periodontol. 2010, 81, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Maria Soardi, C.; Spinato, S.; Zaffe, D.; Wang, H.L. Atrophic maxillary floor augmentation by mineralized human bone allograft in sinuses of different size: An histologic and histomorphometric analysis. Clin. Oral Implant Res. 2010, 22, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, T.; Stacchi, C.; Berton, F.; Traini, T.; Torelli, L.; Di Lenarda, R. Influence of Maxillary Sinus Width on New Bone Formation After Transcrestal Sinus Floor Elevation: A Proof-of-Concept Prospective Cohort Study. Implant Dent. 2017, 26, 209–216. [Google Scholar] [CrossRef]
- Stacchi, C.; Lombardi, T.; Ottonelli, R.; Berton, F.; Perinetti, G.; Traini, T. New bone formation after transcrestal sinus floor elevation was influenced by sinus cavity dimensions: A prospective histologic and histomorphometric study. Clin. Oral Implant Res. 2018, 29, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Jungner, M.; Cricchio, G.; Salata, L.A.; Sennerby, L.; Lundqvist, C.; Hultcrantz, M.; Lundgren, S. On the Early Mechanisms of Bone Formation after Maxillary Sinus Membrane Elevation: An Experimental Histological and Immunohistochemical Study. Clin. Implant Dent. Relat. Res. 2014, 17, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Botticelli, D.; Rangel Jr, I.G.; De Oliveira, J.A.; Okamoto, R.; Lang, N.P. Early healing after elevation of the maxillary sinus floor applying a lateral access: A histological study in monkeys. Clin. Oral Implant Res. 2010, 21, 1320–1326. [Google Scholar] [CrossRef]
- Rosano, G.; Taschieri, S.; Gaudy, J.F.; Weinstein, T.; Del Fabbro, M. Maxillary sinus vascular anatomy and its relation to sinus augmentation surgery. Clin. Oral Implant Res. 2011, 22, 711–715. [Google Scholar] [CrossRef]
- Wallace, S.S.; Mazor, Z.; Froum, S.J.; Cho, S.C.; Tarnow, D.P. Schneiderian membrane perforation rate during sinus elevation using piezosurgery: Clinical results of 100 consecutive cases. Int. J. Periodontics Restor. Dent. 2007, 27, 413–419. [Google Scholar]
- Vlassis, J.M.; Fugazzotto, P.A. A classification system for sinus membrane perforations during augmentation procedures with options for repair. J. Periodontol. 1999, 70, 692–699. [Google Scholar] [CrossRef]
- Black Smith Surgical. Langenbeck Retractor. Available online: https://www.blacksmithsurgical.com/gynecology-instruments/langenbeck-retractor (accessed on 1 March 2019).
- Khoury, F.; Javed, F.; Romanos, G.E. Sinus Augmentation Failure and Postoperative Infections Associated with Prophylactic Clindamycin Therapy: An Observational Case Series. Int. J. Oral Maxillofac. Implant 2018, 33, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.T.; Terheyden, H.; Steinriede, A.; Behrens, E.; Springer, I.; Wiltfang, J. Prospective observation of 41 perforations of the Schneiderian membrane during sinus floor elevation. Clin. Oral Implant Res. 2008, 19, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Wallace, S.S.; Del Fabbro, M.; Taschieri, S.L.M.; Trisi, P.; Capelli, M.; Weinstein, R.L. Repair of large sinus membrane perforations using stabilized collagen barrier membranes: Surgical techniques with histologic and radiographic evidence of success. Int. J. Periodontics Restor. Dent. 2008, 28, 9–17. [Google Scholar]
- Kim, G.S.; Lee, J.W.; Chong, J.H.; Han, J.J.; Jung, S.; Kook, M.S.; Park, H.J.; Ryu, S.Y.; Oh, H.K. Evaluation of clinical outcomes of implants placed into the maxillary sinus with a perforated sinus membrane: A retrospective study. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 50. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.C.; Son, Y.; Hong, J.Y.; Shin, S.I.; Jung, U.W.; Chung, J.H. Sinus floor elevation in sites with a perforated schneiderian membrane: What is the effect of placing a collagen membrane in a rabbit model? Clin. Oral Implant Res. 2018, 29, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Troedhan, A.; Kurrek, A.; Wainwright, M.; Schlichting, I.; Fischak-Treitl, B.; Ladentrog, M. The transcrestal hydrodynamic ultrasonic cavitational sinus augmentation: Results of a 2-year prospective multicentre study on 404 patients, 446 sinus augmentation sites and 637 inserted implants. Open J. Stomatol. 2013, 3, 471–485. [Google Scholar] [CrossRef][Green Version]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Sinus floor augmentation by a lateral approach (height of residual bone less than 5 mm in posterior area) | Implant placement performed subsequently in other clinics |
| Schneiderian membrane perforation during the procedure | Prosthetic restoration performed in other clinics |
| Preoperative and postoperative CBCT (cone beam computed tomography) (6 months after surgery) | Patients who refused to be included in the study |
| Total Surgeries | 172 |
|---|---|
| Perforations | 61 (35%) |
| Unintentional perforation | 45 (74%) |
| Intentional membrane incision | 16 (26%) |
| Schneiderian Membrane Sealing Technique | Failure | Success | Bone Density at 5 Months (Cutting Resistance) | |
|---|---|---|---|---|
| Repair by suturing | 31 (51%) | 5 (16%) | 26 (84%) | D3 |
| Repair by membrane sealing | 30 (49%) | 2 (7%) | 28 (93%) | D4 |
| Sealing Technique | Type of Perforation | Position in the Bony Window Osteotomy | Number |
|---|---|---|---|
| Collagen membrane | large (>10 mm) | center; inferior | 8 |
| medium (5–10 mm) | inferior | 22 | |
| Suture | medium (5–10 mm) | center, superior | 16 |
| small (<5 mm) | center, superior | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbu, H.M.; Iancu, S.A.; Jarjour Mirea, I.; Mignogna, M.D.; Samet, N.; Calvo-Guirado, J.L. Management of Schneiderian Membrane Perforations during Sinus Augmentation Procedures: A Preliminary Comparison of Two Different Approaches. J. Clin. Med. 2019, 8, 1491. https://doi.org/10.3390/jcm8091491
Barbu HM, Iancu SA, Jarjour Mirea I, Mignogna MD, Samet N, Calvo-Guirado JL. Management of Schneiderian Membrane Perforations during Sinus Augmentation Procedures: A Preliminary Comparison of Two Different Approaches. Journal of Clinical Medicine. 2019; 8(9):1491. https://doi.org/10.3390/jcm8091491
Chicago/Turabian StyleBarbu, Horia Mihail, Stefania Andrada Iancu, Iasmin Jarjour Mirea, Michele Davide Mignogna, Nachum Samet, and José Luis Calvo-Guirado. 2019. "Management of Schneiderian Membrane Perforations during Sinus Augmentation Procedures: A Preliminary Comparison of Two Different Approaches" Journal of Clinical Medicine 8, no. 9: 1491. https://doi.org/10.3390/jcm8091491
APA StyleBarbu, H. M., Iancu, S. A., Jarjour Mirea, I., Mignogna, M. D., Samet, N., & Calvo-Guirado, J. L. (2019). Management of Schneiderian Membrane Perforations during Sinus Augmentation Procedures: A Preliminary Comparison of Two Different Approaches. Journal of Clinical Medicine, 8(9), 1491. https://doi.org/10.3390/jcm8091491







