NK Cells as Potential Targets for Immunotherapy in Endometriosis
Abstract
1. Introduction
2. Phenotype and Function of NK Cells
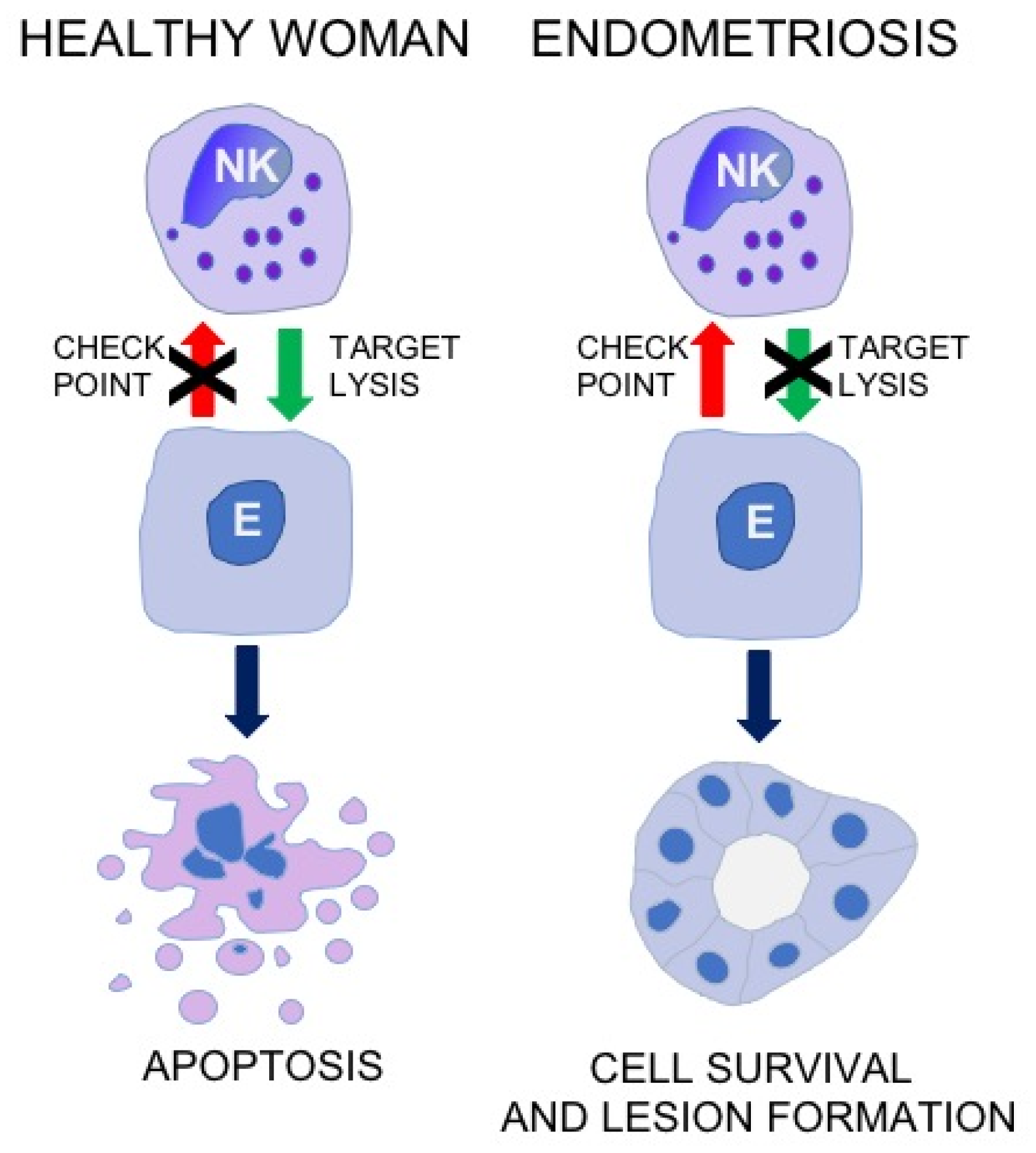
3. Cytotoxic Activity of NK Cell in Patients with Endometriosis
4. Frequency of NK Cell in Patients with Endometriosis
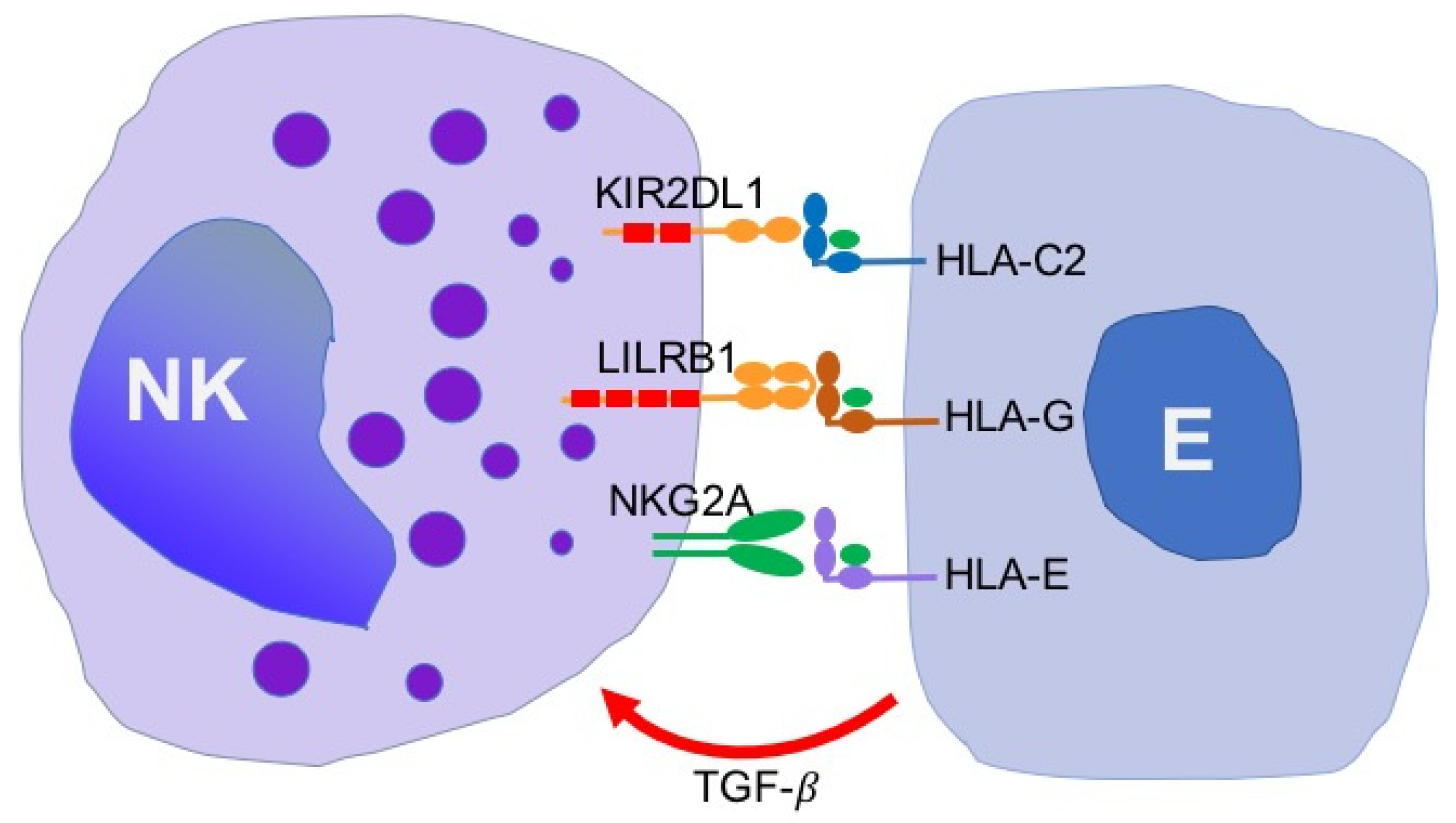
5. Expression of NK Cell Receptors and Their Ligands in Patients with Endometriosis
6. The Role of Local Factors in Regulation of NK Cell Activity in Endometriosis
7. Association of Endometriosis with Haplotypes and Polymorphisms in NK Cell Receptor Genes
8. Conclusions and Prospects for Immunotherapy
Author Contributions
Funding
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Sinaii, N.; Plumb, K.; Cotton, L.; Lambert, A.; Kennedy, S.; Zondervan, K.; Stratton, P. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil. Steril. 2008, 89, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Broi, M.G.D.; Ferriani, R.A.; Navarro, P.A. Ethiopathogenic mechanisms of endometriosis-related infertility. JBRA Assist. Reprod. 2019. [Google Scholar] [CrossRef]
- Tomassetti, C.; D’Hooghe, T. Endometriosis and infertility: Insights into the causal link and management strategies. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Baranov, V.; Malysheva, O.; Yarmolinskaya, M. Pathogenomics of Endometriosis Development. Int. J. Mol. Sci. 2018, 19, 1852. [Google Scholar] [CrossRef]
- Klemmt, P.A.B.; Starzinski-Powitz, A. Molecular and Cellular Pathogenesis of Endometriosis. Curr. Womens Health Rev. 2018, 14, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–467. [Google Scholar] [CrossRef]
- Meyer, R. Ueber den stand der frage der adenomyositis und adenomyome im allgemeinem und insbesondere ueber adenomyositis serosoepithelialis und adenomyometritis sarcomatosa. Zentralbibliothek Gynaecologie 1919, 43, 745–750. [Google Scholar]
- Fujii, S. Secondary mullerian system and endometriosis. Am. J. Obstet. Gynecol. 1991, 165, 219–225. [Google Scholar] [CrossRef]
- Gazvani, R.; Templeton, A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 2002, 123, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ulukus, M.; Arici, A. Immunology of endometriosis. Minerva Ginecol. 2005, 57, 237–248. [Google Scholar] [PubMed]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef]
- Matarese, G.; De Placido, G.; Nikas, Y.; Alviggi, C. Pathogenesis of endometriosis: Natural immunity dysfunction or autoimmune disease? Trends Mol. Med. 2003, 9, 223–228. [Google Scholar] [CrossRef]
- Eisenberg, V.H.; Zolti, M.; Soriano, D. Is there an association between autoimmunity and endometriosis? Autoimmun. Rev. 2012, 11, 806–814. [Google Scholar] [CrossRef]
- Zhang, T.; De Carolis, C.; Man, G.C.W.; Wang, C.C. The link between immunity, autoimmunity and endometriosis: A literature update. Autoimmun. Rev. 2018, 17, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Arici, A. Apoptosis and the pathogenesis of endometriosis. Semin. Reprod. Med. 2003, 21, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.M.; Petraglia, F.; Taylor, R.N. Endometriosis: Hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 2013, 19, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Lagana, A.S.; Salmeri, F.M.; Triolo, O.; Palmara, V.I.; Vitale, S.G.; Sofo, V.; Kralickova, M. Regulation of apoptotic pathways during endometriosis: From the molecular basis to the future perspectives. Arch. Gynecol. Obstet. 2016, 294, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Witz, C.A. Cell adhesion molecules and endometriosis. Semin. Reprod. Med. 2003, 21, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Osteen, K.G.; Yeaman, G.R.; Bruner-Tran, K.L. Matrix metalloproteinases and endometriosis. Semin. Reprod. Med. 2003, 21, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Balkowiec, M.; Maksym, R.B.; Wlodarski, P.K. The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (Review). Mol. Med. Rep. 2018, 18, 3123–3136. [Google Scholar] [CrossRef]
- Cousins, F.L.; O, D.F.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38. [Google Scholar] [CrossRef]
- Yang, Y.M.; Yang, W.X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Sikora, J.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Role of natural killer cell activity in the pathogenesis of endometriosis. Curr. Med. Chem. 2011, 18, 200–208. [Google Scholar] [CrossRef]
- Thiruchelvam, U.; Wingfield, M.; O’Farrelly, C. Natural Killer Cells: Key Players in Endometriosis. Am. J. Reprod. Immunol. 2015, 74, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Jeung, I.; Cheon, K.; Kim, M.R. Decreased Cytotoxicity of Peripheral and Peritoneal Natural Killer Cell in Endometriosis. Biomed. Res. Int. 2016, 2016, 2916070. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, Y.; Liu, L.L.; Lundqvist, A. Strategies to Augment Natural Killer (NK) Cell Activity against Solid Tumors. Cancers 2019, 11, 1040. [Google Scholar] [CrossRef] [PubMed]
- Beldi-Ferchiou, A.; Caillat-Zucman, S. Control of NK Cell Activation by Immune Checkpoint Molecules. Int. J. Mol. Sci. 2017, 18, 2129. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Hamerman, J.A.; Ogasawara, K.; Lanier, L.L. NK cells in innate immunity. Curr. Opin. Immunol. 2005, 17, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Walzer, T.; Dalod, M.; Robbins, S.H.; Zitvogel, L.; Vivier, E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood 2005, 106, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Takeda, K.; Kawano, M.; Takai, T.; Ishii, N.; Ogasawara, K. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 18360–18365. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Zocchi, M.R. NK cell autoreactivity and autoimmune diseases. Front. Immunol. 2014, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Benichou, G.; Yamada, Y.; Aoyama, A.; Madsen, J.C. Natural killer cells in rejection and tolerance of solid organ allografts. Curr. Opin. Organ. Transplant. 2011, 16, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Tosello-Trampont, A.; Surette, F.A.; Ewald, S.E.; Hahn, Y.S. Immunoregulatory Role of NK Cells in Tissue Inflammation and Regeneration. Front. Immunol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, L.M.; Colucci, F. Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front. Immunol. 2017, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Ollert, M.; Zimmer, J. Human CD56bright NK Cells: An Update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Das, A. Organ-specific phenotypic and functional features of NK cells in humans. Immunol. Res. 2014, 58, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, C.; Mikulak, J.; Mavilio, D. On the Way to Become a Natural Killer Cell. Front. Immunol. 2019, 10, 1812. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Lash, G.E. Uterine natural killer cells: Time for a re-appraisal? F1000Res. 2019, 8. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta. 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Lettau, M.; Paulsen, M.; Schmidt, H.; Janssen, O. Insights into the molecular regulation of FasL (CD178) biology. Eur. J. Cell Biol. 2011, 90, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Heller, N.M.; Berga-Bolanos, R.; Naler, L.; Sen, J.M. Natural Killer T (NKT) Cells in Mice and Men. In Signaling Mechanisms Regulating T. Cell Diversity and Function; Soboloff, J., Kappes, D.J., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; pp. 119–146. [Google Scholar] [CrossRef]
- Kumar, S. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology 2018, 154, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Urlaub, D.; Hofer, K.; Muller, M.L.; Watzl, C. LFA-1 Activation in NK Cells and Their Subsets: Influence of Receptors, Maturation, and Cytokine Stimulation. J. Immunol. 2017, 198, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Rajalingam, R. Diversity of Killer Cell Immunoglobulin-Like Receptors and Disease. Clin. Lab. Med. 2018, 38, 637–653. [Google Scholar] [CrossRef]
- Parham, P.; Moffett, A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat. Rev. Immunol. 2013, 13, 133–144. [Google Scholar] [CrossRef] [PubMed]
- van der Touw, W.; Chen, H.M.; Pan, P.Y.; Chen, S.H. LILRB receptor-mediated regulation of myeloid cell maturation and function. Cancer Immunol. Immunother. 2017, 66, 1079–1087. [Google Scholar] [CrossRef]
- Hudson, L.E.; Allen, R.L. Leukocyte Ig-Like Receptors - A Model for MHC Class I Disease Associations. Front. Immunol. 2016, 7, 281. [Google Scholar] [CrossRef]
- Koch, J.; Steinle, A.; Watzl, C.; Mandelboim, O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013, 34, 182–191. [Google Scholar] [CrossRef]
- Hudspeth, K.; Silva-Santos, B.; Mavilio, D. Natural cytotoxicity receptors: Broader expression patterns and functions in innate and adaptive immune cells. Front. Immunol. 2013, 4, 69. [Google Scholar] [CrossRef]
- Stojanovic, A.; Correia, M.P.; Cerwenka, A. The NKG2D/NKG2DL Axis in the Crosstalk Between Lymphoid and Myeloid Cells in Health and Disease. Front. Immunol. 2018, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Molfetta, R.; Fionda, C.; Soriani, A.; Paolini, R.; Cippitelli, M.; Cerboni, C.; Santoni, A. NKG2D and Its Ligands: “One for All, All for One”. Front. Immunol. 2018, 9, 476. [Google Scholar] [CrossRef]
- Bartel, Y.; Bauer, B.; Steinle, A. Modulation of NK cell function by genetically coupled C-type lectin-like receptor/ligand pairs encoded in the human natural killer gene complex. Front. Immunol. 2013, 4, 362. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, C.L.; Carlyle, J.R. Complexity and Diversity of the NKR-P1: Clr (Klrb1:Clec2) Recognition Systems. Front. Immunol. 2014, 5, 214. [Google Scholar] [CrossRef] [PubMed]
- Bialoszewska, A.; Malejczyk, J. Biological and Clinical Significance of Human NKRP1A/LLT1 Receptor/Ligand Interactions. Crit. Rev. Immunol. 2018, 38, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Tomasello, E.; Vivier, E.; Vely, F. Coordination of activating and inhibitory signals in natural killer cells. Mol. Immunol. 2005, 42, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Oosterlynck, D.J.; Meuleman, C.; Waer, M.; Vandeputte, M.; Koninckx, P.R. The natural killer activity of peritoneal fluid lymphocytes is decreased in women with endometriosis. Fertil. Steril. 1992, 58, 290–295. [Google Scholar] [CrossRef]
- Oosterlynck, D.J.; Cornillie, F.J.; Waer, M.; Vandeputte, M.; Koninckx, P.R. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil. Steril. 1991, 56, 45–51. [Google Scholar] [CrossRef]
- Garzetti, G.G.; Ciavattini, A.; Provinciali, M.; Muzzioli, M.; Di Stefano, G.; Fabris, N. Natural killer activity in stage III and IV endometriosis: Impaired cytotoxicity and retained lymphokine responsiveness of natural killer cells. Gynecol. Endocrinol. 1995, 9, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.J.; Hertzog, P.J.; Angus, D.; Munnery, L.; Wood, E.C.; Kola, I. Decreased natural killer cell activity in endometriosis patients: Relationship to disease pathogenesis. Fertil. Steril. 1994, 62, 1086–1088. [Google Scholar] [CrossRef]
- Wong, K.H.; Simon, J.A. In vitro effect of gonadotropin-releasing hormone agonist on natural killer cell cytolysis in women with and without endometriosis. Am J Obstet. Gynecol. 2004, 190, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, M.G.; Porpora, M.G.; Mattioli, B.; Giordani, L.; Libri, I.; Ingelido, A.M.; Cerenzia, P.; Di Felice, A.; Abballe, A.; De Felip, E.; et al. Impaired NK-cell-mediated cytotoxic activity and cytokine production in patients with endometriosis: A possible role for PCBs and DDE. Life Sci. 2006, 79, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Oosterlynck, D.J.; Lacquet, F.A.; Waer, M.; Koninckx, P.R. Lymphokine-activated killer activity in women with endometriosis. Gynecol. Obstet. Investig. 1994, 37, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.N.; Chao, K.H.; Chen, H.F.; Wu, M.Y.; Yang, Y.S.; Lee, T.Y. Peritoneal natural killer cytotoxicity and CD25+ CD3+ lymphocyte subpopulation are decreased in women with stage III-IV endometriosis. Hum. Reprod. 1995, 10, 2671–2675. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Yang, J.H.; Chao, K.H.; Hwang, J.L.; Yang, Y.S.; Ho, H.N. Increase in the expression of killer cell inhibitory receptors on peritoneal natural killer cells in women with endometriosis. Fertil. Steril. 2000, 74, 1187–1191. [Google Scholar] [CrossRef]
- Jeung, I.C.; Chung, Y.J.; Chae, B.; Kang, S.Y.; Song, J.Y.; Jo, H.H.; Lew, Y.O.; Kim, J.H.; Kim, M.R. Effect of helixor A on natural killer cell activity in endometriosis. Int. J. Med. Sci. 2015, 12, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Sendo, F.; Kawagoe, S.; Hiroi, M. Decreased natural killer cell activity in women with endometriosis. Gynecol. Obstet. Investig. 1992, 34, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Izumiya, C.; Kusum, T.; Masumoto, T.; Yamashita, C.; Yamamoto, Y.; Oguri, H.; Fukaya, T. Killer inhibitory receptor CD158a overexpression among natural killer cells in women with endometriosis is undiminished by laparoscopic surgery and gonadotropin releasing hormone agonist treatment. Am. J. Reprod. Immunol. 2004, 51, 364–372. [Google Scholar] [CrossRef]
- Vigano, P.; Vercellini, P.; Di Blasio, A.M.; Colombo, A.; Candiani, G.B.; Vignali, M. Deficient antiendometrium lymphocyte-mediated cytotoxicity in patients with endometriosis. Fertil. Steril. 1991, 56, 894–899. [Google Scholar] [CrossRef]
- Klein, E.; Ben-Bassat, H.; Neumann, H.; Ralph, P.; Zeuthen, J.; Polliack, A.; Vanky, F. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int. J. Cancer 1976, 18, 421–431. [Google Scholar] [CrossRef]
- Lisovsky, I.; Isitman, G.; Bruneau, J.; Bernard, N.F. Functional analysis of NK cell subsets activated by 721.221 and K562 HLA-null cells. J. Leukoc. Biol. 2015, 97, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Melioli, G.; Semino, C.; Semino, A.; Venturini, P.L.; Ragni, N. Recombinant interleukin-2 corrects in vitro the immunological defect of endometriosis. Am. J. Reprod. Immunol. 1993, 30, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Izumiya, C.; Oguri, H.; Kusume, T.; Yamamoto, Y.; Fukaya, T. Aberrant expression of intercellular adhesion molecule-1 and killer inhibitory receptors induces immune tolerance in women with pelvic endometriosis. Fertil. Steril. 2002, 77, 679–683. [Google Scholar] [CrossRef]
- Gagne, D.; Rivard, M.; Page, M.; Shazand, K.; Hugo, P.; Gosselin, D. Blood leukocyte subsets are modulated in patients with endometriosis. Fertil. Steril. 2003, 80, 43–53. [Google Scholar] [CrossRef]
- Matsuoka, S.; Maeda, N.; Izumiya, C.; Yamashita, C.; Nishimori, Y.; Fukaya, T. Expression of inhibitory-motif killer immunoglobulin-like receptor, KIR2DL1, is increased in natural killer cells from women with pelvic endometriosis. Am. J. Reprod. Immunol. 2005, 53, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maeda, N.; Izumiya, C.; Yamamoto, Y.; Kusume, T.; Oguri, H.; Yamashita, C.; Nishimori, Y.; Hayashi, K.; Luo, J.; et al. Killer immunoglobulin-like receptor and human leukocyte antigen expression as immunodiagnostic parameters for pelvic endometriosis. Am. J. Reprod. Immunol. 2006, 55, 106–114. [Google Scholar] [CrossRef]
- Galandrini, R.; Porpora, M.G.; Stoppacciaro, A.; Micucci, F.; Capuano, C.; Tassi, I.; Di Felice, A.; Benedetti-Panici, P.; Santoni, A. Increased frequency of human leukocyte antigen-E inhibitory receptor CD94/NKG2A-expressing peritoneal natural killer cells in patients with endometriosis. Fertil. Steril. 2008, 89, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Ishikawa, N.; Hirata, J.; Imaizumi, E.; Sasa, H.; Nagata, I. Changes of peripheral blood lymphocyte subsets before and after operation of patients with endometriosis. Acta Obstet. Gynecol. Scand. 1993, 72, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Szyllo, K.; Tchorzewski, H.; Banasik, M.; Glowacka, E.; Lewkowicz, P.; Kamer-Bartosinska, A. The involvement of T lymphocytes in the pathogenesis of endometriotic tissues overgrowth in women with endometriosis. Mediators Inflamm. 2003, 12, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.A., Jr.; Podgaec, S.; de Oliveira, R.M.; Carnevale Marin, M.L.; Baracat, E.C.; Abrao, M.S. Patients with endometriosis of the rectosigmoid have a higher percentage of natural killer cells in peripheral blood. J. Minim. Invasive Gynecol. 2012, 19, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Oosterlynck, D.J.; Meuleman, C.; Lacquet, F.A.; Waer, M.; Koninckx, P.R. Flow cytometry analysis of lymphocyte subpopulations in peritoneal fluid of women with endometriosis. Am. J. Reprod. Immunol. 1994, 31, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Tariverdian, N.; Siedentopf, F.; Rucke, M.; Blois, S.M.; Klapp, B.F.; Kentenich, H.; Arck, P.C. Intraperitoneal immune cell status in infertile women with and without endometriosis. J. Reprod. Immunol. 2009, 80, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Jeung, I.C.; Park, A.; Park, Y.J.; Jung, H.; Kim, T.D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Klentzeris, L.D.; Bulmer, J.N.; Liu, D.T.; Morrison, L. Endometrial leukocyte subpopulations in women with endometriosis. Eur J. Obstet. Gynecol. Reprod. Biol. 1995, 63, 41–47. [Google Scholar] [CrossRef]
- Giuliani, E.; Parkin, K.L.; Lessey, B.A.; Young, S.L.; Fazleabas, A.T. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am. J. Reprod. Immunol. 2014, 72, 262–269. [Google Scholar] [CrossRef]
- Witz, C.A.; Montoya, I.A.; Dey, T.D.; Schenken, R.S. Characterization of lymphocyte subpopulations and T cell activation in endometriosis. Am. J. Reprod. Immunol. 1994, 32, 173–179. [Google Scholar] [CrossRef]
- Drury, J.A.; Parkin, K.L.; Coyne, L.; Giuliani, E.; Fazleabas, A.T.; Hapangama, D.K. The dynamic changes in the number of uterine natural killer cells are specific to the eutopic but not to the ectopic endometrium in women and in a baboon model of endometriosis. Reprod. Biol. Endocrinol. 2018, 16, 67. [Google Scholar] [CrossRef]
- Maeda, N.; Izumiya, C.; Yamamoto, Y.; Oguri, H.; Kusume, T.; Fukaya, T. Increased killer inhibitory receptor KIR2DL1 expression among natural killer cells in women with pelvic endometriosis. Fertil. Steril. 2002, 77, 297–302. [Google Scholar] [CrossRef]
- Kawashima, M.; Maeda, N.; Adachi, Y.; Takeuchi, T.; Yamamoto, Y.; Izumiya, C.; Hayashi, K.; Furihata, M.; Udaka, K.; Fukaya, T. Human leukocyte antigen-G, a ligand for the natural killer receptor KIR2DL4, is expressed by eutopic endometrium only in the menstrual phase. Fertil. Steril. 2009, 91, 343–349. [Google Scholar] [CrossRef]
- Rajagopalan, S. Endosomal signaling and a novel pathway defined by the natural killer receptor KIR2DL4 (CD158d). Traffic 2010, 11, 1381–1390. [Google Scholar] [CrossRef]
- Vernet-Tomas Mdel, M.; Perez-Ares, C.T.; Verdu, N.; Molinero, J.L.; Fernandez-Figueras, M.T.; Carreras, R. The endometria of patients with endometriosis show higher expression of class I human leukocyte antigen than the endometria of healthy women. Fertil. Steril. 2006, 85, 78–83. [Google Scholar] [CrossRef]
- De Placido, G.; Alviggi, C.; Di Palma, G.; Carravetta, C.; Matarese, G.; Landino, G.; Racioppi, L. Serum concentrations of soluble human leukocyte class I antigens and of the soluble intercellular adhesion molecule-1 in endometriosis: Relationship with stage and non-pigmented peritoneal lesions. Hum. Reprod. 1998, 13, 3206–3210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matalliotakis, I.M.; Athanassakis, I.; Goumenou, A.G.; Neonaki, M.A.; Koumantakis, E.E.; Vassiliadis, S.; Koumantakis, E.E. The possible anti-inflammatory role of circulating human leukocyte antigen levels in women with endometriosis after treatment with danazol and leuprorelin acetate depot. Mediators Inflamm. 2001, 10, 75–80. [Google Scholar] [CrossRef]
- Kuroki, K.; Maenaka, K. Immune modulation of HLA-G dimer in maternal-fetal interface. Eur. J. Immunol. 2007, 37, 1727–1729. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Colucci, F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol. Rev. 2015, 267, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Rebmann, V.; Switala, M.; Eue, I.; Grosse-Wilde, H. Soluble HLA-G is an independent factor for the prediction of pregnancy outcome after ART: A German multi-centre study. Hum. Reprod. 2010, 25, 1691–1698. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hornung, D.; Fujii, E.; Lim, K.H.; Vigne, J.L.; McMaster, M.T.; Taylor, R.N. Histocompatibility leukocyte antigen-G is not expressed by endometriosis or endometrial tissue. Fertil. Steril. 2001, 75, 814–817. [Google Scholar] [CrossRef]
- Barrier, B.F.; Kendall, B.S.; Ryan, C.E.; Sharpe-Timms, K.L. HLA-G is expressed by the glandular epithelium of peritoneal endometriosis but not in eutopic endometrium. Hum. Reprod. 2006, 21, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wen, Z.; Li, H.; Yang, Z.; Zhao, X.; Yao, X. Human leukocyte antigen-G is expressed by the eutopic and ectopic endometrium of adenomyosis. Fertil. Steril. 2008, 90, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Rached, M.R.; Coelho, V.; Marin, M.L.C.; Pincerato, K.; Fujita, A.; Kalil, J.E.; Abrao, M.S. HLA-G is upregulated in advanced endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 235, 36–41. [Google Scholar] [CrossRef]
- Eidukaite, A.; Tamosiunas, V. Soluble HLA-G in the peritoneal fluid of women with endometriosis. Fertil. Steril. 2008, 89, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Botet, M.; Llano, M.; Navarro, F.; Bellon, T. NK cell recognition of non-classical HLA class I molecules. Semin. Immunol. 2000, 12, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Du, Y.; Liu, X. Platelet-derived TGF-beta1 mediates the down-modulation of NKG2D expression and may be responsible for impaired natural killer (NK) cytotoxicity in women with endometriosis. Hum. Reprod. 2016, 31, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Foruria, I.; Santulli, P.; Chouzenoux, S.; Carmona, F.; Batteux, F.; Chapron, C. Soluble ligands for the NKG2D receptor are released during endometriosis and correlate with disease severity. PLoS ONE 2015, 10, e0119961. [Google Scholar] [CrossRef] [PubMed]
- Bialoszewska, A.; Olkowska-Truchanowicz, J.; Bocian, K.; Osiecka-Iwan, A.; Czop, A.; Kieda, C.; Malejczyk, J. A Role of NKR-P1A (CD161) and Lectin-like Transcript 1 in Natural Cytotoxicity against Human Articular Chondrocytes. J. Immunol. 2018, 200, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Walankiewicz, M.; Grywalska, E.; Polak, G.; Korona-Glowniak, I.; Witt, E.; Surdacka, A.; Kotarski, J.; Rolinski, J. The Increase of Circulating PD-1- and PD-L1-Expressing Lymphocytes in Endometriosis: Correlation with Clinical and Laboratory Parameters. Mediators Inflamm. 2018, 2018, 7041342. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lv, C.; Su, Y.; Li, C.; Zhang, H.; Zhao, X.; Li, M. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol. Endocrinol. 2019, 35, 251–256. [Google Scholar] [CrossRef]
- Vigano, P.; Somigliana, E.; Di Blasio, A.M.; Cozzolino, S.; Candiani, M.; Vignali, M. Suppression of natural killer cell function and production of soluble ICAM-1: Endometrial stroma versus melanoma. Am. J. Reprod. Immunol. 2001, 46, 342–348. [Google Scholar] [CrossRef]
- Somigliana, E.; Vigano, P.; Gaffuri, B.; Guarneri, D.; Busacca, M.; Vignali, M. Human endometrial stromal cells as a source of soluble intercellular adhesion molecule (ICAM)-1 molecules. Hum. Reprod. 1996, 11, 1190–1194. [Google Scholar] [CrossRef]
- Fukaya, T.; Sugawara, J.; Yoshida, H.; Murakami, T.; Yajima, A. Intercellular adhesion molecule-1 and hepatocyte growth factor in human endometriosis: Original investigation and a review of literature. Gynecol. Obstet. Investig. 1999, 47 (Suppl. 1), 11–16, discussion 16–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Yang, H.L.; Shao, J.; Mei, J.; Chang, K.K.; Zhu, R.; Li, M.Q. Anti-inflammatory cytokines in endometriosis. Cell Mol. Life Sci. 2019, 76, 2111–2132. [Google Scholar] [CrossRef] [PubMed]
- Oosterlynck, D.J.; Meuleman, C.; Waer, M.; Koninckx, P.R.; Vandeputte, M. Immunosuppressive activity of peritoneal fluid in women with endometriosis. Obstet. Gynecol. 1993, 82, 206–212. [Google Scholar] [PubMed]
- Somigliana, E.; Vigano, P.; Gaffuri, B.; Candiani, M.; Busacca, M.; Di Blasio, A.M.; Vignali, M. Modulation of NK cell lytic function by endometrial secretory factors: Potential role in endometriosis. Am. J. Reprod. Immunol. 1996, 36, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Wang, H.S.; Kariya, M.; Mori, T. Suppression of natural killer cell activity by sera from patients with endometriosis. Am. J. Obstet. Gynecol. 1992, 167, 257–261. [Google Scholar] [CrossRef]
- Hirata, J.; Kikuchi, Y.; Imaizumi, E.; Tode, T.; Nagata, I. Endometriotic tissues produce immunosuppressive factors. Gynecol. Obstet. Investig. 1994, 37, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Smycz-Kubanska, M.; Mielczarek-Palacz, A.; Bednarek, I.; Kondera-Anasz, Z. The involvement of multifunctional TGF-beta and related cytokines in pathogenesis of endometriosis. Immunol. Lett. 2018, 201, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bellelis, P.; Frediani Barbeiro, D.; Gueuvoghlanian-Silva, B.Y.; Kalil, J.; Abrao, M.S.; Podgaec, S. Interleukin-15 and Interleukin-7 are the Major Cytokines to Maintain Endometriosis. Gynecol. Obstet. Investig. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Sun, H.T.; Zhang, Z.F.; Shi, R.X.; Liu, L.B.; Shang, W.Q.; Wei, C.Y.; Chang, K.K.; Shao, J.; Wang, M.Y.; et al. IL15 promotes growth and invasion of endometrial stromal cells and inhibits killing activity of NK cells in endometriosis. Reproduction 2016, 152, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.A.; Krutmann, J.; Kirnbauer, R.; Urbanski, A.; Schwarz, T.; Klappacher, G.; Kock, A.; Micksche, M.; Malejczyk, J.; Schauer, E.; et al. IFN-beta 2/IL-6 augments the activity of human natural killer cells. J. Immunol. 1989, 143, 1206–1209. [Google Scholar] [PubMed]
- Malejczyk, J.; Malejczyk, M.; Urbanski, A.; Kock, A.; Jablonska, S.; Orth, G.; Luger, T.A. Constitutive release of IL6 by human papillomavirus type 16 (HPV16)-harboring keratinocytes: A mechanism augmenting the NK-cell-mediated lysis of HPV-bearing neoplastic cells. Cell Immunol. 1991, 136, 155–164. [Google Scholar] [CrossRef]
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, D.; Vigano, P.; Di Blasio, A.M.; Sinigaglia, F.; Vignali, M.; Panina-Bordignon, P. Interleukin-12 and its free p40 subunit regulate immune recognition of endometrial cells: Potential role in endometriosis. J. Clin. Endocrinol. Metab. 1998, 83, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Konjevic, G.M.; Vuletic, A.M.; Mirjacic Martinovic, K.M.; Larsen, A.K.; Jurisic, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Martin, M.P.; Carrington, M. The Yin and Yang of HLA and KIR in human disease. Semin. Immunol. 2008, 20, 343–352. [Google Scholar] [CrossRef]
- Manser, A.R.; Weinhold, S.; Uhrberg, M. Human KIR repertoires: Shaped by genetic diversity and evolution. Immunol. Rev. 2015, 267, 178–196. [Google Scholar] [CrossRef]
- Kitawaki, J.; Xu, B.; Ishihara, H.; Fukui, M.; Hasegawa, G.; Nakamura, N.; Mizuno, S.; Ohta, M.; Obayashi, H.; Honjo, H. Association of killer cell immunoglobulin-like receptor genotypes with susceptibility to endometriosis. Am. J. Reprod. Immunol. 2007, 58, 481–486. [Google Scholar] [CrossRef]
- Nowak, I.; Majorczyk, E.; Wisniewski, A.; Pawlik, A.; Magott-Procelewska, M.; Passowicz-Muszynska, E.; Malejczyk, J.; Ploski, R.; Giebel, S.; Barcz, E.; et al. Does the KIR2DS5 gene protect from some human diseases? PLoS ONE 2010, 5, e12381. [Google Scholar] [CrossRef]
- Nowak, I.; Ploski, R.; Barcz, E.; Dziunycz, P.; Kaminski, P.; Kostrzewa, G.; Milewski, L.; Roszkowski, P.I.; Senitzer, D.; Malejczyk, J.; et al. KIR2DS5 in the presence of HLA-C C2 protects against endometriosis. Immunogenetics 2015, 67, 203–209. [Google Scholar] [CrossRef]
- Bylinska, A.; Wilczynska, K.; Malejczyk, J.; Milewski, L.; Wagner, M.; Jasek, M.; Niepieklo-Miniewska, W.; Wisniewski, A.; Ploski, R.; Barcz, E.; et al. The impact of HLA-G, LILRB1 and LILRB2 gene polymorphisms on susceptibility to and severity of endometriosis. Mol. Genet. Genom. 2018, 293, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Kitawaki, J.; Obayashi, H.; Kado, N.; Ishihara, H.; Koshiba, H.; Maruya, E.; Saji, H.; Ohta, M.; Hasegawa, G.; Nakamura, N.; et al. Association of HLA class I and class II alleles with susceptibility to endometriosis. Hum. Immunol. 2002, 63, 1033–1038. [Google Scholar] [CrossRef]
- Moen, M.; Bratlie, A.; Moen, T. Distribution of HLA-antigens among patients with endometriosis. Acta. Obstet. Gynecol. Scand. Suppl. 1984, 123, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Malinak, L.R.; Elias, S.; Carson, S.A.; Radvany, R.A. HLA associations in endometriosis. Am. J. Obstet. Gynecol. 1984, 148, 395–397. [Google Scholar] [CrossRef]
- Memon, H.; Patel, B.M. Immune checkpoint inhibitors in non-small cell lung cancer: A bird’s eye view. Life Sci. 2019, 116713. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, K. Targeting Immune Signaling Checkpoints in Acute Myeloid Leukemia. J. Clin. Med. 2019, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Rosenberg, S.A. Results of clinical trials with the administration of interleukin 2 and adoptive immunotherapy with activated cells in patients with cancer. Immunobiology 1986, 172, 420–437. [Google Scholar] [CrossRef]
- Velasco, I.; Quereda, F.; Bermejo, R.; Campos, A.; Acien, P. Intraperitoneal recombinant interleukin-2 activates leukocytes in rat endometriosis. J. Reprod. Immunol. 2007, 74, 124–132. [Google Scholar] [CrossRef]
- Afolabi, L.O.; Adeshakin, A.O.; Sani, M.M.; Bi, J.; Wan, X. Genetic Reprogramming For NK cell Cancer Immunotherapy with CRISPR/Cas9. Immunology 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dou, M.; Ma, Q.; Yao, R.; Liu, J. Chimeric antigen receptor (CAR)-modified NK cells against cancer: Opportunities and challenges. Int. Immunopharmacol. 2019, 74, 105695. [Google Scholar] [CrossRef] [PubMed]
- Kloess, S.; Kretschmer, A.; Stahl, L.; Fricke, S.; Koehl, U. CAR-Expressing Natural Killer Cells for Cancer Retargeting. Transfus. Med. Hemother. 2019, 46, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Goppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Sosa, A.; Lopez Cadena, E.; Simon Olive, C.; Karachaliou, N.; Rosell, R. Clinical assessment of immune-related adverse events. Ther. Adv. Med. Oncol. 2018, 10, 1758835918764628. [Google Scholar] [CrossRef] [PubMed]
| Receptor Family | Inhibitory Natural Killer (NK) Cell Receptors | Ligands |
|---|---|---|
| Killer immunoglobulin-like receptors (KIR) | KIR2DL1 | Human leukocyte antigen (HLA)-C2 group |
| KIR2DL2/3 | HLA-C1 group | |
| KIR3DL1 | HLA-Bw4 serotypes | |
| KIR3DL2 | HLA-A3/11 serotype | |
| Leucocyte immunoglobulin-like receptors (LILR) | LILRB1 | HLA-A, -B, -C, -G |
| Cluster of differentiation (CD) 94/Natural killer G2 (NKG2) | NKG2A | HLA-E |
| Natural killer receptor P1 (NKRP1) | NKRP1A | Lectin-like transcript 1 (LLT1) |
| Programmed death 1 (PD-1) | Programmed death ligand 1 (PDL-1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścieżyńska, A.; Komorowski, M.; Soszyńska, M.; Malejczyk, J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J. Clin. Med. 2019, 8, 1468. https://doi.org/10.3390/jcm8091468
Ścieżyńska A, Komorowski M, Soszyńska M, Malejczyk J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. Journal of Clinical Medicine. 2019; 8(9):1468. https://doi.org/10.3390/jcm8091468
Chicago/Turabian StyleŚcieżyńska, Aneta, Michał Komorowski, Marta Soszyńska, and Jacek Malejczyk. 2019. "NK Cells as Potential Targets for Immunotherapy in Endometriosis" Journal of Clinical Medicine 8, no. 9: 1468. https://doi.org/10.3390/jcm8091468
APA StyleŚcieżyńska, A., Komorowski, M., Soszyńska, M., & Malejczyk, J. (2019). NK Cells as Potential Targets for Immunotherapy in Endometriosis. Journal of Clinical Medicine, 8(9), 1468. https://doi.org/10.3390/jcm8091468





